Abstract
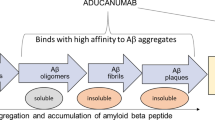
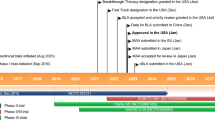
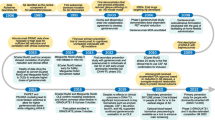
Aducanumab (aducanumab-avwa; Aduhelm™) is a human, immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against aggregated soluble and insoluble forms of amyloid β. It has been co-developed by Biogen and Eisai under license from Neurimmune for the treatment of Alzheimer's disease. In June 2021, aducanumab received its first approval in the USA for the treatment of Alzheimer’s disease. According to the US FDA prescribing information, treatment should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was initiated in clinical trials. There are no safety or effectiveness data on initiating treatment at earlier or later stages of the disease than were studied. Aducanumab is under regulatory review in Japan and in Europe. Its long-term safety and tolerability is being evaluated in a multinational phase 3b clinical study in patients with early Alzheimer’s disease (mild cognitive impairment and mild Alzheimer’s disease). This article summarizes the milestones in the development of aducanumab leading to this first approval for Alzheimer’s disease.
Similar content being viewed by others
Change history
15 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40265-021-01590-2
References
Conway ME. Alzheimer’s disease: targeting the glutamatergic system. Biogerontology. 2020;21(3):257–74.
Wisniewski T, Goni F. Immunotherapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):499–507.
Ferrero J, Williams L, Stella H, et al. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2016;2(3):169–76.
Wisniewski T, Goni F. Immunotherapeutic approaches for Alzheimer’s disease. Neuron. 2015;85(6):1162–76.
Biogen Inc. ADUHELM™ (aducanumab-avwa): US prescribing information 2021. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed 5 Jul 2021.
Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–6.
US Food & Drug Administration. FDA grants accelerated approval for Alzheimer’s drug [media release]. 7 Jun 2021. https://www.fda.gov/.
Biogen Idec, Neurimmune Therapeutics AG. Biogen Idec and Neurimmune Therapeutics announce alliance to develop treatments for Alzheimer's disease [media release]. 20 Nov 2017. http://www.biogenidec.com.
Biogen Idec, Neurimmune Holding AG. Biogen Idec and Neurimmune announce agreement on three neurodegenerative disease programs [media release]. 21 Dec 2010. http://www.biogenidec.com.
Eisai Co Ltd, Biogen Idec. Eisai and Biogen Idec enter collaboration to develop and commercialize Alzheimers disease treatments [media release]. 5 Mar 2014. http://www.eisai.com.
Eisai Co Ltd, Biogen Inc. Biogen and Eisai expand existing collaboration agreement to develop and commercialize investigational Alzheimer's disease treatments including phase 3 aducanumab [media release]. 23 Oct 2017. http://www.eisai.com.
Dunstan R, Bussiere T, Rhodes K, et al. Molecular characterization and preclinical efficacy [abstract no. P2–449]. Alzheimers Dement. 2011;7(4 Suppl):S457.
Arndt JW, Qian F, Smith BA, et al. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β. Sci Rep. 2018;8(1):6412.
Bastrup J, Hansen KH, Poulsen TBG, et al. Anti-Aβ antibody aducanumab regulates the proteome of senile plaques and closely surrounding tissue in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2021;79(1):249–65.
Kastanenka KV, Bussiere T, Shakerdge N, et al. Immunotherapy with aducanumab restores calcium homeostasis in Tg2576 mice. J Neurosci. 2016;36(50):12549–58.
Haeberlein SB, Salloway S, Aisen P, et al. Evaluation of aducanumab efficacy in early Alzheimer’s disease [oral presentation]. AD/PD 2021, Virtual Conference. 2021.
Von Hehn C, Von Rosenstiel P, Tian Y, et al. Baseline characteristics from ENGAGE and EMERGE: two phase 3 studies to evaluate aducanumab in patients with early Alzheimer's disease [abstract no. P4.1-00]. Neurology. 2019;92(15 Suppl).
Haeberlein SB, von Hehn C, Tian Y, et al. EMERGE and ENGAGE topline results: phase 3 studies of aducanumab in early Alzheimer's disease [abstract no. SO3-02-03 + oral presentation]. Alzheimers Dement. 2020;16(Suppl 9).
Cummings J, Aisen P, Lemere C, et al. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimers Res Ther. 2021;13(1):98.
Castrillo-Viguera C, Budd Haeberlein S, Von Rosenstiel P, et al. Interim analyses of fixed-dose and titration cohorts from PRIME: a randomized, double-blind, placebo-controlled phase 1b study of aducanumab [abstract no. S9.006]. Neurology. 2019;92(15 Suppl).
ClinicalTrials.gov. A Study to Evaluate Safety and Tolerability of Aducanumab in Participants With Alzheimer's Disease Who Had Previously Participated in the Aducanumab Studies 221AD103, 221AD301, 221AD302 and 221AD205 2020. https://clinicaltrials.gov/ct2/show/NCT04241068?term=aducanumab&draw=2&rank=2. Accessed 15 Jul 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process Biogen, the manufacturer of the agent under review, was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon is a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The original article has been updated: Due to ESM update.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dhillon, S. Aducanumab: First Approval. Drugs 81, 1437–1443 (2021). https://doi.org/10.1007/s40265-021-01569-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01569-z