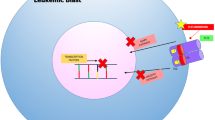
Abstract
Motixafortide (APHEXDATM) is a selective C-X-C motif chemokine receptor 4 (CXCR4) inhibitor being developed by BioLineRx under licence from Biokine Therapeutics for the mobilization of haematopoietic stem cells (HSCs) and the treatment of various cancers. On 11 September 2023, motixafortide was approved in the USA for use in combination with filgrastim [granulocyte colony stimulating factor (G-CSF)] to mobilize HSCs to the peripheral blood for collection and subsequent autologous transplantation in patients with multiple myeloma. Motixafortide has been granted Orphan Drug Designation for the treatment of pancreatic cancer in the EU and the USA, and for the treatment of acute myeloid leukaemia in the USA. Clinical development is ongoing for the mobilization of CD34+ HSCs for gene therapy in patients with sickle cell disease. This article summarizes the milestones in the development of motixafortide leading to this first approval.
Similar content being viewed by others
Change history
07 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40265-023-01984-4
References
Parrondo RD, Ailawadhi S, Sher T, et al. Autologous stem-cell transplantation for multiple myeloma in the era of novel therapies. JCO Oncol Pract. 2020;16(2):56–66.
Giralt S, Costa L, Schriber J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295–308.
Crees ZD, Rettig MP, DiPersio JF. Innovations in hematopoietic stem-cell mobilization: a review of the novel CXCR4 inhibitor motixafortide. Ther Adv Hematol. 2023;14:1–13.
Crees ZD, Rettig MP, Jayasinghe RG, et al. Motixafortide and G-CSF to mobilize hematopoietic stem cells for autologous transplantation in multiple myeloma: a randomized phase 3 trial. Nat Med. 2023;29(4):869–79.
Ladikou EE, Chevassut T, Pepper CJ, et al. Dissecting the role of the CXCL12/CXCR4 axis in acute myeloid leukaemia. Br J Haematol. 2020;189(5):815–25.
Beider K, Begin M, Abraham M, et al. CXCR4 antagonist 4F-benzoyl-TN14003 inhibits leukemia and multiple myeloma tumor growth. Exp Hematol. 2011;39(3):282–92.
BioLineRx Ltd. APHEXDATM (motixafortide) for injection, for subcutaneous use: US prescribing information. 2023. https://www.aphexda.com/files/prescribing-information.pdf. Accessed 13 Sept 2023.
BioLineRx Ltd. BioLineRx announces FDA approval of APHEXDA™ (motixafortide) in combination with filgrastim (G-CSF) to mobilize hematopoietic stem cells for collection and subsequent autologous transplantation in patients with multiple myeloma [media release]. 11 Sep 2023. https://biolinerx.com/.
BioLineRx Ltd. BioLineRx receives Orphan Drug Designation for motixafortide (BL-8040) for the treatment of pancreatic cancer in Europe [media release]. 14 Jan 2020. http://www.biolinerx.com.
BioLineRx Ltd. BioLineRx receives Orphan Drug Designation from the FDA for its lead therapeutic candidate BL-8040 for the treatment of pancreatic cancer [media release]. 4 Feb 2019. http://www.biolinerx.com.
BioLineRx Ltd. BioLineRx's BL-8040 receives Orphan Drug Designation for treatment of AML [media release]. 9 Sep 2013. http://www.biolinerx.com.
BioLineRx Ltd. BioLineRx pipeline. 2023. https://biolinerx.com/pipeline/. Accessed 5 Oct 2023.
Biokine Therapeutics Ltd. Biokine gets $1.2M to kick off phase I/II trial with CXCR4 drug [media release]. 17 Mar 2009. http://it.tmcnet.com.
BioLineRx Ltd. BioLineRx in-licenses a novel, phase II ready drug for the treatment of leukemia and other hematological cancers [media release]. 4 Sep 2012. http://www.biolinerx.com.
BioLineRx Ltd. BioLineRx announces collaboration with MSD to investigate the combination of KEYTRUDA (pembrolizumab) and BL-8040 in pancreatic cancer [media release]. 12 Jan 2016. https://biolinerx.com/.
BioLineRx Ltd. BioLineRx announces expansion of immuno-oncology collaboration in pancreatic cancer [media release]. 30 July 2018. http://www.biolinerx.com.
BioLineRx Ltd. BioLineRx signs second clinical immuno-oncology collaboration agreement to investigate combination of BL-8040 and KEYTRUDA® for pancreatic cancer [media release]. 8 Aug 2016. http://www.biolinerx.com.
BioLineRx Ltd. BioLineRx announces clinical research collaboration to investigate combination of BL-8040 with atezolizumab in multiple oncology indications [media release]. 7 Sept 2016. http://www.biolinerx.com.
BioLineRx Ltd. BioLineRx announces initiation of phase 2 clinical trial in first-line metastatic pancreatic cancer [media release]. 29 Oct 2020. https://biolinerx.com/.
BioLineRx Ltd. BioLineRx announces collaboration agreement with GenFleet Therapeutics to further develop motixafortide in pancreatic ductal adenocarcinoma (PDAC) [media release]. 28 June 2022. http://www.genfleet.com.
BioLineRx Ltd. BioLineRx announces clinical trial collaboration with Washington University School of Medicine to evaluate motixafortide for CD34+ hematopoietic stem cell mobilization for gene therapies in sickle cell disease [media release]. 6 Mar 2023. http://www.biolinerx.com.
BioLineRx Ltd. BioLineRx increases stake in BL-8040, its lead oncology platform in late stage development for multiple oncology indications [media release]. 3 Oct 2018. http://www.biolinerx.com.
BioLineRx Ltd. BioLineRx reports second quarter 2023 financial results and recent corporate and portfolio updates [media release]. 30 Aug 2023. http://www.biolinerx.com.
United States Securities and Exchange Commission. Form 6-K: report of foreign private issuer pursuant to Rule 13a-16 OR 15d-16 of the Securities Exchange Act of 1934 for the month of August 2023 2023. https://ir.biolinerx.com/static-files/eab8decb-7f5a-4e5b-9201-428a561ba689. Accessed 21 Sept 2023.
United States Securities and Exchange Commission. Form 20-F: annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act OF 1934 for the fiscal year ended December 31, 2022. 2022. https://www.sec.gov/ix?doc=/Archives/edgar/data/1498403/000117891323001091/zk2329404.htm. Accessed 25 Sept 2023.
Rebolledo-Bustillo M, Garcia-Gomez D, Dávila EM, et al. Structural basis of the binding mode of the antineoplastic compound motixafortide (BL-8040) in the CXCR4 chemokine receptor. Int J Mol Sci. 2023;24(5):1–14.
Abraham M, Biyder K, Begin M, et al. Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4F-benzoyl-TN14003. Stem Cells. 2007;25(9):2158–66.
Abraham M, Pereg Y, Bulvik B, et al. Single dose of the CXCR4 antagonist BL-8040 induces rapid mobilization for the collection of human CD34(+) cells in healthy volunteers. Clin Cancer Res. 2017;23(22):6790–801.
Crees ZD, Rettig MP, Bashey A, et al. Hematopoietic stem cell mobilization for allogeneic stem cell transplantation by motixafortide, a novel CXCR4 inhibitor. Blood Adv. 2023;7(18):5210–4.
Xia J, Jotte MRM, Sun S, et al. The CXCR4 antagonist, BL8040, is highly active against human T-ALL in preclinical models [abstract]. Blood. 2018;132(Suppl 1).
Crees ZD, Stockerl-Goldstein K, Vainstein A, et al. GENESIS: a phase III randomized double-blind, placebo-controlled, multi-center trial evaluating the safety and efficacy of BL-8040 and G-CSF in mobilization of hematopoietic stem cells for autologous transplantation in multiple myeloma. Lead-in period results [abstract no. 455]. Biol Blood Marrow Transplant. 2019;25(3):S308–9.
Lamotte M, DiPersio JF, Siegel DS, et al. Cost-effectiveness analysis of motixafortide on top of G-CSF for stem cell mobilization for autologous bone marrow transplantation in patients with multiple myeloma [abstract no. EE56]. Value Health. 2022;25(Suppl 7):S346.
Lamotte M, DiPersio JF, Siegel D, et al. Cost-effectiveness analysis of motixafortide versus plerixafor in stem cell mobilization for autologous transplantation in patients with multiple myeloma [abstract]. Blood. 2022;140(Suppl 1):10794–5.
Crees ZD, Stockerl-Goldstein K, Vainstein A, et al. GENESIS: phase III trial evaluating BL-8040 + G-CSF to mobilize hematopoietic cells for autologous transplant in myeloma. Future Oncol. 2019;15(31):3555–63.
Peled A, Abraham M, Avivi I, et al. The high-affinity CXCR4 antagonist BKT140 is safe and induces a robust mobilization of human CD34+ cells in patients with multiple myeloma. Clin Cancer Res. 2014;20(2):469–79.
Bockorny B, Semenisty V, Macarulla T, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26(6):878–85.
Bockorny B, Macarulla T, Semenisty V, et al. Motixafortide and pembrolizumab combined to nanoliposomal irinotecan, fluorouracil, and folinic acid in metastatic pancreatic cancer: the COMBAT/KEYNOTE-202 trial. Clin Cancer Res. 2021;27(18):5020–7.
Borthakur G, Ofran Y, Tallman MS, et al. BL-8040 CXCR4 antagonist is safe and demonstrates antileukemic activity in combination with cytarabine for the treatment of relapsed/refractory acute myelogenous leukemia: an open-label safety and efficacy phase 2a study. Cancer. 2021;127(8):1246–59.
Segura SJ, Wass M, Schaffrath J, et al. Double-blind, placebo controlled, randomized, multicenter, phase II study to assess the efficacy of the high affinity CXCR4 inhibitor BL-8040 as addition to consolidation therapy in AML by the SAL and OSHO Leukemia Study Groups [abstract no. 1446]. Blood. 2022;140(Suppl 1):3338–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sheridan M. Hoy is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The original article has been updated: Due to textual changes.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoy, S.M. Motixafortide: First Approval. Drugs 83, 1635–1643 (2023). https://doi.org/10.1007/s40265-023-01962-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01962-w