Abstract
Cluster headache belongs to the group of trigeminal autonomic headaches. This review summarizes drug therapy of cluster attacks and prophylactic treatment. Neurostimulation methods are not addressed. The therapy for acute cluster attacks includes inhalation of 100% oxygen, subcutaneous administration of sumatriptan, and intranasal application of sumatriptan or zolmitriptan. Bridging therapy, which is used until oral prophylactic therapy is effective, is performed either with oral prednisolone or with a pharmacological block of the major occipital nerves. Best documented drugs for preventive treatment of cluster headache are verapamil and lithium, and possibly effective drugs are gabapentin, topiramate, divalproex sodium, and melatonin. The efficacy of monoclonal antibodies to the calcitonin gene-related peptide so far has been only demonstrated for episodic cluster headache. Several drug therapies are being investigated including ketamine, onabotulinumtoxinA, lysergic acid, and sodium oxybate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cluster headache is a rare trigeminal autonomic headache associated with severe attacks of hemifacial pain. Episodic cluster headache (90%) is distinguished from chronic cluster headache (10%). |
Therapy of single cluster attacks is by inhalation of 100% oxygen using a rebreather mask, subcutaneous administration of sumatriptan, or intranasal application of sumatriptan or zolmitriptan. |
Oral prophylaxis for cluster headache must be increased slowly, to reduce adverse events. Until this medication is effective, bridging therapy with oral prednisolone or an occipital nerve blockade may be useful. |
For prophylaxis of cluster headache, the best scientific evidence is for verapamil and lithium. Based on the results of open trials, topiramate, gabapentin, valproic acid, and melatonin, and for episodic cluster headache, galcanezumab may be effective. |
1 Introduction
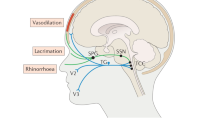
Cluster headache belongs to the group of trigeminal autonomic headaches according to the criteria of the International Headache Society [1]. In addition to the strictly hemicranial headache occurring in attacks, the pain is associated with cranial autonomic symptoms on the side of the pain.
Cluster headache is characterized by strictly unilateral attacks of severe head and facial pain. Cluster headache is a primary headache, i.e., the headache is a syndrome in its own right and not just a symptom of another disease. The attacks reach their maximum within a short time and may also have a rapid offset. The pain is extremely severe. The localization is strictly unilateral with a particular localization orbital, retro-orbital, and frontotemporal. Cranial autonomic symptoms ipsilateral to the pain occur during the attack. These include conjunctival injection and/or lacrimation, nasal congestion and/or rhinorrhea, eyelid edema, forehead and facial sweating, forehead and facial skin redness, ptosis, and or miosis. Attacks are accompanied by physical agitation or restlessness. Attacks last between 15 min and 3 h and can be triggered by the intake of alcohol if the patient is in the active period. Attack frequency ranges from one attack every 2 days to eight attacks per day. For episodic cluster headache, bouts frequently start with one to two attacks per week, may progress to several/day over 1–2 weeks, and then taper over 1–2 weeks after occurring at the high plateau for up to several or more weeks. There is a circadian rhythm with attacks occurring at the same time of the night or day. Cluster attacks are also more frequent in spring and autumn. It is clinically important to highlight that even patients with long-lasting chronic cluster headache show a cycling pattern and that an increase in attack frequency does not necessarily mean that medication is failing but rather that for some time add-on therapy may be necessary. Cluster attacks seem to get less severe once the patient gets older and may disappear altogether in most patients. The male:female ratio of occurrence may range from 2:1 to 6:1 [2].
A distinction is made between episodic and chronic cluster headache. Episodic cluster headache occurs in 90% of patients, with episodes lasting 1–2 months, followed by months to years of remission [1]. In chronic cluster headache, remissions no longer occur, or if they do, they last shorter than 12 weeks at a stretch. In some patients, there is a transition from episodic to chronic cluster headache; in a sub-group, the cluster headache is primarily chronic. In this review, we summarize the most important data on the treatment of cluster attacks and the prevention of cluster headache with recommendations for the management of these patients. Therapy of cluster headache consists of the medical abortion of the single attack, bridging therapy to cover the time until the prophylactic treatment takes effect, and the actual preventive therapy. In the field of preventive treatment of cluster headache, there are unfortunately very few placebo-controlled studies. Therefore, most treatment recommendations are limited to results from open observational studies.
2 Treatment of Cluster Attacks
The individual cluster attacks are rather short and between 15 and 180 min. Therefore, oral medication is not recommended. The cluster attacks are treated with subcutaneous sumatriptan or triptans as nasal sprays. An effective therapy is oxygen inhalation using a rebreather mask. The efficacy of this therapy has been demonstrated in placebo-controlled studies [3, 4] (Table 1).
2.1 Subcutaneous Sumatriptan
By far the most effective therapy for acute cluster attacks is the subcutaneous injection of 6 mg of sumatriptan. In the first randomized placebo-controlled trial with 39 patients, two cluster attacks were treated in random order with 6 mg of sumatriptan subcutaneously or placebo. The primary endpoint was freedom from pain or almost complete relief from headache within 10 or 15 min [5]. This endpoint [15 min] was achieved in 74% of the patients treated with sumatriptan and 26% for placebo. The success rate of 6 mg of sumatriptan subcutaneously for pain free at 10 min was 36% and for placebo 3% [5]. The second study investigated 6 and 12 mg of sumatriptan subcutaneously compared to placebo. Headache improvement to mild or no pain at 15 min was 35% for placebo, 75% for 6 mg of sumatriptan, and 80% for 12 mg of sumatriptan [6]. The pooled analysis of the Cochrane Collaboration reported pain-free results after 15 min for 48% with sumatriptan and 17% with placebo, resulting in a number needed to treat of 3.3 (95% confidence interval [CI] 2.4–5.0). The rates for headache relief after 15 min were 32% and 7%, resulting in a number needed to treat of 2.4 (95% CI 1.9–3.2) [7].
Meanwhile, a 3-mg dose of sumatriptan subcutaneously is also available in some countries and sufficient for some patients [8]. Sumatriptan is well tolerated. Rare adverse events (AEs) include local injection-site reactions, nausea, drowsiness, fatigue, and paresthesia. Contraindications, according to the label, include severe cardiovascular diseases such as coronary artery disease, ischemic stroke and transient ischemic attack, or multiple vascular risk factors. Although many patients with cluster headache have vascular risk factors [9], there have been no reports of stroke or myocardial infarction in patients with cluster headache treating their attacks with subcutaneous sumatriptan. It must be considered that some patients with chronic cluster headache inject sumatriptan three times daily or more for years.
2.2 Sumatriptan Nasal Spray
Sumatriptan nasal spray was investigated in a dose of 20 mg in a randomized double-blind placebo-controlled trial [10]. The study included patients with episodic and chronic cluster headache. Patients treated one attack with a sumatriptan 20-mg nasal spray and another attack with placebo. Headache intensity was measured on a five-point scale at time intervals between 5 and 30 min. The primary outcome was headache response defined as a decrease in pain from very severe, severe, or moderate to mild or none at 30 min. Secondary outcomes were pain-free rates, relief of associated symptoms, and AEs. The study recruited 118 patients who treated 154 cluster attacks. The responder rates at 30 min were 57% for sumatriptan and 26% for placebo (p = 0.002). Pain-free rates at 30 min were 47% for sumatriptan and 18% for placebo (p = 0.003). Sumatriptan was superior to placebo for most of the secondary outcomes. No serious AEs were recorded. Two patients reported chest tightness. The most frequently reported AE was a bitter taste with 21% with sumatriptan and 1% with placebo.
2.3 Intranasal Zolmitriptan
Intranasal zolmitriptan at doses of 5 and 10 mg was studied in two randomized placebo-controlled trials. One study of 92 patients achieved a rate of pain relief from very severe, severe, or moderate to mild or no headache at 30 min of 62% at a dose of 10 mg and 40% at a dose of 5 mg. The comparable result with placebo was 21%. The treatment effect was better in patients with episodic cluster headache than in patients with chronic cluster headache [11]. The second study evaluated 52 patients and the primary endpoint was headache relief at 30 min. This endpoint was met by 63% of patients treated with 10 mg, 50% with 5 mg, and 30% with placebo [12]. Tolerability was good and no serious AEs were reported.
A meta-analysis of the two studies included 121 patients [13]. Headache relief at 30 min was observed in 63% of patients treated with 10 mg of zolmitriptan compared with 48% treated with 5 mg of zolmitriptan and 30% treated with placebo. This corresponds to an odds ratio of 8.68 and 3.48, respectively. The most common side effects of zolmitriptan were taste disturbances, nausea, chest tightness, and fatigue.
In summary, zolmitriptan nasal spray at a preferred dose of 10 mg is effective in the treatment of cluster attacks. In indirect comparisons, the efficacy is inferior to that of the subcutaneous application of sumatriptan. Patients with episodic cluster headache respond usually better than patients with chronic cluster headache. Zolmitriptan nasal spray is only available in a 5-mg dose.
2.4 Ergotamine Derivatives
Because of the fast increase in plasma concentrations, dihydroergotamine is best applied as an aerosol spray, a suppository (no first-pass effect), or a subcutaneous injection [14,15,16]. One publication reported the use of two to three aerosol doses (0.35 mg each) with deep inhalation at the onset of an attack. The treatment did not significantly shorten the duration of the individual attack, neither did it reduce the frequency of attacks but it significantly reduced the intensity of the individual attack [14]. Ergotamine tartrate suppositories are not able to abort attacks because of the slow onset of action, but may be used as short-term prophylaxis. While these studies suggest effectiveness, no prospective randomized controlled trial has confirmed this hypothesis. Dihydroergotamine spray is not available in many European countries.
2.5 Others
A randomized, double-blind, multi-center, parallel-group study investigated the efficacy and safety of the C213 (zolmitriptan microneedle system) compared to placebo for the treatment of cluster attacks (NCT04066023). Results have not been made public.
3 Oxygen Treatment
Inhalation of oxygen is effective in up to 60% of patients. The recommended dosage is the inhalation of at least 12 L/min of 100% oxygen and has been tested in a randomized double-blind trial [17]. In some cases, up to 15 L/min is necessary, for a duration of 20 min using a non-rebreathe mask. Nasal canulae are generally not sufficient [18]. Different protocols and mask types are available [19]. A systematic review and meta-analysis published by the Cochrane Collaboration in 2015 [20] included three trials on normobaric oxygen therapy compared to sham or ergotamine tartrate (145 patients) into the quantitative analysis [17, 21, 22] and eight additional trials into the qualitative synthesis. The authors found a statistically significant effect for the termination of the attack and a 75% responder rate after 15 min. It needs to be noted that in the experience of the authors, in some patients, oxygen treatment may not end the attacks but seem to prolong them. In these cases, oxygen inhalation seems to end the attack but comes back within 1 hour. Patients will therefore report more and shorter attacks, which often leads to (unnecessary) changes in the preventative medication. In these patients, oxygen therapy should be stopped.
4 Bridging or Transitional Therapy
Patients in whom cluster bouts start (bout = active period in episodic cluster headache) and patients with chronic cluster headache require preventive therapy. Because of possible side effects, doses of available drugs have to be increased slowly to prevent AEs. Therefore, treatment with immediate effect is needed to bridge the time until long-term preventive therapy becomes effective. Most bridging studies were performed with corticosteroids. Corticosteroids can be used as oral or intravenous medication or injected into the greater occipital nerves. Corticosteroids should not be used long term because of their AE profile (Table 2).
Jammes reported results from patients with cluster headache in a double-blind placebo-controlled study with a single crossover for prednisone [23]. A single oral dose of oral prednisone in 17 patients produced a short-term improvement. An open trial in Italy assessed high-dose methylprednisolone in 13 patients with episodic cluster headache [24]. On day 8 of a new bout, methylprednisolone was given at an intravenous dose of 30 mg/kg body weight. The daily cluster attack frequency before administration of the corticosteroid was 1.38 ± 0.42 attacks and reduced to 0.83 ± 0.78 attacks 7 days after treatment (p = 0.05). Three patients experienced complete remission from cluster attacks. Prednisone in peak doses of 10–80 mg/day was used in 19 patients with cluster headache in the USA [25]. Fourteen patients (73%) had greater than 50% relief from cluster attacks and 11 (58%) no longer had cluster attacks. Recurrence of cluster headache occurred in most patients when the prednisone dose was reduced to below 10–20 mg daily.
The largest trial with 118 patients was a multicenter placebo-controlled trial conducted in Germany [26]. Patients with episodic cluster headache within a new cluster bout lasting not longer than 30 days were randomized to 100 mg of oral prednisone for 5 days followed by tapering the dose or placebo. All patients received an increasing dose of oral verapamil for long-term prevention. One hundred and nine patients were available for analysis. Patients in the prednisone group had a mean of 7.1 attacks in the first week compared with 9.5 attacks in the placebo group. Seventy-one percent of patients in both groups reported AEs (most common were nausea, dizziness, and headache, and additionally palpitations in the prednisone group).
Corticosteroid injections in the area of the ipsilateral greater occipital nerve were investigated in a double-blind placebo-controlled trial in 16 patients with episodic headache and seven patients with chronic cluster headache [27]. In patients with episodic cluster headache, the bout started less than 1 week prior to the treatment. Thirteen patients in the active arm received a suboccipital injection of both long-acting and short-acting betamethasone and ten patients received physiological saline as a placebo. Eleven patients (85%) in the corticosteroid group became attack free in the first week after the injection compared with no patient in the placebo group. The effect of corticosteroids lasted on average 4 weeks. Remissions of cluster attacks lasted between 4 and 26 months in five patients. Another study used the combination of 120 mg of methylprednisolone with lidocaine for injection into the area of the greater occipital nerve. This resulted in remissions of cluster attacks of 5–73 days [28]. Another randomized, double-blind, placebo-controlled trial enrolled patients with more than two cluster headache attacks per day [29]. Patients were randomly allocated to three suboccipital injections of cortivazol 3.75 mg within 3 days or placebo. In all patients, preventive therapy with verapamil was initiated. The primary outcome was the reduction in the number of daily cluster attacks in the 72 h, 2–4 days after the third injection. The study included 43 patients, 15 with chronic cluster headache and 28 with episodic cluster headache. Twenty of 21 patients who received cortivazol had a mean of two or fewer daily cluster attacks after injections compared with 12 of 22 patients treated with placebo (odds ratio 14.5, 95% CI 1.8–116.9; p = 0.012). Patients who were treated with cortivazol also had fewer cluster attacks in the first 15 days after injections than controls.
In conclusion, corticosteroids are effective to bridge the time interval in new bouts of episodic cluster headache or chronic cluster headache until preventive drug therapy becomes effective (see below) [30]. Corticosteroids can be used in an oral form with dose tapering, as an intravenous injection, and also in the form of ipsilateral injections into the area of the greater occipital nerve. Some patients will experience a relapse of cluster attacks with decreasing doses of corticosteroids.
5 Prevention of Cluster Headache
Patients with episodic cluster headache, in whom the duration of the bout is longer than 4–8 weeks, need preventive treatment. This is also particularly true for patients with chronic cluster headache. The most effective treatment with the best scientific evidence is verapamil, followed by lithium (Table 3).
5.1 Verapamil
Verapamil is the medication of choice as a preventive treatment in cluster headache [18]. The first randomized placebo-controlled trial with 30 patients investigated the efficacy of verapamil compared with placebo in the prophylaxis of episodic cluster headache. Patients received either verapamil 120 mg three times daily or a placebo for 2 weeks. Verapamil resulted in a significant reduction in cluster attack frequency and the need for abortive treatment [31]. Two open trials assessed the efficacy of verapamil. Seventy-two patients with episodic or chronic cluster headache were treated with verapamil starting with 200 mg [32]. Complete relief from cluster attacks was observed in 49 (94%) of 52 patients with episodic cluster headache, and 10 (55%) of 18 patients with chronic cluster headache. Most patients need 200–480 mg of verapamil. Twelve patients needed 520–960 mg. A second open study investigated verapamil in 84 patients with cluster headache [33]. Thirty-three (69%) patients improved more than 75% in terms of attack frequency. No differences with respect to efficacy were observed between episodic and chronic cluster headache. A pair-wise meta-analysis of the open-label studies indicated that 87% of patients reached either a complete response or a more than 50% reduction in attack frequency with verapamil [34].
In clinical practice, treatment with verapamil is started with 40–80 mg three times daily. Some patients require doses up to 960 mg. Verapamil can lead to heart block [35]. Therefore, a baseline electrocardiogram is required and should be repeated after dose increments of 160 mg. Treatment should last for the expected duration of a bout plus a few weeks after cluster attacks have finished. For discontinuation of verapamil, slow tapering is strictly recommended to avoid cardiac complications. Adverse events such as constipation and edema can be dose limiting. In rare cases, verapamil can lead to severe skin reactions [32, 36].
5.2 Lithium
Lithium is the second choice for the prevention of cluster headache. Lithium has more and potentially more dangerous AEs than verapamil. With one exception, the efficacy of lithium has only been investigated in open studies. Early studies involved small patient numbers [37,38,39]. On average, two-thirds of patients responded. A meta-analysis of three open trials [40,41,42] with 103 patients reported that 77% of patients reached either a complete response or a more than a 50% reduction in attack frequency with lithium [34]. A placebo-controlled study in episodic cluster headache was negative, possibly the observation period of 1 week was too short and the lithium concentrations were too low [43]. A prophylactic effect of lithium has been demonstrated in a retrospective case series for episodic cluster headache [40]. In a comparative study of lithium and verapamil, the substances did not differ in their efficacy, but verapamil was better tolerated [44]. The treatment with lithium requires monitoring of lithium plasma concentrations and kidney and thyroid function. These should be in a range of 0.4–0.8 mEq/L. Concomitant use of diuretics and non-steroidal anti-inflammatory drugs should be avoided. Duration of treatment is similar to verapamil.
5.3 Topiramate
There are predominantly case reports on the preventive therapy of cluster headache with topiramate. An initial open-label study of ten patients reported a positive effect within 3 weeks [45]. Another open study of 36 consecutive patients including 26 with episodic cluster headache and ten with chronic cluster headache reported a reduction in cluster attacks of more than 50% in 7/33 patients. Doses of topiramate ranged from 100 to 150 mg daily [46]. A third open study with 12 patients with cluster headache reported a good or moderate effect in 9/12 patients with cluster headache [47]. A prospective study from Spain with 26 patients, 12 with episodic cluster headache and 14 with chronic cluster headache used a maximum dose of topiramate of 200 mg. This resulted in remission of the cluster period in 15 patients and reduced the number of attacks by >50% in six patients [48]. The mean time to remission was 2 weeks. In summary, there are only data on topiramate from open observational studies. Topiramate is a possible alternative in patients in whom verapamil or lithium is not effective or not tolerated. Clinical experience showed promising results if the medication is well tolerated (most common side effect: cognitive disturbances, paresthesia, and weight loss). Topiramate is contra-indicated in nephrolithiasis and glaucoma. The rate of side effects can be reduced by slowly increasing the dosage by 25 mg/week. Topiramate can lead to mood swings and depression. This is relevant because of the increased risk of suicide in patients with cluster headache [49]
5.4 Sodium Valproate
A randomized, double-blind, placebo-controlled study on the effect of sodium valproate in cluster headache included 96 patients (17 chronic cluster headache), 50 in the sodium valproate group and 46 in the placebo group. Treatment was given for 2 weeks with a dose of 1000–2000 mg of sodium valproate per day. The primary study endpoint was at least a 50% reduction in the mean number of cluster attacks per week. For this endpoint, there was no statistical difference between sodium valproate and placebo [50]. However, the authors point out that this could also be due to the relatively high rate of patients in whom cluster attacks resolved spontaneously. An open-label study in patients with cluster headache reported that 73% of patients with cluster headache experienced a reduction in pain [51]. Another open study in 15 patients with cluster headache showed treatment success in 11/15 patients [52]. Cluster attacks subsided in nine patients. The dose of sodium valproate ranged from 600 to 2000 mg/day. In summary, there is no scientific evidence that sodium valproate is effective in cluster headache and the treatment of women of childbearing age is contraindicated.
5.5 Gabapentin
The first report on the use of gabapentin in a patient with refractory chronic cluster headache was published in 2000 [53]. The patient became symptom free with a daily dose of 1800 mg of gabapentin after previous therapies with lithium, verapamil, and pizotifen were unsuccessful. In a small Italian study in eight patients with episodic cluster headache and four patients with chronic cluster headache, all of whom were refractory to traditional prophylactics, a dose of 1000 mg of gabapentin resulted in a significant reduction in the length of the cluster period [54]. Another open-label study examined eight patients with refractory chronic cluster headache, six of whom responded [55]. Another small study of 14 patients treated for 3.5 months with gabapentin resulted in a mean 45% reduction in cluster attacks [56]. In summary, there is evidence only from open-label small observational series that some patients with refractory chronic cluster headache may respond to gabapentin.
5.6 Melatonin
Because of the circadian rhythm of cluster attacks, the use of melatonin for the prevention of cluster headache has also been investigated. A small randomized trial of 20 patients with cluster headache (two with chronic cluster headache) studied in a double-blind placebo-controlled design of 10 mg of melatonin or placebo at night-time for 14 days [57]. Melatonin resulted in a significant reduction in the number of cluster attacks. Five out of ten patients were classified as responders. Other small case series also reported an effect of melatonin in chronic cluster headache [58] and in nine patients as adjunctive treatment [59]. Melatonin has very good tolerability and can be used in patients who do not tolerate lithium or verapamil.
5.7 Monoclonal Antibodies Against Calcitonin Gene-Related Peptide
Calcitonin gene-related peptide (CGRP) plays an important role in the pathophysiology of migraine where both monoclonal antibodies against CGRP or the CGRP receptor are approved for migraine prophylaxis and CGRP receptor antagonists are investigated for short-term therapy of the acute migraine attack and for migraine prophylaxis. Serum levels of CGRP are also elevated in cluster headache during cluster periods [60]. Patients with active cluster headache have increased CGRP levels in tear fluid compared with healthy subjects, which are reduced to control levels after intake of attack abortive medication [61]. Galcanezumab has been investigated for the prophylaxis of both episodic and chronic cluster headache. In the episodic cluster headache trial, 106 patients were randomized to galcanezumab or placebo. In the first 3 weeks of therapy, the frequency of cluster attacks decreased by 52% in the galcanezumab group and 27% in the placebo group [62]. The chronic cluster headache trial included 237 patients [63]. The investigators observed a mean weekly reduction in cluster attacks of 5.4 with galcanezumab compared with 4.6 with placebo. This difference was not statistically significant. Galcanezumab is approved in the USA for the prophylaxis of episodic cluster headache.
Teva Pharmaceutical announced in 2019 the termination of the ENFORCE Phase III development program of fremanezumab for the treatment of cluster headache (https://www.clinicaltrialsarena.com/news/teva-fremanezumab-cluster-headache). A futility analysis of the clinical trial for episodic cluster headache showed that the primary endpoint of a mean change in the weekly average number of cluster headache attacks from baseline during the 4-week treatment duration would not be met. The study program also included a chronic cluster headache trial that was also discontinued. Another CGRP monoclonal antibody study involving fremanezumab was recently discontinued because of a lack of efficacy.
In an ongoing study with intravenous eptinezumab, eligible participants will be randomly assigned to receive treatment, in a blinded manner, of two infusions of either eptinezumab or placebo in a cross-over manner after 12 weeks during a placebo-controlled period and an active treatment period of the study. The total duration of the study is 24 weeks, including a safety follow-up period of 8 weeks (NCT04688775).
In conclusion, only preventive therapy with verapamil and lithium has evidence from randomized studies. The other drugs mentioned here can be used on a trial-and-error basis based on co-morbidities and anticipated AEs. The authors clinical experience is that the combination of verapamil with lithium or with topiramate is helpful if verapamil alone is ineffective.
6 Future Therapy of Cluster Headache
6.1 Ketamine
Ketamine is a non-competitive NMDA receptor antagonist. In a study in 2016, 13 patients with chronic headache and 16 with episodic cluster headache were treated with low doses of intravenous ketamine at 2-week intervals [64]. In patients with episodic cluster headache, this resulted in a suspension of cluster attacks for a period of between 3 and 18 months. Half of the patients with chronic cluster headache also responded to ketamine. However, the study was not placebo controlled. A ketamine-magnesium combination was studied in an open trial in patients with chronic cluster headache who were resistant to at least three preventive treatments [65]. Seventeen patients received a single ketamine infusion (0.5 mg/kg over 2 h) combined with magnesium sulfate (3000 mg). The number of daily cluster attacks decreased from 4.3 ± 2.4 before treatment to 1.3 ± 1.0 after treatment resulting in a difference of − 3.1 (95% CI − 4.5 to − 1.6), p < 0.001). Thirteen of 17 patients were responders. Mild sedation was reported by 41.2% of patients. These results indicate a possible role of ketamine. This needs to be validated by a placebo-controlled trial. As a caveat, ketamine also has a strong potential for addiction. An ongoing trial in Denmark is a proof-of-concept study for the evaluation of the effect of a ketamine intranasal spray in the treatment of chronic cluster headache (EudraCT2019-001260-29).
6.2 Pasireotide (SOM230)
Somatostatin plays an important role in the pathophysiology of pain. Somatostatin has a very short half-life and is therefore not suitable for the treatment of cluster attacks. Pasireotide has a much longer half-life than somatostatin. Patients with episodic and chronic cluster headache were enrolled in a randomized, double-blind, placebo-controlled phase II trial. After the inclusion of 28 patients, the study was discontinued because of a lack of efficacy (NCT02619617).
6.3 OnabotulinumtoxinA
OnabotulinumtoxinA was investigated in an open trial in patients with chronic cluster headache [66]. Seventeen male patients completed the study of 28 weeks. A > 50% reduction in cumulative headache minutes was observed in 59% of the participants. A second open-label trial in ten subjects with injections of onabotulinumtoxinA toward the otic ganglion did not find a statistically significant reduction in the number of attacks per week at month 2 after injection compared to the baseline [67]. Another trial with injections of onabotulinumtoxinA into the sphenopalatine ganglion is ongoing (NCT03944876).
6.4 Other Treatments
Other treatments under investigation are lysergic acid diethylamide and psilocybin. The Clusterbusters.org medication use survey aimed to investigate the effects of both conventional and alternative medications used in cluster headache [68]. The analysis included responses from 496 participants. The indoleamine hallucinogens, psilocybin, lysergic acid diethylamide, and lysergic acid amide, were comparable to or more efficacious than most conventional medications. These agents shortened or aborted a cluster period and were effective in some patients with chronic cluster headache. Sodium oxybate was reported in case reports of patients with cluster headache and sleep disturbances [69, 70].
7 Conclusions
Cluster headache is extremely distressing owing to the high intensity of pain attacks and significantly affects the quality of life of affected patients [71]. For attack therapy, only oxygen, subcutaneous sumatriptan, and intranasal sumatriptan and zolmitriptan are available and approved. Both oxygen and subcutaneous sumatriptan remain very expensive and are not reimbursed in many healthcare systems. For example, in a real-life study in Denmark, of 399 patients with cluster headache, only 30 treated their attacks with subcutaneous sumatriptan. A 100% treatment effect was achieved by 20% and a 50% pain relief by a further 10% [72]. There is a considerable need to develop new fast-acting therapies for the treatment of cluster attacks.
Even more problematic is the prophylactic therapy of cluster headache. Here, there are very few placebo-controlled studies. The best studied substances, verapamil and lithium, have significant adverse drug reactions and many patients discontinue therapy within 6–12 months. In the Danish cohort study, only 20% of patients reported being responders to prophylaxis with verapamil [72]. For the other investigated substances, there are only data from open studies. Therefore, these substances are not approved for the prophylaxis of cluster headache. Here, we would need evidence for efficacy from randomized placebo-controlled trials. However, there are considerable difficulties in designing and conducting these trials: for example, in episodic cluster headache, it is always very difficult to identify whether an improvement in the headache occurs because the cluster period ends spontaneously or whether it is a treatment effect. Moreover, surprisingly, there is a relatively high placebo effect, which makes it very difficult to prove the efficacy of a new therapy. Again, it would be desirable if there were effective and well-tolerated new therapies for the prophylaxis of cluster headache.
References
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders ICHD-3, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Fischera M, Marziniak M, Gralow I, Evers S. The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. Cephalalgia. 2008;28(6):614–8.
Cohen AS, Mathura MS, Burns B, Goadsby PJ. Randomized, double-blind placebo-controlled trial of high-flow inhaled oxygen in acute cluster headache. Cephalalgia. 2007;27:1188.
Petersen AS, Barloese MC, Lund NL, Jensen RH. Oxygen therapy for cluster headache. A mask comparison trial. A single-blinded, placebo-controlled, crossover study. Cephalalgia. 2017;37(3):214–24.
The Sumatriptan Cluster Headache Study Group. Treatment of acute cluster headache with sumatriptan. N Engl J Med. 1991;325:322–6.
Ekbom K, Monstad I, Prusinski A, Cole JA, Pilgrim AJ, Noronha D. Subcutaneous sumatriptan in the acute treatment of cluster headache: a dose comparison study. Acta Neurol Scand. 1993;88:63–9.
Law S, Derry S, Moore RA. Triptans for acute cluster headache. Cochrane Database Syst Rev. 2013;7:CD008042.
Cady RK, Munjal S, Cady RJ, Manley HR, Brand-Schieber E. Randomized, double-blind, crossover study comparing DFN-11 injection (3 mg subcutaneous sumatriptan) with 6 mg subcutaneous sumatriptan for the treatment of rapidly-escalating attacks of episodic migraine. J Headache Pain. 2017;18(1):17.
Lasaosa SS, Diago EB, Calzada JN, Benito AV. Cardiovascular risk factors in cluster headache. Pain Med. 2017;18(6):1161–7.
Van Vliet JA, Bahra A, Martin V, Ramadan N, Aurora SK, Mathew NT, et al. Intranasal sumatriptan in cluster headache: randomized placebo-controlled double-blind study. Neurology. 2003;60:630–3.
Cittadini E, May A, Straube A, Evers S, Bussone G, Goadsby PJ. Effectiveness of intranasal zolmitriptan in acute cluster headache: a randomized, placebo-controlled, double-blind crossover study. Arch Neurol. 2006;63(11):1537–42.
Rapoport AM, Mathew NT, Silberstein SD, Dodick D, Tepper SJ, Sheftell FD, et al. Zolmitriptan nasal spray in the acute treatment of cluster headache: a double-blind study. Neurology. 2007;69(9):821–6.
Hedlund C, Rapoport AM, Dodick DW, Goadsby PJ. Zolmitriptan nasal spray in the acute treatment of cluster headache: a meta-analysis of two studies. Headache. 2009;49(9):1315–23.
Andersson PG, Jespersen LT. Dihydroergotamine nasal spray in the treatment of attacks of cluster headache: a double-blind trial versus placebo. Cephalalgia. 1986;6(1):51–4.
Mathew NT. Dosing and administration of ergotamine tartrate and dihydroergotamine. Headache. 1997;37(Suppl. 1):S26-32.
Ward TN, Scott G. Dihydroergotamine suppositories in a headache clinic. Headache. 1991;31(7):465–6.
Cohen AS, Burns B, Goadsby PJ. High-flow oxygen for treatment of cluster headache: a randomized trial. JAMA. 2009;302(22):2451–7.
Hoffmann J, May A. Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol. 2018;17(1):75–83.
Oude Nijhuis JC, Haane DY, Koehler PJ. A review of the current and potential oxygen delivery systems and techniques utilized in cluster headache attacks. Cephalalgia. 2016;36(10):970–9.
Bennett MH, French C, Schnabel A, Wasiak J, Kranke P, Weibel S. Normobaric and hyperbaric oxygen therapy for the treatment and prevention of migraine and cluster headache. Cochrane Database Syst Rev. 2015;12:CD005219.
Fogan L. Treatment of cluster headache: a double blind comparison of oxygen v air inhalation. Arch Neurol. 1985;42:362–3.
Kudrow L. Response of cluster headache attacks to oxygen inhalation. Headache. 1981;21:1–4.
Jammes JL. The treatment of cluster headaches with prednisone. Dis Nerv Syst. 1975;36(7):375–6.
Antonaci F, Costa A, Candeloro E, Sjaastad O, Nappi G. Single high-dose steroid treatment in episodic cluster headache. Cephalalgia. 2005;25(4):290–5.
Couch JR, Ziegler DK. Prednisone therapy for cluster headache. Headache. 1978;18:219–21.
Obermann M, Nägel S, Ose C, Sonuc N, Scherag A, Storch P, et al. Safety and efficacy of prednisone versus placebo in short-term prevention of episodic cluster headache: a multicentre, double-blind, randomised controlled trial. Lancet Neurol. 2020;20(1):29–37.
Ambrosini A, Vandenheede M, Rossi P, Aloj F, Sauli E, Pierelli F, et al. Suboccipital injection with a mixture of rapid- and long-acting steroids in cluster headache: a double-blind placebo-controlled study. Pain. 2005;118(1–2):92–6.
Anthony M. Arrest of attacks of cluster headache by local steroid injection of the occipital nerve. In: Rose FC, editor. Migraine: clinical and research advances. London: Karger; 1985. p. 169–73.
Leroux E, Valade D, Taifas I, Vicaut E, Chagnon M, Roos C, et al. Suboccipital steroid injections for transitional treatment of patients with more than two cluster headache attacks per day: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2011;10(10):891–7.
Ducros A. Oral steroids for episodic cluster headache. Lancet Neurol. 2021;20(1):19–20.
Leone M, D’Amico D, Frediani F, Moschiano F, Grazzi L, Attanasio A, et al. Verapamil in the prophylaxis of episodic cluster headache: a double-blind study versus placebo. Neurology. 2000;54(6):1382–5.
Blau JN, Engel HO. Individualizing treatment with verapamil for cluster headache patients. Headache. 2004;44(10):1013–8.
Gabai IJ, Spierings EL. Prophylactic treatment of cluster headache with verapamil. Headache. 1989;29(3):167–8.
Pompilio G, Migliore A, Integlia D. Systematic literature review and Bayesian network meta-analysis of episodic cluster headache drugs. Eur Rev Med Pharmacol Sci. 2021;25(3):1631–40.
Cohen AS, Matharu MS, Goadsby PJ. Electrocardiographic abnormalities in patients with cluster headache on verapamil therapy. Neurology. 2007;69(7):668–75.
Lin AY, Baker BA. Verapamil-associated Stevens-Johnson syndrome. DICP. 1989;23(12):987–8.
Kudrow L. Lithium prophylaxis for chronic cluster headache. Headache. 1977;17:15–8.
Mathew N. Clinical subtypes of cluster headache and response to lithium therapy. Headache. 1978;18:26–30.
Manzoni GC, Terzano MG, Bono G, Micieli G, Martucci N, Nappi G. Cluster headache: clinical findings in 180 patients. Cephalalgia. 1983;3:21–30.
Stochino ME, Deidda A, Asuni C, Cherchi A, Manchia M, Del Zompo M. Evaluation of lithium response in episodic cluster headache: a retrospective case series. Headache. 2012;52(7):1171–5.
Damasio H, Lyon L. Lithium carbonate in the treatment of cluster headache. J Neurol. 1980;224:1–8.
Savoldi F, Bono G, Manzoni GC, Micieli G, Lanfranchi M, Nappi G. Lithium salts in cluster headache treatment. Cephalalgia. 1983;3(Suppl. 1):79–84.
Steiner TJ, Hering R, Couturier EG, Davies PT, Whitmarsh TE. Double-blind placebo-controlled trial of lithium in episodic cluster headache. Cephalalgia. 1997;17(6):673–5.
Bussone G, Leone M, Peccaresi C, Micieli G, Granella F, Magri M, et al. Double blind comparison of lithium and verapamil in cluster headache prophylaxis. Headache. 1990;30:411–7.
Wheeler SD, Carrazana EJ. Topiramate-treated cluster headache. Neurology. 1999;53(1):234–6.
Leone M, Dodick D, Rigamonti A, D’Amico D, Grazzi L, Mea E, et al. Topiramate in cluster headache prophylaxis: an open trial. Cephalalgia. 2003;23:1001–2.
Mathew NT, Kailasam J, Meadors L. Prophylaxis of migraine, transformed migraine, and cluster headache with topiramate. Headache. 2002;42(8):796–803.
Lainez M, Pascual J, Pascual A, Santonja J, Ponz A, Salvador A. Topiramate in the prophylactic treatment of cluster headache. Headache. 2003;43:784–9.
Ji Lee M, Cho SJ, Wook Park J, Kyung Chu M, Moon HS, Chung PW, et al. Increased suicidality in patients with cluster headache. Cephalalgia. 2019;39(10):1249–56.
El Amrani M, Massiou H, Bousser M. A negative trial of sodium valproate in cluster headache: methodological issues. Cephalalgia. 2002;22(3):205–8.
Gallagher RM, Mueller LL, Freitag FG. Divalproex sodium in the treatment of migraine and cluster headaches. J Am Osteopath Assoc. 2002;102(2):92–4.
Hering R, Kuritzky A. Sodium valproate in the treatment of cluster headache: an open clinical trial. Cephalalgia. 1989;9(3):195–8.
Ahmed F. Chronic cluster headache responding to gabapentin: a case report. Cephalalgia. 2000;20:252–3.
Leandri M, Luzzani M, Cruccu G, Gottlieb A. Drug-resistant cluster headache responding to gabapentin: a pilot study. Cephalalgia. 2001;21:744–6.
Schuh-Hofer S, Israel H, Neeb L, Reuter U, Arnold G. The use of gabapentin in chronic cluster headache patients refractory to first-line therapy. Eur J Neurol. 2007;14(6):694–6.
Vuković V, Lovrencić-Huzjan A, Budisić M, Demarin V. Gabapentin in the prophylaxis of cluster headache: an observational open label study. Acta Clin Croat. 2009;48(3):311–4.
Leone M, D’amico D, Moschiano F, Fraschini F, Bussone G. Melatonin versus placebo in the prophylaxis of cluster headache: a double-blind pilot study with parallel groups. Cephalalgia. 1996;16(7):494–6.
Peres MF, Rozen TD. Melatonin in the preventive treatment of chronic cluster headache. Cephalalgia. 2001;21(10):993–5.
Pringsheim T, Magnoux E, Dobson CF, Hamel E, Aube M. Melatonin as adjunctive therapy in the prophylaxis of cluster headache: a pilot study. Headache. 2002;42(8):787–92.
Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache - neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117:427–34.
Kamm K, Straube A, Ruscheweyh R. Baseline tear fluid CGRP is elevated in active cluster headache patients as long as they have not taken attack abortive medication. Cephalalgia. 2021;41(1):69–77.
Goadsby PJ, Dodick DW, Leone M, Bardos JN, Oakes TM, Millen BA, et al. Trial of galcanezumab in prevention of episodic cluster headache. N Engl J Med. 2019;381(2):132–41.
Dodick DW, Goadsby PJ, Lucas C, Jensen R, Bardos JN, Martinez JM, et al. Phase 3 randomized, placebo-controlled study of galcanezumab in patients with chronic cluster headache: results from 3-month double-blind treatment. Cephalalgia. 2020;40(9):935–48.
Granata L, Niebergall H, Langner R, Agosti R, Sakellaris L. Ketamine i. v. for the treatment of cluster headaches: an observational study. Schmerz. 2016;30(3):286–8.
Moisset X, Giraud P, Meunier E, Conde S, Perie M, Picard P, et al. Ketamine-magnesium for refractory chronic cluster headache: a case series. Headache. 2020;60(10):2537–43.
Lampl C, Rudolph M, Brautigam E. OnabotulinumtoxinA in the treatment of refractory chronic cluster headache. J Headache Pain. 2018;19(1):45.
Crespi J, Bratbak D, Dodick DW, Matharu M, Solheim O, Gulati S, et al. Open-label, multi-dose, pilot safety study of injection of onabotulinumtoxinA toward the otic ganglion for the treatment of intractable chronic cluster headache. Headache. 2020;60(8):1632–43.
Schindler EA, Gottschalk CH, Weil MJ, Shapiro RE, Wright DA, Sewell RA. Indoleamine hallucinogens in cluster headache: results of the Clusterbusters Medication Use Survey. J Psychoactive Drugs. 2015;47(5):372–81.
Hidalgo H, Uhl V, Gantenbein AR, Sándor PS, Kallweit U. Efficiency of sodium oxybate in episodic cluster headache. Headache. 2013;53(9):1490–1.
Khatami R, Tartarotti S, Siccoli MM, Bassetti CL, Sándor PS. Long-term efficacy of sodium oxybate in 4 patients with chronic cluster headache. Neurology. 2011;77(1):67–70.
Sjöstrand C, Alexanderson K, Josefsson P, Steinberg A. Sickness absence and disability pension days in patients with cluster headache and matched references. Neurology. 2020;94(21):e2213–21.
Petersen AS, Lund N, Jensen RH, Barloese M. Real-life treatment of cluster headache in a tertiary headache center: results from the Danish Cluster Headache Survey. Cephalalgia. 2021;41(5):525–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL.
Conflicts of interest/Competing interests
HCD received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from: Ipsen, Lilly, Lundbeck, Novartis, Pfizer, and Teva. The German Research Council (DFG), the German Ministry of Education and Research (BMBF), and the European Union support his headache research. HCD serves on the editorial boards of Cephalalgia, Drugs, and Lancet Neurology. HCD is a member of the Clinical Trials Committee of the IHS. AM is the editor of Cephalalgia and reports no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
HCD and AM contributed equally to the review. HCD and AM approved the final version of the manuscript and agree to be accountable for the work presented.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Diener, H.C., May, A. Drug Treatment of Cluster Headache. Drugs 82, 33–42 (2022). https://doi.org/10.1007/s40265-021-01658-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01658-z