Abstract
Teprotumumab (TEPEZZA®), a monoclonal antibody that inhibits the insulin-like growth factor 1 receptor (IGF-1R), is the first disease-modifying therapy approved for the treatment of thyroid eye disease (TED) in the USA. In phase II and III clinical trials in adults with active, moderate-to-severe TED, intravenous teprotumumab significantly improved proptosis response rate and a range of other TED outcomes, including overall response rate, Clinical Activity Score, diplopia and disease-specific quality of life. The clinical benefit of teprotumumab was maintained for up to 51 weeks post-treatment in the majority of patients. Teprotumumab was generally well tolerated; adverse events with the greatest risk difference compared with placebo were muscle spasms, hearing loss and hyperglycaemia. Early real-world experience suggests teprotumumab may also be beneficial in a more diverse TED population. Teprotumumab is the first approved treatment for TED and is effective at reducing symptoms which are often unamenable to historical pharmacological interventions. While further data are required, current evidence suggests teprotumumab represents an important advance in the treatment of TED.
Plain Language Summary
Thyroid eye disease (TED) is an inflammatory disease that involves expansion of the soft tissue surrounding and behind the eye. It can lead to bulging of the eye(s), double vision, optic nerve compression and vision loss. Traditional treatments are often unsatisfactory. Insulin-like growth factor 1 receptor (IGF-1R) signalling is implicated in the progression of TED, leading to the development of teprotumumab (TEPEZZA®). Teprotumumab, administered intravenously, is a first-in-class monoclonal antibody that inhibits IGF-1R. In clinical trials, teprotumumab was effective at improving bulging of the eye, inflammation, double vision and TED-related quality of life. Almost one year after the cessation of treatment, clinical benefits endured in most patients. Teprotumumab was generally well tolerated, with most adverse events being mild or moderate in severity. Adverse events included muscle spasms, hearing loss and hyperglycaemia. Teprotumumab is the first targeted therapy approved for TED and represents an important advance in the management of this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.21375867 |
First-in-class monoclonal antibody that inhibits IGF-1R; administered intravenously |
Effectively reduces proptosis and other manifestations of TED, with long-term responses in most patients |
Generally well tolerated; adverse events are generally mild or moderate in severity |
1 Introduction
Thyroid eye disease (TED), also known as Graves’ ophthalmopathy or Graves’ orbitopathy, is a rare but debilitating condition that involves inflammation and expansion of the orbital tissue during the active phase of the disease [1,2,3]. It is associated with Graves’ disease, an autoimmune disorder that results in hyperthyroidism, in ≈ 90% of cases [2] and can cause pain, proptosis (bulging of the eye), diplopia (double vision), compression of the optic nerve and, in severe cases, loss of sight [1, 2]. TED is often associated with decreased quality of life, even in mild cases. The active phase of TED, which may last 1–3 years, is followed by a chronic, inactive phase where inflammation appears to resolve and the disease stabilizes [1, 3]. However, symptoms, including proptosis and diplopia, can persist and continue to affect quality of life [4, 5].
Preferred treatment options depend on the severity of the disease [1]. Mild TED can be managed with local treatments, such as eye drops, with or without selenium supplementation. For active moderate-to-severe TED, off-label treatments to suppress inflammation, such as glucocorticoids, have been used historically. Response to these treatments is often unsatisfactory, particularly with respect to proptosis, requiring further pharmacological intervention or rehabilitative surgery [1,2,3]. Safety concerns with such treatments may also be an issue [1, 3].
Insulin-like growth factor 1 receptor (IGF-1R) is overexpressed on orbital fibroblasts and fibrocytes in both active and inactive TED [6]. IGF-1R forms physical and functional complexes with the thyroid-stimulating hormone receptor (TSHR, the receptor implicated in Graves’ disease), contributing to speculation that IGF-1R has a pathogenic role in TED [7].
Teprotumumab (TEPEZZA®), a fully human immunoglobulin (Ig) G1 monoclonal antibody that inhibits IGF-1R, is the first drug to be approved for the treatment of TED in the USA [8, 9]. This article summarises the pharmacological properties of teprotumumab and reviews its efficacy and tolerability in the treatment of this potentially disfiguring and sight-threatening disease.
2 Pharmacological Properties
Teprotumumab is an IGF-1R antagonist that inhibits receptor signalling [8]. It is presumed that by blocking IGF-1R activation, teprotumumab prevents signalling through IGF-1R/TSHR complexes, thereby inhibiting autoimmune activation [7]. Cell-based studies indicate that teprotumumab inhibits IGF-1R/TSHR crosstalk in orbital fibroblasts [10], and attenuates pro-inflammatory protein expression on the surface of cultured circulating fibrocytes [11]. Circulating fibrocytes from patients with TED treated with teprotumumab also showed reduced levels of these proteins, with these reductions being associated with decreased inflammatory cytokine expression in circulating T cells [11].
The pharmacokinetics of intravenous (IV) teprotumumab are generally consistent with those of other IgG1 antibodies [12]. Population pharmacokinetic modelling in TED patients receiving the therapeutic dosage of teprotumumab (10 mg/kg followed by 20 mg/kg every 3 weeks; Sect. 5) showed that the central volume of distribution was 3.26 L and the peripheral volume of distribution was 4.32 L [8]. Teprotumumab followed a two-compartment model with a mean clearance of 0.27 L/day and mean elimination half-life of 20 days. At the therapeutic dosage of teprotumumab, a trough concentration of > 20 mg/ml is achieved, which is predicted to maintain > 90% saturation of IGF-1R [12]. At this dosage, there was a significant correlation between steady-state trough concentration and proptosis response in two TED clinical trials (Sect. 3.1) [12].
Baseline characteristics, including sex, age, body weight, ethnicity, race, smoking status and hepatic and kidney function biomarker levels, did not have a clinically meaningful effect on teprotumumab pharmacokinetics [12]. Anti-drug antibodies were detected in four (2.3%) patients in the phase II and III TED clinical trials at low levels and did not alter the pharmacokinetics of teprotumumab [13]. Teprotumumab is expected to be degraded by proteolytic enzymes and no drug interactions are expected [12]; no formal drug interaction studies have been conducted with teprotumumab [8].
3 Therapeutic Efficacy of Teprotumumab
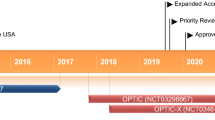
The efficacy of teprotumumab was evaluated in two randomized, double-masked, placebo-controlled, multicentre trials in patients with active, moderate-to-severe TED: the phase III OPTIC trial [14] and an earlier phase II trial [15] (Sect. 3.1). Efficacy data are also available from the open-label extension of OPTIC (OPTIC-X; Sect. 3.1.1), which enrolled patients from OPTIC who did not achieve a proptosis response (or who experienced a flare during the post-treatment follow-up) [16]. Early real-world experience with teprotumumab in the treatment of TED has also been reported (Sect. 3.2).
3.1 In Clinical Trials
The phase II and III clinical trials enrolled patients aged 18–75 years [15] or 18–80 years [14] with diagnosed Graves’ disease and active, moderate-to-severe TED that had manifested in the 9 months prior to baseline assessment. Participants were required to be euthyroid and have a Clinical Activity Score (CAS) of ≥ 4 in the more proptotic eye. CAS is a measure of inflammation (range 0–7), with a score of 0 or 1 considered inactive TED and a score of ≥ 3 considered active TED [1]. Patients who had received previous treatment for TED (except limited glucocorticoids) or who had decreased best corrected visual acuity due to optic neuropathy were excluded [14, 15].
Patients in both trials were randomized to receive a total of eight IV infusions of teprotumumab (10 mg/kg of body weight for the first dose followed by 20 mg/kg thereafter) or placebo, administered at 3-week intervals over a period of 21 weeks [14, 15]. Baseline characteristics were generally similar across the teprotumumab and placebo groups in both studies, including sex (73% female in the pooled trial populations), age (mean ≈ 51 years), tobacco use (27% current tobacco users), duration of TED (median 5.7–6.8 months since diagnosis), degree of proptosis (mean 23 mm), CAS (mean 5) and thyroid hormone levels [13]. Although patients were stratified by tobacco use, an imbalance occurred in the phase II trial (26% of teprotumumab recipients vs 41% placebo recipients were smokers) [15]. Also in the phase II trial, a greater number of patients treated with teprotumumab had diplopia at baseline (90% vs 69%).
In both trials, outcomes were measured in the intention-to-treat (ITT) population at week 24 (3 weeks after the final infusion) and were reported for the more proptotic eye at baseline (the study eye) [14, 15]. The primary outcome in OPTIC was the proportion of patients who had a proptosis response [14], while the primary outcome in the phase II trial was the proportion of patients who had an overall response i.e. both a proptosis response and a CAS response (responses are defined in Table 1) [15]. Patients in the phase II study and in OPTIC who were not included in the OPTIC-X extension study (Sect. 3.1.1) entered a 48-week off-treatment follow-up period to assess the durability of teprotumumab efficacy [14, 15].
In OPTIC, significantly more patients receiving teprotumumab than placebo experienced a proptosis response at week 24 (primary endpoint; Table 1) [14]. Sensitivity analyses in the per-protocol population were consistent with this result. In the ITT population, 56% of teprotumumab recipients experienced a proptosis response by week 6 (median time to response was 6.4 weeks) compared with 7% of placebo recipients [14]. While proptosis response was not a primary endpoint in the phase II trial [15], the US Food & Drug Administration considered it to be the preferred efficacy endpoint [17] and a comparable response rate to that in OPTIC was reported (Table 1) [8]. A pooled data analysis revealed that the proportion of patients who experienced a proptosis response across both studies was 77% with teprotumumab versus 15% with placebo (p < 0.0001) and the number-needed-to-treat (NNT) for a proptosis response was 1.6 [13]. Proptosis response results were supported by the mean changes in proptosis (mm), which indicated significant reductions with teprotumumab versus placebo (secondary endpoint; Table 1) [14, 15].
Similarly, teprotumumab was effective in producing overall responses in patients with TED. The proportion of patients who achieved an overall response at week 24 was significantly greater with teprotumumab than with placebo in both OPTIC (secondary endpoint) and the phase II trial (primary endpoint; Table 1). This treatment difference was apparent from week 6 in the phase II study (p < 0.001) [15]. The NNT for an overall response across both trials was 1.7 [13].
Additional secondary outcomes were significantly improved with teprotumumab versus placebo in each study (Table 1). These included the proportion of patients with a CAS of 0 or 1 [14] or the mean change in CAS [15], the proportion of patients who achieved a diplopia response [14], and the mean change in overall score on the Graves’ ophthalmopathy-specific quality-of-life questionnaire (GO-QOL) [14, 15]. Diplopia response was measured in the phase II study as a prespecified exploratory endpoint, with results suggesting that teprotumumab improved diplopia (Table 1) [15]. Across the two trials, the NNT for CAS of 0 or 1 and diplopia response were both 2.5 [13]. Secondary outcomes in the phase II trial also included the least-squares mean change from baseline in the GO-QOL visual-functioning subscale (21.7 with teprotumumab vs 7.5 with placebo; p < 0.001) and GO-QOL appearance subscale (12.9 vs 6.6; p = 0.10) [higher scores indicate improved quality of life] [15]. Across both trials, the least-squares mean improvements in overall GO-QOL score, the visual-functioning subscale and the appearance subscale were 19.0, 19.7 and 17.7 with teprotumumab (vs 6.3, 7.0 and 5.6 with placebo; all p ≤ 0.0003) [13].
Subgroup analyses using data pooled from the two trials showed that the rate of proptosis response with teprotumumab was greater than that with placebo across all subgroups analysed, including those based on current tobacco use, sex, age < 65 years or older, time to diagnosis of TED or Graves’ disease, baseline CAS and baseline serum thyrotropin binding inhibiting immunoglobulin (TBII) levels (p ≤ 0.0015 for all) [13]. Diplopia response was also consistent across almost all subgroups (p < 0.05 with teprotumumab vs placebo for all except current tobacco users and patients with baseline TBII levels < 10 IU/L) [13].
Post hoc analyses of ophthalmic composite outcome response (improvements from baseline of at least two of the following: resolution of eyelid swelling, CAS ≥ 2, proptosis ≥ 2 mm, lid aperture ≥ 2 mm, diplopia resolution or grade change, or globe motility ≥ 8 degrees, in at least one eye without deterioration in either eye) demonstrated that 68 (81%) teprotumumab recipients in both studies achieved a composite response [vs 38 (44%) placebo recipients; p < 0.0001], with an NNT of 2.5 [13]. A post hoc analysis in a subset of phase II and III patients with asymmetric TED (difference in exophthalmometry of ≥ 3 mm; n = 10 and 12 teprotumumab and placebo recipients) showed that teprotumumab also improved proptosis, diplopia and CAS in the non-study eye (p < 0.05 vs baseline) [18]. As the improvements in proptosis and CAS were smaller than those in the more proptotic study eye (p < 0.05), symmetry was improved [18].
Six patients in OPTIC underwent orbital imaging before and after teprotumumab treatment [19]. At week 24, these teprotumumab recipients had significantly decreased extraocular muscle (EOM) volume (p < 0.01 vs baseline) and orbital fat volume (p < 0.05). Post-treatment EOM volume did not significantly differ from a comparative control group of 12 patients without TED, although post-treatment orbital fat volume did significantly differ between teprotumumab recipients and the control group (p < 0.05). In teprotumumab recipients, overall EOM inflammation was also reduced from baseline in all evaluated orbits (eight orbits in four patients; p < 0.01) [19].
The benefits of teprotumumab for patients with TED appear to be long lasting. Follow-up data from both trials indicated there was no evidence of disease rebound 7 weeks after the last dose of teprotumumab, and the majority of responders maintained proptosis (67%; 38/57), diplopia (69% of those with baseline diplopia; 33/48) and ophthalmic composite outcome (83%; 48/58) responses 51 weeks after the last dose [13].
3.1.1 OPTIC-X Open-Label Extension
Patients enrolled in OPTIC-X following lack of proptosis response in OPTIC received a first course of teprotumumab (if they had previously received placebo) or a second course (if they had received teprotumumab), with a course consisting of eight infusions [16]. Ten of 34 patients (29%) who had a proptosis response in OPTIC experienced a relapse (a proptosis increase of ≥ 2 mm and/or CAS increase of ≥ 2 points) during the 48-week follow-up period and were also eligible to receive a course of teprotumumab in OPTIC-X [16].
Amongst 37 patients who had initially received placebo, 33 (89%) had a proptosis response after teprotumumab treatment [16]. This response rate was similar to that of the initial OPTIC trial even though patients had a longer duration of TED (median 12.9 months in OPTIC-X vs 6.3 months in OPTIC). Secondary outcomes were also similar to results in the OPTIC study. Clinical benefits appeared to be durable, with > 85% of responders maintaining proptosis (29/32 patients), overall (22/24), diplopia (12/14) and CAS 0 or 1 (20/21) responses 27 weeks after the last infusion. In patients who did not respond to a first course of teprotumumab, two of five patients had a proptosis response after retreatment. Of patients who initially responded to teprotumumab and entered the extension study after a relapse, five of eight patients achieved a proptosis response. These results indicate that retreatment with a second course of teprotumumab may be effective in TED [16].
3.2 In Real-World Studies
Currently available real-world data on the use of teprotumumab in the treatment of TED are limited, but several studies including ≥ 10 patients [20,21,22,23,24,25,26,27,28,29] suggest that teprotumumab may be effective in a more diverse disease population than that included in clinical trials. For example, in observational cohort studies of patients with heterogeneous TED (n = 74 [20] and 21 [21]), teprotumumab was associated with proptosis response rates of 71–73%, significantly improving outcomes such as proptosis (change in mm; p < 0.01 vs baseline) regardless of TED activity [20, 21] or severity [21]. A retrospective study specifically in chronic (> 2 years), stable TED showed that teprotumumab was associated with a 90% proptosis response rate, as well as significant improvements in proptosis (mm), CAS and diplopia (all p < 0.05 vs baseline; n = 31), and EOM volume and orbital fat volume (both p < 0.01; n = 15) [22]. In a case series of ten patients with TED and dysthyroid optic neuropathy, teprotumumab was associated with a significant improvement in visual function (p = 0.02 for best corrected visual acuity in affected eyes); however, improvement was limited in three patients who had severe vision loss due to long-standing optic nerve compression [23]. The patients also had a significant improvement in proptosis and CAS (both p < 0.00001 vs baseline), but not diplopia [23]. In prospective studies in patients with TED, teprotumumab reduced proptosis, CAS, orbital fat volume and EOM volume (all p < 0.01 vs baseline, n = 21) [24], and was associated with reductions in measures of facial soft tissue volume (n = 43) [25] and markers of eyelid position (n = 23) [26].
4 Tolerability of Teprotumumab
Teprotumumab was generally well tolerated in patients with TED participating in clinical trials [14, 15]. In the phase II [15] and phase III OPTIC [14] trials, 88% and 95% of teprotumumab recipients completed the treatment course, respectively. In both trials (pooled safety data), adverse events (AEs) occurred in 67 (80%) teprotumumab recipients compared with 60 (70%) placebo recipients during the randomized controlled periods [13]. Most AEs were of grade 1 or 2 severity (94% and 98% of AEs with teprotumumab and placebo, respectively), limited in duration and manageable without interrupting therapy. The incidences of the most common AEs are shown in Fig. 1. Risk difference analyses indicated that teprotumumab increased the risk of muscle spasm (by 18%; 95% CI 7.3–28.7), hearing loss (by 10%) and hyperglycaemia (by 8%; 95% CI 1.7–15.0) relative to placebo [13].
During the randomized controlled periods of the two trials, seven (8%) teprotumumab recipients experienced a serious AE, compared with one (1%) placebo recipient [13]. Three (4%) teprotumumab recipients experienced a serious AE that was deemed to be treatment-related or possibly treatment-related and led to discontinuation of teprotumumab. These were diarrhoea, an infusion-related reaction and Hashimoto’s encephalopathy (characterized by transient confusion). The case of diarrhoea occurred in a patient with pre-existing ulcerative colitis in the phase II trial [15]. Also in this trial, one patient with pre-existing ileitis and colitis developed inflammatory bowel disease (IBD), which was considered serious and led to treatment discontinuation. IBD was included in the exclusion criteria of OPTIC [14].
In the 48-week off-treatment follow-up periods of both trials (pooled data), 29 (39%) teprotumumab recipients had an AE compared with nine (20%) placebo recipients [13]. The only AE to occur at a ≥ 5% incidence in the teprotumumab group during follow-up was onychoclasis (nail breaking), occurring in seven (9%) patients compared with none in the placebo group. Treatment-related or possibly treatment-related AEs in teprotumumab recipients included skin and subcutaneous tissue disorders (five patients; most commonly onychoclasis), diabetes, worsening of hyperglycaemia, muscle spasms, and abnormal levels of lactate dehydrogenase and alkaline phosphatase (one patient each). No serious AEs related to teprotumumab occurred during this time. No deaths occurred during either the study or follow-up periods [13].
Hyperglycaemia and hearing impairment AEs may be related to teprotumumab’s mechanism of action (Sect. 2) [15, 30]. During randomized treatment with teprotumumab, hyperglycaemia was generally mild and the majority of patients with AEs of hyperglycaemia had pre-existing diabetes [13, 31]. While patients with pre-existing diabetes were more likely to have hyperglycaemia that was moderate in severity, no AEs of hyperglycaemia were serious or led to discontinuation [13, 15]. Most hearing impairment during clinical trials was transient and resolved without treatment [14, 15], but there have been case reports of long-term and potentially irreversible hearing loss [30, 32, 33].
The safety profile of teprotumumab during the OPTIC-X extension study was similar to that described above and no additional safety concerns were apparent following a second course of treatment [16]. However, one recipient of a second teprotumumab course experienced a life-threatening cerebral haemorrhage. Although this event may have been related to underlying medical conditions rather than teprotumumab, pharmacovigilance is required [16]. Since approval of teprotumumab for the treatment of TED, new AEs have been reported, including two cases of new-onset ulcerative colitis [34, 35] and one case of hyperosmolar hyperglycaemia requiring hospitalisation [36].
5 Dosage and Administration
Teprotumumab is approved in the USA for the treatment of TED and is administered as an IV infusion every 3 weeks for a total of eight infusions [8]. The first dose is administered at 10 mg/kg body weight with the following seven infusions administered at 20 mg/kg. The infusion time is initially 90 min, which can be reduced to 60 min after the first two infusions if teprotumumab is well tolerated [8].
Patients should be monitored for infusion-related reactions, which can occur during and up to 1.5 h after infusion, and hyperglycaemia during treatment with teprotumumab (Sect. 4) [8]. Patients with IBD should be monitored for exacerbation and discontinuation of teprotumumab treatment should be considered if a flare occurs. As teprotumumab may have harmful effects on foetuses, females of reproductive potential should be advised to use effective contraception. Local prescribing information should be referred to for further details on dosage and administration, warnings and precautions, and use in special population groups [8].
6 Current Status of Teprotumumab in the Management of Thyroid Eye Disease
Teprotumumab is the first approved agent in the USA specifically indicated for the treatment of TED, targeting IGF-1R, which is pathogenic in this disease [8]. Teprotumumab is not currently approved in the EU [1].
The efficacy of teprotumumab in adults with active, moderate-to-severe TED was demonstrated in two randomized, placebo-controlled trials that together included 171 patients (Sect. 3.1), one of the largest controlled trial populations of patients with TED thus far [13]. Teprotumumab treatment led to clinically meaningful improvements across a range of TED manifestations, including proptosis, inflammation (based on CAS) and diplopia, as well as in disease-specific quality of life (based on GO-QOL score) [Sect. 3.1]. Limited data from the OPTIC-X open-label extension indicate that a second course of teprotumumab may be effective in non-responders or those who experience a relapse after successful teprotumumab treatment (Sect. 3.1.1) [16]. Although clinical trial data show that most patients have an enduring response to teprotumumab ≈ 1 year after cessation of treatment (Sect. 3.1), longer-term data on the durability of clinical benefit and the need for rehabilitative surgery would be useful [1]. In a study of claims data over 2 years, a low (4.9%; 286/5,845) proportion of patients who completed a full, uninterrupted course of teprotumumab were prescribed an additional course of teprotumumab [37].
Teprotumumab is generally well tolerated, with the vast majority of patients in clinical trials completing the treatment course (Sect. 4). AEs with the greatest risk difference versus placebo were muscle spasms, hearing loss and hyperglycaemia. Patients receiving teprotumumab should be observed in case of infusion reactions, monitored for hyperglycaemia and, where indicated, worsening of IBD (Sect. 5). Pharmacovigilance monitoring around the potential for serious AEs such as cerebral haemorrhage is ongoing and further safety data, particularly around the potential for hearing loss, are needed (Sect. 4). Audiological monitoring has been recommended by some authors [30, 32].
Current, but limited, real-world data suggest that teprotumumab has potential in the treatment of chronic TED (TED traditionally considered to be inactive but still overexpressing IGF-1R [6]) and dysthyroid optic neuropathy (a serious complication of TED that can result in permanent loss of vision [23]) [Sect. 3.2]; however, data from clinical trials are lacking. Further data on the use of teprotumumab in these patient groups are awaited with interest, including the results of an ongoing phase IV study in chronic, inactive TED [38].
While no randomized trials have directly compared teprotumumab and IV glucocorticoids, a meta-analysis and matching-adjusted indirect comparison favoured teprotumumab over a glucocorticoid regime for proptosis change and diplopia response [39]. A recent consensus paper from 15 TED experts based in the USA recommended that teprotumumab be considered as the first-line treatment in adults with significant TED (i.e. CAS ≥ 4, proptosis ≥ 3 mm above normal, intermittent or constant diplopia, moderate or severe soft tissue involvement, or orbital pain and/or pressure) [40]. Recommendations also included the consideration of teprotumumab in the treatment of patients with CAS < 3, lid retraction ≥ 2 and mild compressive optic neuropathy (CON) with close monitoring. Teprotumumab was considered suitable for patients with TED for longer than 16 months and may be appropriate as a single therapy for the primary treatment of mild CON with timely initiation and close monitoring, but not for the primary treatment of severe CON [40]. As the cost of teprotumumab is manyfold greater than historical treatments for TED and may be a limiting factor in its use, cost analyses may be of benefit [41].
In conclusion, teprotumumab is the first approved therapy that targets the pathogenic mechanism underlying TED. It is an effective and generally well-tolerated treatment that reduces symptoms of TED that are often unamenable to traditional pharmacological intervention. While further data on long-term durability of benefits, safety and use in disease subpopulations outside of those included so far in clinical trials are needed, current evidence suggests that teprotumumab represents an important advance in the treatment of this debilitating, vision-threatening condition.
Data Selection Teprotumumab: 193 records identified
Duplicates removed | 8 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 101 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 43 |
Cited efficacy/tolerability articles | 24 |
Cited articles not efficacy/tolerability | 17 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were teprotumumab, Graves’ ophthalmopathy, thyroid eye disease. Records were limited to those in English language. Searches last updated 28 Oct 2022 | |
Change history
02 December 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40265-022-01823-y
References
Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves’ Orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(4):G43–67.
Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–421.
Patel A, Yang H, Douglas RS. A new era in the treatment of thyroid eye disease. Am J Ophthalmol. 2019;208:281–8.
Cockerham KP, Padnick-Silver L, Stuertz N, et al. Quality of life in patients with chronic thyroid eye disease in the United States. Ophthalmol Ther. 2021;10(4):975–87.
Wang Y, Padnick-Silver L, Francis-Sedlak M, et al. Inflammatory and noninflammatory thyroid eye disease: comparison of disease signs, symptoms, and quality of life in patients in the United States. Endocr Pract. 2022;28(9):842–6.
Ugradar S, Shi L, Wang Y, et al. Teprotumumab for non-inflammatory thyroid eye disease (TED): evidence for increased IGF-1R expression. Eye (Lond). 2021;35(9):2607–12.
Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181(6):4397–405.
Horizon Therapeutics USA Inc. TEPEZZA - teprotumumab injection, powder, lyophilized, for solution: US prescribing information. 2022. https://dailymed.nlm.nih.gov/dailymed/. Accessed 28 Oct 2022.
US Food & Drug Administration. FDA approves first treatment for thyroid eye disease [media release]. 21 Jan 2020. https://www.fda.gov/.
Krieger CC, Sui X, Kahaly GJ, et al. Inhibition of TSH/IGF-1 receptor crosstalk by teprotumumab as a treatment modality of thyroid eye disease. J Clin Endocrinol Metab. 2022;107(4):e1653–60.
Fernando R, Caldera O, Smith TJ. Therapeutic IGF-I receptor inhibition alters fibrocyte immune phenotype in thyroid-associated ophthalmopathy. Proc Natl Acad Sci USA. 2021;118(52):1–10.
Xin Y, Xu F, Gao Y, et al. Pharmacokinetics and exposure-response relationship of teprotumumab, an insulin-like growth factor-1 receptor-blocking antibody, in thyroid eye disease. Clin Pharmacokinet. 2021;60(8):1029–40.
Kahaly GJ, Douglas RS, Holt RJ, et al. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021;9(6):360–72.
Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341–52.
Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–61.
Douglas RS, Kahaly GJ, Ugradar S, et al. Teprotumumab efficacy, safety, and durability in longer-duration thyroid eye disease and re-treatment: OPTIC-X study. Ophthalmology. 2022;129(4):438–49.
US Food & Drug Administration. Teprotumumab: summary review. 2020. https://www.accessdata.fda.gov/. Accessed 28 Oct 2022.
Ugradar S, Wang Y, Mester T, et al. Improvement of asymmetric thyroid eye disease with teprotumumab. Br J Ophthalmol. 2021;106(6):755–9.
Jain AP, Gellada N, Ugradar S, et al. Teprotumumab reduces extraocular muscle and orbital fat volume in thyroid eye disease. Br J Ophthalmol. 2022;106(2):165–71.
Ho TC, Maamari RN, Kossler AL, et al. Outcomes of patients with thyroid eye disease partially treated with teprotumumab. Ophthalmic Plast Reconstr Surg. 2022. https://doi.org/10.1097/IOP.0000000000002267.
Diniz SB, Cohen LM, Roelofs KA, et al. Early experience with the clinical use of teprotumumab in a heterogenous thyroid eye disease population. Ophthalmic Plast Reconstr Surg. 2021;37(6):583–91.
Ugradar S, Kang J, Kossler AL, et al. Teprotumumab for the treatment of chronic thyroid eye disease. Eye (Lond). 2022;36(8):1553–9.
Sears CM, Wang Y, Bailey LA, et al. Early efficacy of teprotumumab for the treatment of dysthyroid optic neuropathy: a multicenter study. Am J Ophthalmol Case Rep. 2021;23:101111.
Ugradar S, Zimmerman E, Parunakian E, et al. Teprotumumab reduces orbital fat and muscle volume in patients with thyroid eye disease [abstract no. LBSAT238]. In: ENDO. 2022.
Ugradar S, Zimmerman E, Parunakian E, et al. Reversal of Graves’ disease-associated facial volume expansion following teprotumumab therapy [abstract no. LBSAT238]. In: ENDO. 2022.
Ugradar S, Braun J, Wang Y, et al. Facial and eyelid changes in thyroid eye disease are reversed by teprotumumab. Plast Reconstr Surg Glob Open. 2021;9(e3809):1–10.
Wang Y, Mester T, Ugradar S, et al. Teprotumumab for the treatment of thyroid eye disease: clinical experience from expanded access program (EAP). Invest Ophthalmol Vis Sci. 2021;62(8):3339 (abstract).
Adetunji MO, Nguyen BJ, Meer E, et al. Effect of teprotumumab on intraocular pressure in thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2021;62(8):3327 (abstract).
Douglas RS, Wang Y, Bruch J, et al. Teprotumumab use in a real-world setting: expanded access program findings. Invest Ophthalmol Vis Sci. 2021;62(8):3345.
Belinsky I, Creighton FX Jr, Mahoney N, et al. Teprotumumab and hearing loss: case series and proposal for audiologic monitoring. Ophthalmic Plast Reconstr Surg. 2022;38(1):73–8.
Smith TJ, Bhattacharya RK, Hsu K, et al. Blood glucose in thryoid eye disease (TED) in patients treated with teprotumumab: clinical trials data [abstract no. PSAT265]. In: ENDO. 2022.
Chow A, Silkiss RZ. Teprotumumab-associated chronic hearing loss screening and proposed treatments. BMJ Case Rep. 2022;15(4): e248335.
Sears CM, Azad AD, Amarikwa L, et al. Hearing dysfunction after treatment with teprotumumab for thyroid eye disease. Am J Ophthalmol. 2022;240:1–13.
Ashraf DC, Jankovic I, El-Nachef N, et al. New-onset of inflammatory bowel disease in a patient treated with teprotumumab for thyroid associated ophthalmopathy. Ophthalmic Plast Reconstr Surg. 2021;37(5):e160–4.
Safo MB, Silkiss RZ. A case of ulcerative colitis associated with teprotumumab treatment for thyroid eye disease. Am J Ophthalmol Case Rep. 2021;22(101069):1–4.
Shah K, Charitou M. A novel case of hyperglycemic hyperosmolar state after the use of teprotumumab in a patient with thyroid eye disease. AACE Clin Case Rep. 2022;8(4):148–9.
Ugradar S, Qashqai A, Holt RJ, et al. Evaluation of United States thyroid eye disease patients receiving an additional course of teprotumumab treatment over 2 years [abstract and poster]. In: 91st Annual Meeting of the American Thyroid Association. 2022.
US National Institutes of Health. ClinicalTrials.gov identifier NCT04583735. 2022. https://clinicaltrials.gov/. Accessed 28 Oct 2022.
Douglas RS, Dailey R, Subramanian PS, et al. Proptosis and diplopia response with teprotumumab and placebo vs the recommended treatment regimen with intravenous methylprednisolone in moderate to severe thyroid eye disease: a meta-analysis and matching-adjusted indirect comparison. JAMA Ophthalmol. 2022;140(4):328–35.
Douglas RS, Kossler AL, Abrams J, et al. Expert consensus on the use of teprotumumab for the management of thyroid eye disease using a modified-delphi approach. J Neuroophthalmol. 2022;42(3):334–9.
Winn BJ, Kersten RC. Teprotumumab: interpreting the clinical trials in the context of thyroid eye disease pathogenesis and current therapies. Ophthalmology. 2021;128(11):1627–51.
Acknowledgments
During the peer review process, the manufacturer of teprotumumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of Interest
Tina Nie and Yvette Lamb are salaried employees of Adis International Ltd/Springer Nature, and declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethical Approval, Consent to Participate, Consent to Publish, Availability of Data and Material, Code Availability
Not applicable.
Additional information
The manuscript was reviewed by: C. C. Krieger, Laboratory of Endocrinology and Receptor Biology, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA; B. Vaidya, Department of Endocrinology, University of Exeter Medical School, Royal Devon & Exeter Hospital, Exeter, UK.
The original article has been revised due to retrospective open choice order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nie, T., Lamb, Y.N. Teprotumumab: A Review in Thyroid Eye Disease. Drugs 82, 1663–1670 (2022). https://doi.org/10.1007/s40265-022-01804-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01804-1