Abstract
After more than 2 decades of recommending an escalating strategy for the treatment of most patients with multiple sclerosis, there has recently been considerable interest in the use of high-efficacy therapies in the early stage of the disease. Early intervention with induction/high-efficacy disease-modifying therapy may have the best risk-benefit profile for patients with relapsing-remitting multiple sclerosis who are young and have active disease, numerous focal T2 lesions on spinal and brain magnetic resonance imaging, and no irreversible disability. Although we have no curative treatment, at least seven classes of high-efficacy drugs are available, with two main strategies. The first strategy involves the use of high-efficacy drugs (e.g., natalizumab, sphingosine 1-phosphate receptor modulators, or anti-CD20 drugs) to achieve sustained immunosuppression. These can be used as a first-line therapy in many countries. The second strategy entails the use of one of the induction drugs (short-term use of mitoxantrone, alemtuzumab, cladribine, or autologous hematopoietic stem cell transplant) that are mainly recommended as a second-line or third-line treatment in patients with very active or aggressive multiple sclerosis disease. Early sustained immunosuppression exposes patients to heightened risks of infection and cancer proportionate to cumulative exposure, and induction drugs expose patients to similar risks during the initial post-treatment period, although these risks decrease over time. Their initial potential safety risks should now be revisited, taking account of long-term data and some major changes in their regimens: natalizumab with the long-term monitoring of John Cunningham virus; use of monthly courses of mitoxantrone with maximum cumulative doses of 36–72 mg/m2, followed by a safer disease-modifying drug; cladribine with only 2-weekly treatment courses required in years 1 and 2 and no systematic treatment for the following 2 years; alemtuzumab, whose safety and clinical impacts have now been documented for more than 6 years after the last infusion; and autologous haematopoietic stem cell transplant, which dramatically reduces transplantation-related mortality with a new regimen and guidelines. Escalation and induction/high-efficacy treatments need rigorous magnetic resonance imaging monitoring. Monitoring over the first few years, using the MAGNIMS score or American Academy of Neurology guidelines, considerably improves prediction accuracy and facilitates the selection of patients with relapsing-remitting multiple sclerosis requiring aggressive treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Early intervention with induction/high-efficacy disease-modifying therapy may have the best risk-benefit profile for patients with relapsing-remitting multiple sclerosis who are young and have active disease, numerous focal T2 lesions on spinal and brain magnetic resonance imaging, and no irreversible disability. |
Although we have no curative treatment, at least seven classes of high-efficacy drugs are now available, with two main strategies: either the use of high-efficacy drugs (natalizumab, anti-sphingosine 1-phosphate receptors, or anti-CD20 drugs) to achieve sustained immunosuppression or the short-term use of induction drugs (mitoxantrone, alemtuzumab, cladribine, or autologous haematopoietic stem cell transplant). |
1 Concept of Early Treatment Initiation and Importance of Age at Treatment Onset
Most consensus groups advocate early treatment initiation for patients diagnosed with relapsing-remitting multiple sclerosis (RRMS) [1, 2]. One key goal of treatment is to avert the accumulation of irreversible neurological disabilities, and more particularly to prevent conversion to secondary progressive multiple sclerosis (SPMS). The disease-modifying drugs (DMDs) that are currently available are not effective once conversion has occurred, for by that stage, acute focal inflammatory activity is less relevant. More specifically, randomized clinical trials have shown that this type of treatment is largely, if not wholly, ineffectual in primary progressive multiple sclerosis (MS) and SPMS where there is no relapse and no disease activity on magnetic resonance imaging (MRI) [3,4,5,6,7,8,9,10]. Long-term epidemiological data indicate that disability progression takes place in two stages in MS: the first stage is dependent on current focal and acute central nervous system (CNS) inflammatory events (relapses and new T2 focal lesions on MRI), while the second is independent of current focal and acute CNS inflammatory lesions [11]. Patients with MS who experience more relapses or have more focal T2 lesions within 2–5 years of disease onset develop disability more rapidly [11,12,13,14,15]. This is doubtless owing to the nature of the immune disturbances that characterize changes over time, moving from a peripheral immunopathological profile, prevailing at the early stage to a compartmentalized CNS profile, present early in the disease, but prevailing at the late stage of the disease [16, 17]. As the purpose of all currently approved treatments is to avoid the acute focal CNS inflammatory events that predominantly occur at the beginning of the disease course, it is all the more logical to focus therapeutic efforts on the early disease phase. The other key determinant of DMD efficacy is patient age at treatment onset, as the lower the age, the more DMDs reduce disability worsening. This was suggested by Weideman et al. [18], who extracted disability data from 12 placebo-controlled, phase III trials among patients with RRMS and, using a mathematical modeling paradigm, tracked the correlation between age and disability progression over 2 years, comparing patients who received high-efficacy or low-efficacy drugs or a placebo. Results indicated that the impact of DMDs versus placebo on the risk of disability worsening over 2 years was only significant before age 53 years, and the impact of high-efficacy versus low-efficacy DMDs was only significant before 40.5 years.
2 Long-Term Impact of High-Efficacy Disease-Modifying Drugs on Disability Worsening: Escalation Versus Early Use
There are two contrasting treatment regimens in MS: escalation versus early use of high-efficacy DMDs [19,20,21,22]. The justification for escalating therapy is that treatment starts with safe drugs known to have low-to-moderate clinical efficacy, and patients are only moved on to more aggressive high-efficacy DMDs in the event of treatment failure. Glatiramer acetate, interferon-beta, teriflunomide, and dimethyl fumarate are regarded as first-line drugs with moderate efficacy, and immunosuppressants (mitoxantrone, natalizumab, fingolimod, alemtuzumab, anti-CD20 drugs) as high-efficacy second-line drugs. Clinicians should not wait until severe and irreversible disability has set in to move patients who respond poorly from a first-line therapy to a second-line therapy. Many patients do well on relatively safe drugs and never have to be escalated to more aggressive drugs. However, some patients may waste precious years taking a treatment that is not potent enough, potentially leading to increased disability. The key to success with escalation therapy is to engage with each patient beforehand in order to define a suboptimal response threshold beyond which the next-level therapeutic option is adopted. Patients who do not exhibit any disease activity under DMDs during a longitudinal follow-up should be classified as probable DMD responders, even if they experience a gradual worsening of disability. The scoring proposed by the MAGNIMS group [23] therefore seems adequate for the yearly classification of DMD responders and non-responders: 0 (no relapse and 0–2 new T2 lesions per year); 1 (1 relapse or > 2 new T2 lesions per year); and 2 (2 relapses and > 2 new T2 lesions per year). This scoring was initially assessed in patients with RRMS treated with interferon [23], and more recently in patients treated with teriflunomide, dimethyl fumarate, or fingolimod [24]. In the USA, American Academy of Neurology guidelines suggest that more than one MRI lesion or a clinical attack or disability worsening in a year should trigger a discussion about switching treatment [25].
Early intensive therapy entails starting with a strong immune intervention, in the form of high-efficacy DMDs. This results in the earlier achievement of no evidence of disease activity, which is the gold standard for MS treatment according to some schools of thought. The disadvantage is that some patients may be needlessly exposed to serious side effects for a longer period of their lives. Six randomized clinical trials comparing high-efficacy versus moderate-efficacy DMDs [26,27,28,29,30,31] found that the former brought about a relative reduction in disability worsening. However, none of these trials lasted more than 3 years, so given the complexity of the mechanisms underlying disability progression [16, 17], long-term observational studies are needed to gauge the lasting impact of early use of high-efficacy versus moderate-efficacy DMDs (Table 1 [32,33,34,35,36,37]).
Brown et al. [32] explored associations between the use, type, and timing of DMDs, comparing initial high-efficacy treatments (fingolimod, alemtuzumab, natalizumab) and initial low-efficacy or moderate-efficacy treatments (interferon-beta or glatiramer acetate) in matched cohorts of patients with MS followed for a mean treatment period of 5.8 years. Those who initially received high-efficacy DMDs (n = 235) had a significantly lower risk of conversion to SPMS than matched patients who initially received moderate-efficacy DMDs (n = 380): at 5 years, 7% versus 12% had converted to SPMS, and at 9 years, these figures were 16% versus 27%.
Buron et al. [33] assessed the impact on disability worsening of high-efficacy (70%, natalizumab 29% fingolimod) versus moderate-efficacy (77% interferon, 13% teriflunomide, 9% glatiramer acetate) DMDs as the first treatment choice in two cohorts of 194 matched treatment-naive patients with MS, followed for a mean treatment period of 5.3 years. Results for 6-month confirmed disability worsening on the Expanded Disability Status Scale (EDSS; ≥ 1-point increase) were better for the high-efficacy group: 11.5% versus 18.3% at 2 years; 16.5% versus 30.1% at 4 years.
Harding et al. [34] analyzed long-term outcomes in a population-based MS cohort according to initial treatment strategy: high efficacy (67% alemtuzumab, 34% natalizumab) or moderate efficacy (interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, fingolimod). Models were adjusted for sex, age at treatment onset, year of DMD initiation, and escalation to high-efficacy treatment. Among the 592 patients, 104 (17.2%) received high-efficacy DMDs as a first-line treatment (early intensive therapy group), while 488 (82%) began with moderate-efficacy DMDs (escalating group), with 11.9% subsequently going on to receive high-efficacy DMDs (74% natalizumab, 26% alemtuzumab). They were followed for a mean treatment period of 5.8 years (early intensive) or 6.9 years (escalating). The primary endpoint was the change in EDSS scores 5 years after treatment onset. This change was smaller in the early intensive group than in the escalating group (0.3 [standard deviation: 1.5] vs 1.2 [standard deviation 1.5]). Median time to sustained accumulation of disability was 6 years for the early intensive group, and 3.1 years for the escalating group. Moreover, the median annualized relapse rate (ARR) was 0 in the early intensive group, and 0.19 in the escalating group (p = 0.02).
He et al. [35] compared patients who started high-efficacy DMDs within 2 years of disease onset (n = 213) with those who started them 4–6 years after disease onset (n = 253) on long-term disability outcomes. They matched patients using propensity scores calculated on the basis of their baseline clinical and demographic data. The high-efficacy DMDs were mainly natalizumab (75%) and mitoxantrone (15%). The primary endpoint was the disability score 6–10 years after disease onset. The EDSS score was lower in the group that commenced high-efficacy DMDs within 2 years of disease onset than in the group that commenced them later in the disease course (EDSS: 2.2 vs 2.9 at year 5; 2.3 vs 3.5 at year 10).
Iaffaldano et al. [36] assessed disability trajectories in patients with RRMS who received either early intensive treatment or moderate-efficacy treatment followed by escalation to higher efficacy DMDs. After a propensity score matching procedure, they compared 393 pairs of patients: one group with initial high-efficacy DMDs (41% natalizumab, 39% mitoxantrone, 14% fingolimod) and one with initial moderate-efficacy DMDs (84% interferon, 9% glatiramer acetate) followed (after a median interval of 6.3 years) by a high-efficacy DMD (43% natalizumab, 39% fingolimod, 14% mitoxantrone). The primary endpoint was the disability score (EDSS) at 10 years after treatment initiation. Although mean EDSS scores were similar at treatment onset (EDSS = 2.5), the mean delta-EDSS difference between the two groups increased substantially, from 0.1 at 1 year to 0.30 at 5 years and 0.67 at 10 years.
Prosperini et al. [37] compared induction immunosuppression versus escalation strategies on the risk of reaching EDSS scores ≥ 6.0 within 10 years in previously untreated patients with RRMS. They assessed 75 pairs of matched patients: one group received induction treatment with high-efficacy DMDs (73% mitoxantrone, 27% cyclophosphamide) followed by a maintenance treatment with moderate-efficacy DMDs (85% interferon), while the other group initially received moderate-efficacy treatment (interferon) followed (after a median interval of 3.5 years) by high-efficacy treatment (natalizumab, fingolimod, mitoxantrone). The proportion of patients requiring subsequent escalation to fingolimod or other monoclonal antibodies was lower in the induction group than in the escalation group (34.7% vs 53.4%). At 10 years, a lower proportion of patients had reached an EDSS score ≥ 6 after induction (21/75, 28%) than after escalation (29/75, 38.7%).
The results of these observational studies were also in line with two other studies [38, 39] comparing between cohorts of national MS registries the two alternatives strategies for the initiation of a first DMD. Tacking advantages that the relative frequency of each strategy differs markedly between the Swedish registry cohorts and the Danish and Czech registry cohorts (initiation with high-efficacy treatment: 34% and 42% for the two Swedish cohorts, 7.6% for the Danish cohort, 3.8% for the Czech cohort), these two studies showed better long-term outcomes (reduction of the risk to reach EDSS scores of 3 or 4) in the Swedish cohorts and suggested that escalation of treatment was inferior to using a more efficacious DMD as initial treatment.
It is worth pointing out the demographic and clinical characteristics of the patients with MS in these studies at treatment onset [32,33,34,35,36,37,38,39]: they were young (mean age < 40 years), with a short disease duration (< 5 years), active disease (one to two relapses within the previous 12 months), and no irreversible disability (mean EDSS score of 2). The clinical and demographic features of the best candidates for initial use of high-efficacy treatments are summarized in Table 2.
3 Early Intensive Treatment Strategies: Induction Drugs Versus High-Efficacy Drugs to Achieve Sustained Immunosuppression
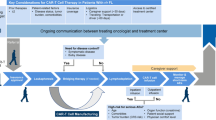
All currently available immunosuppressants have potentially serious side effects, thus early intensive treatment has usually only been envisaged for young patients with highly active disease. These patients have a known risk of early disability, and once they have lost neurological function, they cannot recover it. For these patients, we can assume that this disease-inherent risk outweighs the risk of using powerful immunosuppressants. This treatment strategy involves the use of these drugs in two different treatment regimens: long-term versus induction (Table 3). Long-term use of high-efficacy drugs, possibly over several decades, exposes patients to infections and cancer risks that rise proportionally with cumulative exposure. At least two drugs with anti-cell migration activity (natalizumab and sphingosine-1-phosphate [S1P] receptor modulators) expose patients to the risk of reactivation/rebound at withdrawal. Induction drugs are used for the shortest amount of time needed to achieve sufficient control over disease activity, after which patients can either switch to maintenance therapy with a better tolerated drug or stop treatment altogether. Patients treated with induction drugs undergo rigorous clinical and MRI monitoring for signals of disease reactivation (relapse or new focal T2 lesions on MRI). This may be a useful and conservative approach to using these highly effective therapies whilst minimizing exposure and the attendant safety risks.
3.1 High-Efficacy Treatment to Achieve Sustained Immunosuppression
Three classes of high-efficacy drugs have been authorized as first-line therapies in many countries (natalizumab, S1P receptor modulators, anti-CD20 drugs). High-efficacy treatments are here defined as DMDs whose superiority to a first-line therapy (interferon-beta, teriflunomide) has been clearly documented.
3.1.1 Natalizumab
Patients with RRMS (n = 942) in the AFFIRM study [40] were randomized to receive either monthly intravenous natalizumab (300 mg) or a placebo over a 2-year period. Results revealed a 68% reduction in the ARR at year 1, and a reduction of 42% in the relative risk of disability progression at year 2. A post hoc analysis [26] established the efficacy of natalizumab in patients who met the criteria (i.e., two or more relapses in the year before study entry and more than one gadolinium-enhancing [Gd+] lesion on MRI) for highly active disease: 148 patients receiving natalizumab and 61 patients receiving placebo. In these treatment-naive patients with highly active disease, there was a 2-year cumulative probability of confirmed disability worsening over 3 months of 14% for the natalizumab group and 29% for the placebo group. Natalizumab therefore reduced the 2-year cumulative probability of relapse by 81%, compared with placebo (0.28 vs 1.46), demonstrating the strong impact of this drug on disease activity and thus on short-term disability worsening.
Regarding more long-term effects, the real-world TOP study [41] reported the clinical data of 6148 patients with MS for up to 10 years after initiation of natalizumab (mean follow-up: 5.2 years; mean time on study drug: 3.3 years, with drug discontinuation in 27% of the cohort and loss to follow-up of 34.4%). At drug initiation, mean age was 37 years, mean disease duration was 7.8 years, number of relapses within 12 months before starting natalizumab was 2, and the mean EDSS score was 3.5. During the follow-up, the ARR was low (0.15), with 45.8% of patients remaining relapse free. The 6-month confirmed disability worsening was 27.8%, and the 6-month confirmed disability improvement was 23.4% over a mean period of approximately 6 years, showing a clear impact on disease course with natalizumab use.
The chief concern with natalizumab is the high cumulative risk of progressive multifocal leukoencephalopathy, which can be up to 3% over 6 years for patients who are John Cunningham virus positive [42]. This underscores the importance of regular (every 3–6 months) John Cunningham virus blood tests, in order to switch treatments if a patient becomes John Cunningham virus positive. As natalizumab does not have a residual effect after withdrawal, patients risk experiencing a rapid rebound with potentially severe clinical consequences [43, 44]. To minimize this risk, a therapeutic gap should clearly be avoided, by switching to a high-efficacy drug [45].
3.1.2 Sphingosine 1-Phosphate Receptor Modulators
Four S1P receptor modulators can be used to treat patients with MS: fingolimod, siponimod, ponesimod, and ozanimod. In the TRANSFORMS trial [27], patients were randomized to either daily fingolimod 0.5 mg (n = 431) or weekly intramuscular interferon-beta 1-a 30 μg (n = 435) for 1 year [27]. To be included, patients had to have had a relapse the previous year, or two relapses within the previous 2 years, and an EDSS score between 0 and 5.5. A total of 45% of patients had already received a DMD. The main clinical results were a reduction of 0.16 in the ARR for the fingolimod arm versus 0.33 for the interferon-beta 1-a arm (52% reduction), 82.6% of relapse-free patients in the fingolimod arm versus 69.3% in the interferon-beta-1-a arm, and no difference on disability worsening after 1 year.
In FREEDOMS 2 [46], patients were randomized to receive either fingolimod 0.5 mg (n = 358) or placebo (n = 355) once a day. Over 2 years, the mean ARR was 0.40 for patients given placebo, and 0.21 for patients given fingolimod 0.5 mg, corresponding to a reduction of 48% for fingolimod versus placebo. There was no statistically significant between-group difference on confirmed disability worsening (hazard rate 0.83 for fingolimod vs placebo; 95% confidence interval [CI] 0.61–1.12).
The real-world LONGTERMS study [47] reported the clinical data of 4086 patients with MS after fingolimod initiation for up to 14 years (mean follow-up duration: 8.6 years; mean time on the study drug: 4.2 years, with drug discontinuation in 64% of patients and a loss to follow-up of 14.8% of the cohort). At drug initiation, mean age was 38 years, mean disease duration was 8.7 years, the number of relapses within the 12 months preceding fingolimod initiation was 1.3, and the mean EDSS score was 2.4. During follow-up, the ARR was low (0.19), with 45.5% of patients remaining free of relapse, while 6-month confirmed disability worsening was 37% over 8 years.
Compared with placebo, fingolimod 0.5 mg caused more of the following adverse events: lymphopenia (8% vs 0%), increased alanine aminotransferase (8% vs 2%), herpes zoster infection (3% vs 1%), hypertension (9% vs 3%), first-dose bradycardia (1% vs < 0.5%), first-degree atrioventricular block (5% vs 2%), basal-cell carcinoma (3% vs 1%), macular edema (1% vs < 1%), infections (3% vs 1%), and neoplasms (4% vs 2%). As fingolimod has no residual effect after withdrawal, patients were exposed to the risk of experiencing a rapid rebound [48]. Research has confirmed the superiority of the other S1P receptor modulators (i.e., siponimod [49], ponesimod [50], ozanimod [51]) over first-line immunomodulators (interferon-beta or teriflunomide), and although there are some differences in terms of targeting S1P receptors, no clear safety differences have been documented.
3.1.3 Anti-CD20 Drugs
Three anti-CD20 drugs have been approved by the health authorities: ocrelizumab, ofatuzumab, and ublituximab. The phase III development program for ocrelizumab in patients with RRMS consisted of two identical trials (OPERA I and OPERA II) [52]. The inclusion criterion was at least one relapse within the previous 2 years. A total of 1656 patients were randomized to receive either ocrelizumab or Rebif 44. In each arm, 71% and 73% patients were treatment naive. Pooled analyses showed that the mean ARR over 2 years was 0.16 (ocrelizumab) versus 0.29 (Rebif 44), and the proportion of patients who exhibited confirmed disability worsening at 12 weeks was 9.1% versus 13.6%.
The phase III program for ofatumumab [53] also comprised two identical trials (ASCLEPIOS I and ASCLEPIOS II). A total of 1882 patients were randomized to receive either subcutaneous ofatumumab 20 mg every 4 weeks (n = 946) or teriflunomide (n = 936). The median follow-up duration was 1.6 years. The ARR was 0.11 for the ofatumumab group, and 0.22 for the teriflunomide group in ASCLEPIOS I (0.10 and 0.25 in ASCLEPIOS II). The percentage of patients with confirmed disability worsening at 6 months was 8.1% for the ofatumumab group versus 12% for the teriflunomide group.
A third anti-CD20 drug, ublituximab [54], has been approved in late 2022 and was available in 2023 on the basis of two phase III trials comparing intravenous ublituximab 450 mg every 6 months (ULTIMATE 1 and 2) and oral teriflunomide, showing a 49–59% reduction in the ARR and a 96–97% reduction in the mean number of Gd+ lesions, but no significant difference on disability worsening. In a single-center, double-blind, controlled phase II study, Honce et al. showed that short-term use of rituximab followed by glatiramer acetate had only short-term efficacy, which waned within 3 years [55], suggesting that anti-CD20 is not an induction drug.
The short-term use of anti-CD20 drugs in patients with MS seems relatively safe [52,53,54], exposing them mainly to infusion reactions at initiation or injection reactions, with a slightly higher risk of infections. There are currently no data on the long-term use of anti-CD20 drugs in MS, although their long-term safety in autoimmune conditions has been assessed in patients with underlying rheumatic diseases treated with rituximab [56]. In published studies, the rate of serious infections ranges from 2.2 to 9.8/100 person-years. Quantitative immunoglobulins are typically being checked in subjects with MS receiving an anti-CD20 drug. Lower immunoglobulin G levels (< 6 g/L) at baseline and during treatment appear to be predictive of serious infection, suggesting that there should be close monitoring of patients with low baseline immunoglobulin levels [57]. Hepatitis B virus (HBV) reactivation is known to occur in patients receiving anti-CD20 drugs who are either HBV surface antigen negative and HBV core antibody positive (8–42%) or HBV surface antigen positive (30–60%), putting them at risk of high morbidity and mortality. Screening for HBV is mandatory prior to initiating anti-CD20 drugs. Regarding the effect of anti-CD20 drugs on COVID-19, concerns have been raised about viral clearance, increased disease severity, and a poor antibody response to vaccination [56].
3.2 Induction Drugs
Two emerging concepts for managing MS with induction drugs are immune reconstitution therapy and pulsed immune reconstitution therapy [59]. Induction drugs (mitoxantrone, alemtuzumab, cladribine, autologous hematopoietic stem cell transplant [AHSCT]) are mainly recommended as a second-line or third-line treatment in patients with very active or aggressive MS disease. However, initial potential safety risks should now be revisited, in the light of long-term data and some major changes in drug regimens: monthly courses of mitoxantrone with maximum cumulative doses of 36–72 mg/m2 followed by long-term interferon-beta, and not the US-used (and approved) regimen consisting of every 3-month courses of mitoxantrone [60] of up to 120–140 mg/m2; cladribine with only 2-weekly treatment courses required in years 1 and 2 and no systematic treatment for the following 2 years (i.e., not long-term use); alemtuzumab, whose safety and clinical impacts are now documented for more than 6 years after the last infusion; and AHSCT, which dramatically reduces transplantation-related mortality with a new regimen and guidelines.
3.2.1 Mitoxantrone as an Induction Drug (Monthly for 3–6 Months) Before Other Safer Long-Term Disease-Modifying Drugs
The strong and rapid reduction of the inflammatory process in a pivotal trial [61] suggested a potential role for mitoxantrone as an induction drug before the long-term use of other DMDs in very active cases of MS. This concept was assessed in a randomized controlled 3-year trial of mitoxantrone followed by a maintenance therapy with interferon-beta-1b [31], which included patients with RRMS who had experienced at least two relapses with incomplete recovery the previous year and had displayed Gd+ lesions on MRI. Fifty-five patients were randomly allocated to a 6-month mitoxantrone induction regimen, followed by a 3-month wash-out period, then interferon-beta-1b. Patients (n = 54) in the other study arm received interferon-beta-1b for 3 years, combined with methylprednisolone 1 g per month for the first 6 months. Time to confirmed disability worsening was longer in the mitoxantrone group than in the interferon-beta group (p < 0.012).The 3-year risk of confirmed disability worsening was reduced by 65% (12% vs 34%). There was also a smaller raw percentage of patients with confirmed disability worsening (9.1% vs 25.9%). At the final observation point (M36), patients’ mean EDSS scores had improved by 0.45 points (from 4.16 to 3.66; p = 0.007) in the mitoxantrone group, but remained virtually unchanged (from 3.86 to 3.76, p = 0.771) in the interferon-beta group. The proportion of patients who remained relapse free throughout the follow-up period was 53% in the induction group and 26% in the monotherapy group (p < 0.01).
Mitoxantrone as an induction drug, followed by glatiramer acetate as maintenance therapy [62] was assessed in a controlled study among 40 patients with relapsing MS who had 1–15 Gd+ lesions on MRI and EDSS scores of 0–6.5. Participants were randomized to receive either mitoxantrone as a short-term induction therapy in the form of 3-monthly infusions (12 mg/m2), followed by 12 months of subcutaneous glatiramer acetate (20 mg/day) [n = 21], or glatiramer acetate (20 mg/day) for the whole 15-month period (n = 19). Compared with glatiramer acetate alone, induction mitoxantrone plus glatiramer acetate resulted in an 89% greater reduction (relative risk = 0.11, p = 0.0001) in the number of Gd+ lesions at months 6 and 9, and a 70% greater reduction (relative risk = 0.30, p = 0.0147) at months 12 and 15. The mean ARR for the mitoxantrone-glatiramer acetate group was 0.16, compared with 0.32 for the glatiramer acetate-alone group. Short-term immunosuppression using mitoxantrone, then daily glatiramer acetate for 12 months, was both safe and effective, and resulted in an early and sustained decrease in disease activity on MRI.
Lefort et al. reported the 10-year clinical follow-up and safety of mitoxantrone as an induction drug followed by maintenance therapy in patients with early highly active RRMS and an EDSS score < 4 1 year prior to mitoxantrone initiation [63]. In total, 100 consecutive patients with highly active RRMS from the Rennes EDMUS database received monthly mitoxantrone (20 mg) combined with methylprednisolone (1 g) for either 3 (n = 75) or 6 (n = 25) months, followed by a first-line DMD. At 10 years, 24% were relapse free, and the mean ARR was 0.2. The mean EDSS score remained significantly improved for up to 10 years, falling from 3.5 at mitoxantrone initiation to 2.7 at 10 years. The probability of disability worsening versus improvement between mitoxantrone initiation and the final observation point was 27% versus 58%. Just 13% converted to SPMS. Over the 10-year follow-up, the probability of receiving a second-line or third-line treatment, excluding mitoxantrone, was 11% (95% CI 5–17) at 5 years and 42% (95% CI 32–51) at 10 years.
On average, patients only remained untreated or treated solely with a first-line maintenance DMD for 6.5 years. In this cohort, mitoxantrone was generally safe. There were no cases of leukemia, but six patients developed neoplasms, including four solid cancers.
The long-term safety profile for the short-term use of mitoxantrone was assessed in a prospective French multicenter (n = 12) study [64], with at least five annual assessments after mitoxantrone initiation. A total of 802 patients (308 with RRMS, 352 with SPMS, and 142 with primary progressive multiple sclerosis) received mitoxantrone infusions either monthly (87%) or every 3 months (13%), for a mean cumulative dose of 72 mg/m2. The cohort was followed for a total of 5354 patient-years. One patient (0.1%) developed acute congestive heart failure, and two cases of treatment-related leukemia (0.25%) were diagnosed 20 months after mitoxantrone initiation (one death). Other published studies examining the risk of acute leukemia have yielded either similar [65] or higher [66] percentages, but both were retrospective and involved a different mitoxantrone regimen. Of the 317 women treated before age 45 years, 17.3% were diagnosed with persistent age-dependent amenorrhea (5.4% before 35 years, 30.7% at 35+ years). For the younger fertile women, there was a lower risk of sterility (0% before 25 years, 4.5% at 25–30 years). These safety profile considerations justify monitoring the blood cell count every 3 months and the left ventricular ejection fraction by an echocardiogram every year for 5 years after the last course of mitoxantrone [64].
3.2.2 Alemtuzumab
Alemtuzumab should be considered for induction therapy in MS, as it is more effective than interferon-beta in patients with early active RRMS. This was clearly demonstrated in three controlled trials [29, 30, 67] conducted among 334, 581, and 638 patients with RRMS. Alemtuzumab was compared with subcutaneous interferon-beta-1a (44 μg 3 times per week for 2 years). Lower rates of sustained accumulation of disability were recorded for patients in the alemtuzumab groups (9%, 8%, and 13%) than for patients in the interferon-beta-1a groups (26%, 11%, and 20%) [p < 0.001, p = 0.70, and p = 0.008]. The ARR was also lower in the alemtuzumab groups (0.10, 0.19, and 0.26) than in the interferon-beta-1a groups (0.36, 0.38, and 0.52; all p values < 0.001). There were 79%, 78%, and 65% relapse-free patients in the alemtuzumab groups, compared with 60%, 59%, and 47% in the interferon-beta-1a groups. As for the pooled populations in the CARE-MS trials [68,69,70], the efficacy of alemtuzumab remained stable across 9 years of treatment [70] and 48% of patients did not require retreatment with alemtuzumab or other DMDs.
Regarding the safety profile [71, 72], infections were more common with alemtuzumab than with interferon-beta-1a in years 1 (58.7% vs 41.3%) and 2 (52.6% vs 37.7%), but the incidence diminished over time among recipients of alemtuzumab. Oral anti-herpes prophylaxis should be started at alemtuzumab initiation. Post-marketing studies found an incidence of 0.72% for idiopathic thrombocytopenic purpura. In clinical trials, thyroid disorders occurred in 36.8% of patients with MS (median follow-up 6.1 years), while there was an incidence of 0.34% for autoimmune nephropathies (median follow-up 6.1 years). Owing to these safety profiles, platelet counts and renal function should be monitored monthly, and thyroid function checked every 3 months for 4 years after the final course of alemtuzumab. Multiple sclerosis clinical trials have reported rare autoimmune hepatitis (including fatal cases) after alemtuzumab treatment, as well as suspected autoimmune cytopenias (neutropenia, hemolytic anemia, and pancytopenia). In April 2019, the European Medicines Agency launched a safety review of alemtuzumab [73], drawing on a safety database covering 55,431 patient-years of exposure. The main focus was on fatalities, cardiovascular adverse events occurring shortly after alemtuzumab infusion, autoimmune diseases, and hepatic injury. Postmarketing studies found a mortality rate of 0.42% among alemtuzumab recipients in the European Union: 24 fatal events occurred within 30 days of the final infusion, with cardiac arrest and sepsis each causing death in two patients. Myocardial ischemia (2 cases), myocardial infarction (27 cases), potential myocardial ischemia or myocardial infarction (22 cases), cerebrovascular accidents including arterial dissection (25 cases), pulmonary hemorrhage (7 cases), pulmonary embolism (20 cases,) and transient nonimmune-mediated thrombocytopenia (68 cases) were all found to have occurred shortly after (≤ 30 days) alemtuzumab infusion.
3.2.3 Cladribine
Cladribine tablets are an oral high-efficacy DMD for RRMS, with only 2-weekly treatment courses required in years 1 and 2 [74]. Efficacy was assessed in the CLARITY 2-year, placebo-controlled, phase III trial [74], which compared 433 patients receiving cladribine 3.5 mg/kg, 436 receiving 5.25 mg/kg, and 437 receiving a placebo. Compared with placebo, the approved dose of cladribine (3.5 mg/kg) brought about a significant reduction in the ARR (0.14 vs 0.33), together with a lower risk of 3-month confirmed disability worsening (hazard ratio: 0.67, 95% CI 0.48–0.93), and a relative reduction of around 75% in brain lesion counts.
In the 2-year CLARITY extension study [75,76,77], cladribine recipients were re-randomized to cladribine 3.5 mg/kg (186 patients) or placebo (98 patients), with the blind maintained. The results of this extension were crucial for the development of cladribine, not only because of its reassuring safety profile, but also because efficacy data for the group of patients who received cladribine 3.5 mg/kg for the first 2 years, then a placebo for the third and fourth years, pointed to an induction action. During the 2-year extension, there were lasting clinical benefits, with approximately 75% of patients remaining relapse free and 72.4% free of 3-month confirmed disability worsening, plus a low risk of severe lymphopenia [75,76,77]. Multicenter (N = 17) data on 80 patients with MS in Italy who were given cladribine tablets in randomized clinical trials were obtained from the Italian MS Registry, and reported by the CLARINET-MS study [78]. The latter yielded an indirect measure of long-term disease control with cladribine tablets, showing that more than half of patients remained relapse free and did not experience any disability progression over the 60-month follow-up after the final dose had been administered.
We did not find any clear guidelines on the type of treatment strategy that should be adopted after year 4 and in the event of relevant activity after year 2 [79]. Lymphopenia was the most frequent adverse event, with a frequency of 11.4% for grade 3 or 4 lymphopenia in the first 2 years, falling to 5% (grade 4 <1%) during the extension, and a return to grade 0–1 by the end of the study. There was a reassuringly low occurrence of opportunistic infections, with no reported cases of progressive multifocal leukoencephalopathy, while there was a frequency of 2% for herpes zoster infections in the 3.5-mg/kg group. Malignancy frequency was similar to that of the general population, at 1.4% [75, 77,78,79,80]. Many patients who opt for oral cladribine do so because of its low treatment burden (oral, 2 weeks to 2 years), suitability for pregnancy planning (6 months after last tablet), and the recent observation that it does not affect vaccine responses [58].
3.2.4 Autologous Hematopoietic Stem Cell Transplantation
Over the past 30 years, AHSCT has been performed in more than 1800 patients with MS [81], with an increase in recent years to about 200/year. Sormani et al. [82] conducted a meta-analysis of 15 studies (764 patients with MS) and revealed a strong impact on disease activity and disability worsening, with 67% of patients (range 59–70%) with a 5-year no evidence of disease activity 3 status. Mean transplantation-related mortality was 2.1%, with higher figures in older studies and for patients with higher EDSS scores, and lower figures for patients with RRMS aged below 32 years. The best risk-benefit profile was for patients with an RRMS course who had not yet accumulated a high level of disability.
Boffa et al. [83] reported the impact of AHSCT in 210 patients with MS from five Italian centers. The proportion of patients with a 5-year no evidence of disease activity 3 status was 62% at year 5 and 40% at year 10, and was better in patients with RRMS (67%) than in patients with progressive disease (55%). Among the latter, disability worsening-free survival was 85.5% at 5 years and 71.3% at 10 years. Transplant-related mortality was 1.4% (0% in those with the BEAM [(BCNU), Etoposide, Ara-C, Melphalan] + ATG (Anti-Thymocyte-Globulin) conditioning regimen). In a retrospective analysis, the same team [84] compared the effect of AHSCT (79 patients ) with that of other disease-modifying treatments (1975 patients) on long-term disability worsening in active SPMS. Results suggested that patients who underwent AHSCT were more likely to experience a sustained improvement in disability: 34.7% of patients had a lower EDSS score than at baseline 3 years after transplant versus 4.6% of patients with other treatments (p < 0.001).
In addition, AHSCT in patients previously treated with alemtuzumab, cladribine, or rituximab was safe and efficacious [85]. Recent guidelines of the National Sclerosis Society have been published [86]. However, to gain a clear understanding of the role of AHSCT in the treatment of patients with inflammatory active forms of MS, comparative trials are needed. Several are ongoing (BEAT-MS NCT04047628, US; STAR-MS, UK; RAM-MS, Norway; COAST-MS, Germany; NET-MS, Italy).
4 Conclusions
The current challenge in treating MS is to identify the most effective drug and most effective strategy at each specific phase of the disease for each individual patient. Physicians’ risk perceptions when switching between high-efficacy and non-high efficacy DMDs seem to be a lack of efficacy and not the risk of malignancies and infection, excluding PML [87]. Both escalating and early high-efficacy drug strategies can be successfully implemented on the basis of clinical and imaging data. Disability data extracted from 12 placebo-controlled, phase III trials among patients with RRMS [18] and the results of long-term observational studies [32,33,34,35,36,37] suggest that the patients with RRMS who are liable to benefit most from the initial use of high-efficacy DMDs have the following characteristics: early (age < 5 years after clinical onset), younger (age < 40 years), with high clinical and MRI activity, EDSS score < 4, and more focal lesions (more than ten) on brain MRI (Table 2). Early high-efficacy treatment can follow one of two possible scenarios: either sustained immunosuppression, potentially over several decades, although there is currently a dearth of longer term data, or induction. Escalating strategies and post-induction/high-efficacy treatments need rigorous MRI monitoring, as recommended by expert groups [88, 89].
Monitoring over the first few years, using the MAGNIMS score [24] or American Academy of Neurology [25] guidelines considerably improves prediction accuracy and facilitates the selection of patients with RRMS requiring aggressive treatment. Magnetic resonance imaging is a key prognostic marker. Minimal MRI activity for the first few years after treatment onset should be a key objective when defining the therapeutic strategy. New MRI techniques (brain and spinal cord imaging) should make it easier to identify patients eligible for early and more aggressive treatment, especially those without any real disability who are at greater risk of developing destructive CNS lesions with or without first-line therapy.
References
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96–120.
Rae-Grant A, Day G, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–88.
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–20.
Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391:1263–73.
Edan G. Natalizumab in secondary progressive multiple sclerosis. Lancet Neurol. 2018;17(5):384–5. https://doi.org/10.1016/S1474-4422(18)30108-X. (Epub 2018 Mar 12).
Panitch H, Miller A, Paty D, et al. North American Study Group on interferon beta 1b in secondary progressive MS: results from a 3 year controlled study. Neurology. 2004;63:1788–95.
Coles AJ, Cox E, Le Page E, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253:98–108.
Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66:460–71.
Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:1075–84.
Kapoor R, Ho P-R, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo controlled trial with an open-label extension. Lancet Neurol. 2018;17:405–15.
Leray E, Yaouanq J, Le Page E, et al. Evidence of a two stage disability progression in multiple sclerosis. Brain. 2010;133:1900–13.
Confavreux C, Vukusic S, Moreau T, et al. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343:1430–8.
Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis, a geographically based study. 10. Relapses and long-term disability. Brain. 2010;133:1914–29.
Tremlett H, Zhao Y, Rieckmann P, et al. New perspectives in the natural history of multiple sclerosis. Neurology. 2010;74:2004–15.
Tintore M, Rovira A, Rio J, et al. Developing high, medium and low impact prognostic factors for developing multiple sclerosis. Brain. 2015;138:1863–74.
Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann Neurol. 2009;65:239–48.
Haider L, Fisher MT, Frisher JM, et al. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–24.
Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
Comi G. Induction vs. escalating therapy in multiple sclerosis: practical implications. Neurol Sci. 2008;29:S253–5.
Le Page E, Edan G. Induction or escalation therapy for patients with multiple sclerosis? Rev Neurol (Paris). 2018;174(6):449–57. https://doi.org/10.1016/j.neurol.2018.04.004.
Casanova B, Quintanilla-Bordàs C, Gascon F. Escalation vs. early intense therapy in multiple sclerosis. J Pers Med. 2022;12:119. https://doi.org/10.3390/jpm12010119.
Filippi M, Danes R, Derfus T, et al. Early and unrestricted access to high-efficacy disease-modifying therapies: a consensus to optimize benefits for people living with multiple sclerosis. J Neurol. 2022;269:1670–7.
Sormani MP, Gasperini C, Romeo M, et al. Assessing response to interferon-b in a multicenter dataset of patients with MS. Neurology. 2016;87:134–40.
Ruggieri S, Prosperini L, Al-Araji S, et al. Assessing treatment response to oral drugs for multiple sclerosis in real world setting: a MAGNIMS study. Poster communication. ECTRIMS Congress, Amsterdam, 27 October 2022; 2022.
Corboy JR, Weinshenker BG, Wingerchuk DW. Comment on 2018 American Academy of Neurology guidelines on disease-modifying therapies in MS. Neurology. 2018;90(24):1106–12.
Hutchinson M, Kappos L, Calabresi P, et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol. 2009;256:405–15.
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intra-muscular interferon beta for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15.
Hauser SL, Ba-Or A, Comi G, et al. Ocrelizumab versus interferon beta 1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221–34. https://doi.org/10.1056/NEJMoa1601277.
Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon-beta-1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–28.
Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised phase 3 trial. Lancet. 2012;380(9856):1829–39.
Edan G, Comi G, Le Page E, et al. Mitoxantrone prior to interferon beta-1b in aggressive relapsing multiple sclerosis: a 3-year randomised trial. J Neurol Neurosurg Psychiatry. 2011;82:1344–50.
Brown JWL, Coles A, Horakova D, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA. 2019;321(2):175–87.
Buron MD, Chalmer TA, Sellebjerg F, et al. Initial high-efficacy disease-modifying therapy in multiple sclerosis: a nationwide cohort study. Neurology. 2020;95(8):e1041–51.
Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76:536–41.
He A, Merkel B, Brown W, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19:307–16.
Iaffaldano P, Lucisano G, Caputo F, et al. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther Adv Neurol Disord. 2021;14:17562864211019574.
Prosperini L, Mancinelli CR, Solaro CM, et al. Induction versus escalation in multiple sclerosis: a 10-year real world study. Neurotherapeutics. 2020;17:994–1004.
Spelman T, Magyari M, Piehl F, et al. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis: data from 2 different national strategies. JAMA Neurol. 2021;78(10):1197–204.
Hrnciarova T, Drhota J, Spelman T, et al. Does initial high efficacy therapy in multiple sclerosis surpass escalation treatment strategy? A comparison of patients with relapsing-remitting multiple sclerosis in the Czech and Swedish national multiple sclerosis registries. Mult Scler Relat Disord. 2023. https://doi.org/10.1016/j.msard.2023.104803.
Polman C, O’Connor PW, Havrdora E, et al. A randomized, placebo controlled trial of natalizumab of relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910.
Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry. 2020;91:660–8.
Ho PR, Koendgen H, Campbell N, et al. Risk of natalizumab-associated progressive multifocal encephalopathy with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol. 2017;16(11):925–93.
Kerbrat A, Le Page E, Leray E, et al. Natalizumab and drug holiday in clinical practice: an observational study in very active relapsing-remitting multiple sclerosis patients. J Neurol Sci. 2011;308(1–2):98–102.
Roos I, Malpas C, Leray E, et al. Disease reactivation after cessation of disease modifying therapy in patients with relapsing remitting multiple sclerosis. Neurology. 2022;99(17):e1926–44.
Ferre L, Moiola L, Sangalli F, et al. Recurrence of disease activity after repeated natalizumab withdrawals. Neurol Sci. 2015;36:465–7.
Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomized, placebo-controlled phase 3 trial. Lancet Neurol. 2014;13(6):545–56.
Cohen JA, Tenenbaum N, Bhatt A, et al. Extended treatment with fingolimod for relapsing multiple sclerosis: the 14-year LONGTERMS study results. Ther Adv Neurol Disord. 2019. https://doi.org/10.1177/1756286419878324.
Hatcher SE, Waubant E, Noubakhsh B, et al. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol. 2016;73(7):790–4.
Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263–73.
Kappos L, Fox RJ, Burcklen M, et al. Ponesimod compared with teriflunomide in patients with relapsing multiple sclerosis in the active-comparator phase 3 OPTIMUM study. JAMA Neurol. 2021;78(5):1–10.
Comi G, Kappos L, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18(11):1009–20.
Hauser SL, Ba-or A, Comi G, et al. Ocrelizumab versus interferon beta 1a in relapsing multiple sclerosis. N Engl J Med. 2016. https://doi.org/10.1056/NEJMoa1601277.
Hauser SL, Bar-Or A, Cohen JA. ASCLEPIOS I and ASCLEPIOS II Trial Groups: ofatunumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546–57.
Steinman L, Fox E, Hartung HP, et al. Ublituximab versus teriflunomide in relapsing multiple sclerosis. N Engl J Med. 2022;387:704–14.
Honce JM, Nair KV, Sillau S, et al. Rituximab vs placebo induction prior to glatiramer acetate monotherapy in multiple sclerosis. Neurology. 2019;92:1–10.
Varley CD, Winthrop KL. Long-term safety of rituximab (risks of viral and opportunist infections). Curr Rheumatol Rep. 2021;23:74.
Yusof MYM, Vital EM, McElvenny DM, et al. Predicting severe infection and effects of hypogammaglobulinemia during therapy with rituximab in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. 2019;71(11):1812–23.
Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14:1–8.
Sorensen PS, Sellebjerg F. Pulsed immune reconstitution therapy in multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1–16.
Hartung P, Hartung HP, Gonsette R, et al. Mitoxantrone in prtogressive multiple sclerosis: a placebo-controlled double-blind, randomized, multicenter trial. Lancet. 2002;360(9350):2018–25.
Edan G, Miller D, Clanet M, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicenter study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry. 1997;62:112–8.
Vollmer T, Panitch H, Bar-Or A, et al. Glatiramer acetate after induction therapy with mitoxantrone in relapsing multiple sclerosis. Mult Scler. 2008;14:663–70.
Lefort M, Le Corre G, Le Page E, et al. Ten-year follow-up after mitoxantrone induction for early highly active relapsing-remitting multiple sclerosis: an observational study of 100 consecutive patients. Rev Neurol (Paris). 2022;178(6):569–79.
Le Page E, Leray E, Edan G, et al. Long-term safety profile of mitoxantrone in a French cohort of 802 multiple sclerosis patients: a 5-year prospective study. Mult Scler. 2011;17:867–75.
Stroet A, Hemmelmann C, Starck M, et al. Incidence of therapy-related acute leukaemia in mitoxantrone-treated multiple sclerosis patients in Germany. Ther Adv Neurol Disord. 2012;5(2):75–9.
Martinelli V, Cocco E, Capra R, et al. Acute myeloid leukemia in Italian patients with multiple sclerosis treated with mitoxantrone. Neurology. 2011;77(21):1887–95.
CAMMS223 Trial Investigators. Alemtuzumab vs. interferon-beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–801.
Coles AJ, Fox E, Vladic A, et al. Alemtuzumab more effective than interferon b-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology. 2012;78(14):1069–78.
Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology. 2017;89(11):1117–26.
Ziemssen T, Bass AD, Berkovich R, et al. Efficacy and safety of alemtuzumab through 9 years of follow-up in patients with highly active disease: post hoc analysis of CARE-MS I and II patients in the TOPAZ extension study. CNS Drugs. 2020. https://doi.org/10.1007/s40263-020-00749-x.
Wray S, Havrdova E, Snydman DR, et al. Infection risk with alemtuzumab decreases over time: pooled analysis of 6-year data from the CAMMS223, CARE-MS I, and CARE-MS II studies and the CAMMS03409 extension study. Mult Scler. 2019;25(12):1605–17.
Syed YY. Alemtuzumab: a review in relapsing remitting multiple sclerosis. Drugs. 2021;81:157–68.
European Medicines Agency. Lemtrada assessment report: procedure under article 20 of regulation (EC) No 726/2004 resulting from pharmacovigilance data. 2019. https://www.ema.europa.eu/. Accessed 10 Sept 2023.
Giovannoni G, Comi G, Cook S, et al. A placebo controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–26.
Giovannoni G, Soelberg Sorensen P, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. 2017. https://doi.org/10.1177/1352458517727603.
Giovannoni G, Singer BA, Issard D, et al. Durability of no evidence of disease activity-3 (NEDA-3) in patients receiving cladribine tablets: the CLARITY extension study. Mult Scler. 2021. https://doi.org/10.1177/13524585211049392.
Giovannoni G, Comi G, Rammohan K, et al. Long-term disease stability assessed by the Expanded Disability Status Scale in patients treated with cladribine tablets 3.5 mg/kg for relapsing multiple sclerosis: an exploratory post hoc analysis of the CLARITY and CLARITY Extension studies. Adv Ther. 2021;38(9):4975–85.
Patti F, Visconti A, Capacchione A, et al. Long-term effectiveness in patients previously treated with cladribine tablets: a real-world analysis of the Italian multiple sclerosis registry (CLARINET-MS). Ther Adv Neurol Disord. 2020;13:1756286420922685.
EMC. Mavenclad 10 mg tablets summary of product characteristics. https://www.medicines.org.uk/emc/product/8435. Accessed 10 Sept 2023.
Giovannoni G, Mathews J. Cladribine. Tablets for relapsing-remitting multiple sclerosis: a clinician’s review. Neurol Ther. 2022;11:571–95.
Sharrack B, Saccardi R, Alexander T et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommandations from EBMT Autoimmune Diseases Working Party and the Joint Accreditation Committee of EBMT and ISCT. Bone Marrow Transplant. 2020;55(2):283-306.
Sormani MP, Muraro PA, Schiaverri I, et al. Autologous hematopoietic stem cell transplantation: a meta analysis. Neurology. 2017;88:2115–22.
Boffa G, Massacesi L, Inglese M, et al. Long-term clinical outcomes of hematopoietic stem cell transplantation in multiple sclerosis. Neurology. 2021. https://doi.org/10.1212/WNL.0000000000011461.
Boffa G, Signori A, Massaceci L, et al. Hematopoietic stem cell transplantation in people with active secondary progressive secondary multiple sclerosis. Neurology. 2023;100(11):e1109–22.
Strokke Kvistad SA, Burman J, Lehmann AK, et al. Impact of previous disease-modifying treatment on safety and efficacy in patients with MS treated with AHSCT. J Neurol Neurosurg Psychiatry. 2022;93(8):844–8.
Miller AE, Chitnis T, Cohen BA, et al. Autologous hematopoietic stem cell transplant in multiple sclerosis: recommendations of the National Multiple Sclerosis Society. JAMA Neurol. 2021;78(2):241–6.
Seifer G, Arun T, Capela C, et al. Influence of physicians’ risk perception on switching treatments between high-efficacy and non-high-efficacy disease modifying therapies in multiple sclerosis. Mult Scler Relat Disord. 2023;76: 104770.
Brisset JC, Kremer S, Hannoun S, et al. New OFSEP recommendations for MRI assessment of multiple sclerosis patients: special consideration for gadolinium deposition and frequent acquisitions. J Neuroradiol. 2020;47(4):250–8.
Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No specific funding was recieved for the preparation of this review.
Conflicts of Interest
Both authors were involved as investigators in most of the phase 3 trials of approved DMDs and our institution received occasionally research grants from the industrials : Biogen, merck, Teva, Bayer, Novartis, Sanofi and Roche.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Data availability
This manuscript has no associated data.
Author contributions
Both authors contributed to this review.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Edan, G., Le Page, E. Escalation Versus Induction/High-Efficacy Treatment Strategies for Relapsing Multiple Sclerosis: Which is Best for Patients?. Drugs 83, 1351–1363 (2023). https://doi.org/10.1007/s40265-023-01942-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01942-0