Abstract
Netupitant/palonosetron (NEPA; Akynzeo®), available in oral and intravenous (IV) formulations, is a fixed-dose combination of the neurokinin 1 (NK1) receptor antagonist netupitant (or the prodrug, fosnetupitant, in the IV formulation) and the second-generation serotonin 3 (5-HT3) receptor antagonist palonosetron. Administered as a single dose, (fos)netupitant/palonosetron (in combination with dexamethasone) is indicated for the prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV) in adults. In clinical trials, (fos)netupitant/palonosetron plus dexamethasone was associated with high complete response rates (no emesis and no rescue medication) in the acute, delayed and overall phases in patients receiving highly or moderately emetogenic chemotherapy, with efficacy maintained over multiple cycles. Further, oral netupitant/palonosetron was found to be superior to palonosetron and non-inferior to aprepitant plus granisetron in preventing CINV in individual trials. Both the oral and IV formulations of the drug combination are well tolerated. The fixed-dose combination is concordant with guideline recommendations and provides a simple and convenient option for prophylaxis against acute and delayed CINV in patients receiving highly or moderately emetogenic chemotherapy.
Plain Language Summary
Chemotherapy-induced nausea and vomiting (CINV) is a common problem during cancer treatment. Netupitant/palonosetron (NEPA; Akynzeo®) is a fixed-dose combination of two drugs (netupitant, a neurokinin 1 receptor antagonist; and palonosetron, a serotonin 3 receptor antagonist) which target two different signalling pathways involved in the induction of vomiting. Approved for use in the prevention of acute and delayed CINV in adults, netupitant/palonosetron is given orally or via intravenous infusion as a single dose prior to chemotherapy. In clinical trials, high proportions of patients who received netupitant/palonosetron (used in combination with the corticosteroid dexamethasone) prior to chemotherapy reported no vomiting, no requirement for rescue medication, and no significant nausea in the 5 days post chemotherapy. Both the oral and intravenous formulations of the drug combination are well tolerated. In conclusion, netupitant/palonosetron is a simple, convenient and effective drug combination for the prevention of acute and delayed CINV in patients receiving chemotherapy that has a moderate to high potential to cause nausea and vomiting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.14782410 |
A fixed-dose combination of the NK1 receptor antagonist netupitant and the 5-HT3 receptor antagonist palonosetron |
Available in oral and IV formulations; administered as a single dose prior to chemotherapy |
Efficacious in the prevention of acute and delayed CINV, with efficacy maintained over multiple chemotherapy cycles |
Both formulations are well tolerated |
1 Introduction
Chemotherapy-induced nausea and vomiting (CINV) is mediated by an interaction of neurotransmitter pathways, including serotonin and substance P pathways [1]. Guidelines for the prevention of CINV recommend that patients scheduled to receive highly emetogenic chemotherapy (HEC) or moderately emetogenic chemotherapy (MEC) should be given combination prophylaxis with drugs targeting different pathways [2,3,4,5]. Different options for use in CINV prophylaxis include the dopamine and serotonin 2 (5-HT2) receptor antagonist olanzapine, neurokinin 1 (NK1) receptor antagonists [e.g. (fos)aprepitant, (fos)netupitant, rolapitant], serotonin 3 (5-HT3) receptor antagonists (e.g. dolasetron, granisetron, ondansetron, palonosetron) and corticosteroids (typically dexamethasone).
Netupitant/palonosetron (NEPA; Akynzeo®) is a fixed-dose combination of the NK1 receptor antagonist netupitant and the second-generation 5-HT3 receptor antagonist palonosetron [6, 7], with the rationale that the convenience of administering the two drugs in a fixed combination (as a single dose) has the potential to improve adherence to CINV prevention guidelines [8]. Netupitant/palonosetron is approved in the USA [6] and the EU [7] for use (in combination with dexamethasone) in the prevention of CINV in adults, and is available in oral and, more recently, intravenous (IV) formulations (with fosnetupitant, a water-soluble prodrug of netupitant, used in the IV formulation). This article reviews therapeutic efficacy and tolerability data relating to the use of (fos)netupitant/palonosetron in the prevention of CINV, and also summarises pharmacological data relating to the drug combination.
2 Pharmacodynamic Properties
Netupitant, a potent and selective NK1 receptor antagonist, acts in the prevention of nausea and vomiting by inhibiting the interaction between substance P and NK1 receptors, thereby disrupting signalling that has been linked with delayed emesis [6, 7]. Six hours after the administration of a single dose of netupitant 300 mg in healthy male subjects, NK1 receptor occupancy was ≥ 90% in several regions of the brain (including the occipital cortex, the frontal cortex and the striatum) [9]. Monitoring up to 96 h showed a slow decline in receptor occupancy.
Palonosetron, a highly potent and highly selective 5-HT3 receptor antagonist [10], primarily acts by inhibiting serotonin signalling, which is involved in the development of acute emesis [6, 7]. In addition, palonosetron displays activity in the prevention of delayed emesis [11]. Not only does palonosetron bind 5-HT3 receptors with higher affinity than the first generation 5-HT3 receptor antagonists ondansetron and granisetron [10], in vitro studies have shown that, in contrast to ondansetron and granisetron, palonosetron exhibits allosteric binding and positive cooperativity when binding to the 5-HT3 receptor [12]. Again in contrast to ondansetron and granisetron, there is evidence that palonosetron inhibits cross-talk between the NK1 and 5-HT3 receptor pathways, a characteristic that is proposed to be responsible (at least in part) for the observed effects of palonosetron in preventing delayed emesis [13].
There is also evidence that netupitant and palonosetron act synergistically, with enhanced inhibition of the substance P-mediated effects at NK1 receptors when both antagonists are present [14]. Mechanistic studies show that netupitant [15] and palonosetron [16] trigger internalisation of the NK1 and 5-HT3 receptors, respectively. Furthermore, netupitant-triggered NK1 receptor internalisation is enhanced in the presence of palonosetron in a 5-HT3 receptor-dependent manner [15].
3 Pharmacokinetic Properties
(Fos)netupitant/palonosetron is a fixed-dose combination comprised of netupitant 300 mg and palonosetron 0.5 mg in the oral formulation and fosnetupitant 235 mg and palonosetron 0.25 mg in the IV formulation [6, 7]. Unless otherwise indicated, these are the doses used for the data discussed in this section. Fosnetupitant is a water-soluble, phosphorylated prodrug of netupitant [17].
Netupitant and palonosetron pharmacokinetics are not altered to any clinically relevant extent under co-administration [6, 7]. Administration of netupitant/palonosetron with food or under fasting conditions has no clinically relevant effects on netupitant and palonosetron exposure [6, 7, 18].
3.1 (Fos)netupitant Pharmacokinetics
Following oral administration of netupitant/palonosetron, measurable concentrations of netupitant are attained in 15 min to 3 h, with netupitant reaching a maximum plasma concentration (Cmax) of ~ 400–500 ng/mL in ~ 4–5 h [6, 7]. Netupitant exposure increases greater than dose proportionally over the dose range of 10–300 mg and dose proportionally from 300 mg to 450 mg.
Following IV administration of fosnetupitant/palonosetron, fosnetupitant is rapidly converted to netupitant via metabolic hydrolysis [6, 7]. In cancer patients, fosnetupitant and netupitant each reach Cmax (3478 ng/mL and 590 ng/mL, respectively) near the end of the 30-min infusion, with the fosnetupitant concentration decreasing to < 1% of Cmax within 30 min of completion of the infusion. Over the fosnetupitant dose range of 17.6–353 mg, systemic exposure increases dose proportionally.
Netupitant has a large volume of distribution (mean Vz/F of 1982 L after a single oral dose of netupitant/palonosetron in cancer patients; mean Vz of 2627 L after a single IV dose of fosnetupitant/palonosetron), indicating wide distribution throughout the body [6]. At clinically relevant concentrations, netupitant and its major metabolites (see below) are highly (> 97%) bound to plasma proteins [6, 7].
Netupitant undergoes extensive metabolism, mediated primarily by CYP3A4 (with smaller contributions from CYP2C9 and CYP2D6), to form three major metabolites (a desmethyl-, an N-oxide- and a hydroxymethyl-derivative), each of which has pharmacological activity [6, 7].
Following a single oral dose of netupitant/palonosetron, netupitant has a mean estimated systemic clearance of ~ 20 L/h and an apparent elimination half-life of 80–88 h in cancer patients [6, 7]. Following IV infusion of fosnetupitant/palonosetron, netupitant has a mean total body clearance of 14.1 L/h and a terminal half-life of 144 h. Netupitant is primarily excreted via the faeces.
3.2 Palonosetron Pharmacokinetics
Following oral administration, palonosetron has an absolute bioavailability of ~ 97% [6, 7]. In cancer patients administered a single oral dose of netupitant/palonosetron, a Cmax for palonosetron of ~ 0.95 ng/mL was reached in ~ 5 h [6]. Palonosetron exposure is dose proportional over the dose range of 0.25–6.8 mg following single oral doses in healthy subjects. Following IV administration of fosnetupitant/palonosetron in cancer patients, palonosetron reaches a Cmax of 0.8 ng/mL near the end of the 30-min infusion [6, 7].
Palonosetron is widely distributed in the body (Vz/F of 663 L after a single oral dose of netupitant/palonosetron in cancer patients; Vz of 594 L after a single IV dose of fosnetupitant/palonosetron) [6]. In vitro plasma protein binding was 62% [6, 7].
Approximately half of administered palonosetron is metabolised to two inactive metabolites, N-oxide- and 6-S-hydroxy-palonosetron [6, 7]. In vitro studies indicate that palonosetron metabolism involves CYP2D6 (and to a lesser extent CYP3A4 and CYP1A2), although palonosetron pharmacokinetic parameters are not significantly different between poor and extensive metabolisers of CYP2D6 substrates.
Following oral administration of netupitant/palonosetron in cancer patients, palonosetron has a mean total body clearance of 10.0 L/h and a half-life of 50 h [6]. Following IV infusion of fosnetupitant/palonosetron, palonosetron has a mean total body clearance of 7.6 L/h and a terminal half-life of 58 h. Palonosetron is primarily excreted via the urine [6, 7].
3.3 Use in Specific Populations
No (fos)netupitant/palonosetron dosage adjustment is required in patients with mild to moderate hepatic impairment [6, 7]. Limited data are available on the use of (fos)netupitant/palonosetron in patients with severe hepatic impairment, and the agent should be used with caution [7] or avoided [6] in these patients.
No (fos)netupitant/palonosetron dosage adjustment is required for use in patients with mild to moderate kidney impairment under the US label [6], or with mild to severe kidney impairment under the EU label [7]. Use of (fos)netupitant/palonosetron should be avoided in patients with severe kidney impairment or end-stage renal disease (ESRD) according to the US label [6], or in patients with ESRD requiring haemodialysis according to the EU label [7].
(Fos)netupitant/palonosetron can be used without dosage adjustment in elderly patients, although caution is advised [6], particularly in patients aged > 75 years [7].
3.4 Potential Drug Interactions
Netupitant is a moderate inhibitor of CYP3A4 and there is the potential for clinically relevant drug interactions between drugs that are metabolised by CYP3A4 and (fos)netupitant/palonosetron [6, 7]. With its long half-life (Sect. 3.1), netupitant can cause increased exposure to concomitantly administered CYP3A4 substrates for 6 days after a single dose of (fos)netupitant/palonosetron. Given its metabolism by CYP3A4, dexamethasone administered in combination with (fos)netupitant/palonosetron should be used at a reduced dose (see Sect. 6). Also of particular note, patients receiving chemotherapy agents that are primarily metabolised by CYP3A4 (e.g. docetaxel, paclitaxel, etoposide, cyclophosphamide) should be closely monitored for potential adverse events caused by increased exposure to the chemotherapy agent. Co-administration of netupitant/palonosetron with oral contraceptives containing levonorgestrel and ethinyl estradiol had no clinically significant effects on their efficacy [19].
Given the metabolism of netupitant by CYP3A4 (Sect. 3.1), concomitant use of a strong CYP3A4 inducer (e.g. rifampin) can decrease the exposure to and efficacy of (fos)netupitant/palonosetron [6, 7, 19]. Similarly, concomitant use of (fos)netupitant with a strong CYP3A4 inhibitor (e.g. ketoconazole) can increase exposure to the netupitant component of the fixed-dose combination.
In vitro data have shown that (fos)netupitant is an inhibitor of the P-glycoprotein (P-gp) efflux transporter [6, 7]. However, concomitant administration of netupitant and digoxin (a P-gp substrate) did not significantly affect digoxin pharmacokinetics in healthy subjects, and a clinically relevant interaction between (fos)netupitant/palonosetron and P-gp substrates in vivo is not expected (although one could be more likely in cancer patients, especially those with kidney function impairment) [6, 7].
Local prescribing information should be consulted for full details regarding potential drug interactions involving (fos)netupitant/palonosetron.
4 Therapeutic Efficacy of NEPA
Evidence for the efficacy of (fos)netupitant/palonosetron in the prevention of CINV is available from an extensive clinical development programme, including a phase II dose-finding trial (Sect. 4.1.1) and several phase III trials involving both the oral (Sects. 4.1.2, 4.1.3 and 4.1.4) and IV (Sect. 4.2) formulations. The effectiveness of the oral formulation in the real-world setting has also been evaluated in a large, prospective, non-interventional study (Sect. 4.1.5).
In the trials, (fos)netupitant/palonosetron was administered as a single dose before the start of each chemotherapy cycle. Unless otherwise specified, the oral formulation was used as a fixed-dose combination of netupitant/palonosetron 300 mg/0.5 mg and the IV formulation was used as a fixed-dose combination of fosnetupitant/palonosetron 235 mg/0.25 mg [17, 20,21,22,23,24,25]. In addition, all patients received dexamethasone (see Tables 1, 2, 3 and 4 for details). Efficacy was assessed based on patient-reported emetic episodes, nausea severity and rescue medications intake. Key efficacy outcomes included rates of complete response (CR; defined as no emesis and no use of rescue medication), no significant nausea (defined as a maximum score of < 25 mm on a 0–100 mm visual analogue scale) and complete protection (defined as a CR and no significant nausea). Outcomes were assessed over the acute (hours 0–24), delayed (hours 25–120) and overall (hours 0–120) phases post each chemotherapy cycle.
4.1 Efficacy of Oral NEPA
4.1.1 Phase II Dose-Finding Trial in Patients Receiving HEC
A randomised, double-blind, multinational, phase II dose-finding trial was conducted to establish the optimal dose of oral netupitant for use with oral palonosetron 0.5 mg in the fixed-dose combination [20]. Included in the trial were chemotherapy-naïve patients (n = 694) aged ≥ 18 years with solid tumours who were scheduled to receive their first course of cisplatin-based HEC. Patients were randomised to receive a single dose of netupitant 100, 200 or 300 mg in combination with palonosetron 0.5 mg or a single dose of palonosetron 0.5 mg alone. The trial also included an exploratory arm in which patients received a regimen comprising oral aprepitant for 3 days, a single IV dose of ondansetron, and dexamethasone (see Table 2). The primary endpoint was the CR rate during the overall phase of chemotherapy cycle 1. Based on the results of this trial, a fixed-dose combination of netupitant/palonosetron 300 mg/0.5 mg was selected for evaluation in phase III trials. Data for the netupitant 100 and 200 mg dose groups are not presented or discussed here.
Oral netupitant/palonosetron 300 mg/0.5 mg demonstrated good efficacy in the prevention of CINV in patients receiving their first course of cisplatin-based HEC (Table 1) [20]. Compared with patients receiving palonosetron, patients receiving netupitant/palonosetron had a significantly higher CR rate during the overall phase, as well as during the acute and delayed phases, post chemotherapy. Netupitant/palonosetron was also efficacious in reducing the rates of patients experiencing significant nausea and emesis relative to palonosetron. Rates of complete protection were also higher among netupitant/palonosetron recipients compared with palonosetron recipients (Table 1). Furthermore, efficacy outcomes were similar between patients who received netupitant/palonosetron 300 mg/0.5 mg (as a single dose) and those who received the 3-day aprepitant plus ondansetron regimen, with a slight (yet consistent) numerical advantage in favour of netupitant/palonosetron (Table 2); however, no formal comparisons were made between these groups [20].
4.1.2 Phase III Trial in Patients Receiving AC Chemotherapy
The efficacy of netupitant/palonosetron versus palonosetron was further evaluated in a randomised, double-blind, multicentre, phase III trial (NCT01339260) in patients (n = 1455) receiving their first course of an anthracycline-cyclophosphamide (AC) chemotherapy regimen [22]. In the trial, chemotherapy-naive adults (~ 98% women) with solid tumours (~ 97% with breast cancer) were randomised to receive netupitant/palonosetron 300 mg/0.5 mg or palonosetron 0.5 mg. For approximately two-thirds of patients in the trial, the chemotherapy regimen consisted of doxorubicin plus cyclophosphamide, with the remaining one-third receiving epirubicin plus cyclophosphamide. The primary endpoint of the trial was the CR rate during the delayed phase of chemotherapy cycle 1.
Similar to the findings of the phase II dose-finding trial in patient receiving HEC (Sect. 4.1.1), oral netupitant/palonosetron was superior to palonosetron in preventing CINV in patients receiving their first course of AC chemotherapy [22]. CR rates were significantly higher in netupitant/palonosetron recipients than in palonosetron recipients during the acute, delayed and overall phases following cycle 1 of chemotherapy (Table 1). Compared with palonosetron, netupitant/palonosetron was also associated with significantly higher rates of no emesis, no significant nausea, and complete protection in each of the acute, delayed and overall phases, with the exceptions of no significant nausea and complete protection in the acute phase where between-group differences were non-significant (Table 1).
In an extension to the trial (with 88% of patients participating) [21], the efficacy of netupitant/palonosetron in preventing CINV was maintained over four cycles of AC chemotherapy, with CR rates and the percentages of patients experiencing no significant nausea significantly higher in netupitant/palonosetron recipients than in palonosetron recipients in the overall phase for each of the cycles (Table 3).
4.1.3 Phase III Trial in Patients Receiving MEC or HEC
Further evidence for the efficacy of netupitant/palonosetron in preventing CINV over multiple cycles of chemotherapy is available from a randomised, double-blind, multinational phase III trial (NCT01376297) [23]. Primarily designed to assess safety, this trial evaluated a single oral dose of netupitant/palonosetron 300 mg/0.5 mg versus a 3-day oral regimen of aprepitant (see Table 2 for details) plus a single dose of palonosetron 0.5 mg in chemotherapy-naïve patients (n = 413) diagnosed with a malignant tumour and scheduled to receive repeated consecutive courses of HEC or MEC. Patients with breast cancer scheduled to receive AC chemotherapy were excluded. In cycle 1, 76% of patients received MEC (primarily carboplatin or oxaliplatin) and 24% received HEC (primarily cisplatin). Seventy-five percent of patients in the trial completed at least four cycles of chemotherapy and 40% completed six cycles [23].
In both antiemetic treatment groups, high overall CR rates were observed in cycle 1 of chemotherapy (Table 2) and were maintained across multiple cycles (Table 3). Although no statistical analyses were reported, a small and consistent numerical advantage in overall CR rates was observed for netupitant/palonosetron over the aprepitant plus palonosetron regimen across cycles [23]. In a subgroup analysis, overall CR rates were similar among netupitant/palonosetron-treated patients receiving HEC (79–91% across cycles) and MEC (80–93%) [23].
4.1.4 Phase III Non-Inferiority Trial Versus Aprepitant Plus Granisetron
The efficacy of netupitant/palonosetron versus aprepitant plus granisetron (i.e. another combination of an NK1 receptor antagonist and a 5-HT3 receptor antagonist) was evaluated in a randomised, double-blind phase III non-inferiority trial conducted in Asia [24]. The trial included chemotherapy-naïve adults who were scheduled to receive their first course of cisplatin-based HEC for the treatment of a solid tumour malignancy. Patients (n = 834) were randomised to receive a single oral dose of netupitant/palonosetron 300 mg/0.5 mg or a 3-day regimen of oral aprepitant (see Table 2 for details) plus a single IV dose of granisetron 3 mg. All patients in the trial were Asian; 81% were from China, 71% were male, and lung cancer (58%) was the most common malignancy.
In the trial, a single oral dose of netupitant/palonosetron was non-inferior to a 3-day aprepitant plus granisetron regimen, with overall CR rates (primary endpoint) of 73.8% and 72.4%, respectively (between-group difference 1.5%; 95% CI − 4.5 to 7.5%), meeting the margin for non-inferiority (− 10%) [24]. Similar efficacy rates between the two antiemetic treatment groups were also observed in key secondary endpoints, including the CR rate during the acute and delayed phases, and the percentages of patients experiencing no emesis and no significant nausea during the acute, delayed and overall phases (Table 2). Further, whereas daily rates of patients with CINV events (i.e. emesis and/or use of rescue medication) were largely stable (between 13% and 15%) among patients receiving the aprepitant regimen, a steady numerical decline (from 16% on day 1 to 8% on day 5) was observed for netupitant/palonosetron recipients, with the difference between netupitant/palonosetron and aprepitant recipients reaching statistical significance on day 5 (8.0% vs 13.9%; p = 0.0063) [24].
4.1.5 Effectiveness in the Real-World Setting
Evidence for the effectiveness of oral netupitant/palonosetron in preventing CINV in the real-world setting is available from a prospective, non-interventional study conducted in Germany in 2429 adults receiving HEC or MEC for three cycles [27]. Netupitant/palonosetron was administered according to the EU label (Sect. 6). The primary outcome of the study was quality of life as measured using the Functional Living Index-Emesis (FLIE) questionnaire. Among patients in the full analysis set (n = 2173), 85% were female, breast cancer (66%) was the most common cancer type, approximately two-thirds received HEC (predominantly AC) and approximately one-third received MEC (predominantly carboplatin).
In the HEC and MEC groups, respectively, 84–88% and 82–87% of patients reported no impact on daily life (NIDL) due to vomiting across cycles 1–3, 54–58% and 59–66% reported NIDL due to nausea, and 64–66% and 67–74% reported NIDL for the combined domain of nausea and vomiting [27]. Overall, high CR rates were also observed across cycles 1–3 (89.2–90.9%, 86.9–87.1% and 82.5–83.6% in the acute, delayed and overall phases, respectively).
4.2 Efficacy of IV NEPA
Fosnetupitant 235 mg was shown to be bioequivalent (in terms of exposure) to oral netupitant 300 mg (Sect. 3) [28, 29], and palonosetron 0.25 mg is the recommended IV palonosetron dose (as a bolus administered over 30 s) when used as monotherapy for CINV [30, 31]. Furthermore, a randomized, double-blind, phase III non-inferiority trial in chemotherapy-naïve patients (n = 440) with solid tumours receiving HEC found that, based on CR rates in the acute phase (primary endpoint), palonosetron 0.25 mg given as a 30-min IV infusion was non-inferior to palonosetron 0.25 mg given as a 30-second IV bolus [32]. Thus, it was concluded that a palonosetron 0.25-mg IV infusion was appropriate for use as part of the fosnetupitant/palonosetron IV formulation.
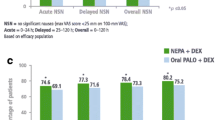
Evidence for the efficacy of IV fosnetupitant/palonosetron in the prevention of CINV is drawn from two randomised, double-blind, parallel group, multinational phase III trials (NCT02517021 [17] and NCT03403712 [25]), both of which were primarily conducted to evaluate the safety of the IV formulation. The two trials were of similar design, each comparing IV fosnetupitant/palonosetron (administered as a 30-min infusion) with oral netupitant/palonosetron (taken 60 min before chemotherapy) in chemotherapy-naïve adults. NCT02517021 enrolled adults with solid tumours (n = 404) who were scheduled to receive up to four cycles of non-AC HEC (with 96% of patients receiving cisplatin-based HEC) [17]; NCT03403712 enrolled women with breast cancer (n = 404) who were scheduled to receive up to four cycles of AC-based chemotherapy (with 60% of patients receiving epirubicin plus cyclophosphamide and 40% receiving doxorubicin plus cyclophosphamide) [25].
Although neither of these studies was designed or powered to evaluate and compare efficacy between the IV and oral (fos)netupitant/palonosetron formulations [17, 25], data from the trials provide good evidence for the efficacy of the IV formulation in the prevention of CINV (Table 4). In cycle 1, overall CR rates in IV and oral (fos)netupitant/palonosetron recipients, respectively, were 76.8% and 84.1% in NCT02517021 and 73.0% and 77.3% in NCT03403712. Furthermore, efficacy was sustained in subsequent chemotherapy cycles (Table 5).
5 Tolerability of NEPA
(Fos)netupitant/palonosetron is well tolerated based on available evidence, with the safety and tolerability being similar between the oral and IV formulations [17, 20,21,22,23,24,25, 27, 33]. The fixed-dose combination (administered with concomitant dexamethasone) has been evaluated in controlled clinical trials in patients receiving HEC or MEC (including AC-based and non-AC chemotherapy regimens), and used over single or multiple cycles, with the profile of treatment-emergent adverse events (TEAEs) observed being generally consistent with what would be expected in patient populations receiving chemotherapy [17, 20,21,22,23,24,25, 33]. Furthermore, a non-interventional study found that the safety and tolerability profile of oral netupitant/palonosetron in the real-world setting was consistent with that observed in clinical trials [27].
In clinical trials, treatment-related adverse events (TRAEs) were reported in ~ 6–15% of (fos)netupitant/palonosetron recipients, similar to the incidence observed in patient groups receiving palonosetron (without netupitant) or those receiving aprepitant-based regimens [17, 20,21,22,23,24,25, 33]. In a pooled analysis of patients receiving oral netupitant/palonosetron, the most commonly reported (incidence ≥ 1%) TRAEs were headache (3.6%), constipation (3.0%) and fatigue (1.2%) [7]. Across two phase III trials investigating the safety of IV fosnetupitant/palonosetron, infusion-site TEAEs were reported in 2% of fosnetupitant/palonosetron recipients; no events were considered to be treatment-related [17, 25]. In all key clinical trials of (fos)netupitant/palonosetron, serious TRAEs were infrequent (incidence < 1% where reported) [17, 20,21,22,23,24,25, 33]. Furthermore, no increase in the incidence, severity or general range of TEAEs was observed when (fos)netupitant/palonosetron was used over multiple (up to four, or more) cycles of chemotherapy [17, 21, 23, 25].
Although some safety concerns have been raised about potential cardiac safety issues associated with 5-HT3 receptor antagonists, no significant cardiac safety issues have been identified with (fos)netupitant/palonosetron [17, 20,21,22,23,24,25]. However, it should be noted that patients with a history of serious cardiovascular disease or predisposition to cardiac conduction abnormalities were excluded from clinical trials. In a randomised, placebo- and positively- (moxifloxacin) controlled thorough QT study in 197 subjects, netupitant/palonosetron was associated with no significant effects on individually heart rate-corrected QT interval, heart rate, PR interval, QRS interval or cardiac morphology, even at supratherapeutic doses (600 mg, not used in clinic) [34]. The development of serotonin syndrome (including fatal cases) has been reported in association with 5-HT3 receptor antagonists, mostly during concomitant use of serotonergic drugs [6, 7]. Finally, hypersensitivity reactions (including very rare cases of anaphylaxis) have been reported in patients who received palonosetron [6, 7].
6 Dosage and Administration of NEPA
In the USA, oral netupitant/palonosetron and IV fosnetupitant/palonosetron are each indicated for use in combination with dexamethasone in adults for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of cancer chemotherapy [6]. IV fosnetupitant/palonosetron is approved for use in the prevention nausea and vomiting associated with HEC; oral netupitant/palonosetron is approved for use in the prevention of nausea and vomiting associated with chemotherapy, including, but not limited to, HEC [6]. In the EU, oral netupitant/palonosetron and IV fosnetupitant/palonosetron are each indicated for use in adults for the prevention of acute and delayed nausea and vomiting associated with highly emetogenic cisplatin-based cancer chemotherapy or with MEC [7].
For the oral formulation, one capsule of netupitant/palonosetron 300 mg/0.5 mg should be taken (with or without food) approximately 1 h prior to the start of chemotherapy [6, 7]. For the IV formulation, one single-dose vial of fosnetupitant/palonosetron 235 mg/0.25 mg lyophilised powder should be reconstituted in 50 mL of 5% dextrose injection or 0.9% saline solution for injection and administered as a 30-min infusion starting approximately 30 min prior to the start of chemotherapy. In the USA, fosnetupitant/palonosetron is also available as a 20-mL solution (in a single-dose vial) which can be added to 30 mL of 5% dextrose or 0.9% saline for infusion as a 50-mL total volume [6]. Given that limited data are available on the compatibility of fosnetupitant/palonosetron with other IV substances (with the exception of IV dexamethasone), other IV substances should not be added to the IV fosnetupitant/palonosetron solution or infused simultaneously [6, 7]. Fosnetupitant/palonosetron is incompatible with any solution containing divalent cations [6, 7].
Based on animal studies, use of (fos)netupitant/palonosetron may be associated with a risk of foetal harm [6, 7], and use of the agent during pregnancy is contraindicated in the EU [7]. Local prescribing information should be consulted for full details regarding the administration of (fos)netupitant/palonosetron, including further information on limitations of use, warnings, precautions, potential drug interactions and use of the agent in specific populations.
7 Place of NEPA in the Management of CINV
Combination therapy with drugs targeting different pathways has become the standard-of-care for CINV prophylaxis [2,3,4,5]. For patients receiving HEC, current guidelines from the Multinational Association of Supportive Care in Cancer (MASCC)/European Society of Medical Oncology (ESMO) [2], the American Society of Clinical Oncology (ASCO) [3] and the National Comprehensive Cancer Network (NCCN) [4] recommend using a four-drug regimen involving olanzapine, an NK1 receptor antagonist, a 5-HT3 receptor antagonist and dexamethasone. According to the MASCC/ESMO guidelines, the inclusion of olanzapine in the regimen is optional but should be considered if nausea is an issue [2]. The NCCN guidelines also recommend as options three-drug combinations of an NK1 receptor antagonist, a 5-HT3 receptor antagonist and dexamethasone, or olanzapine, palonosetron and dexamethasone [4]. For patients receiving MEC, the guidelines generally recommend a two-drug combination of a 5-HT3 receptor antagonist and dexamethasone [3,4,5]. Alternatively, three-drug combinations of olanzapine, palonosetron and dexamethasone, or an NK1 receptor antagonist, a 5-HT3 receptor antagonist and dexamethasone are recommended by the NCCN guidelines for patients with additional patient-related risk factors or patients with previous treatment failure with the recommended two-drug combination [4]. The three-drug combination of an NK1 receptor antagonist, a 5-HT3 receptor antagonist and dexamethasone is also recommended by the MASCC/ESMO and ASCO guidelines for patients receiving carboplatin chemotherapy at any dose [5] or at an area under the curve of ≥ 4 mg/mL/min [3].
As a fixed-dose combination of an NK1 receptor antagonist and a 5-HT3 receptor antagonist, (fos)netupitant/palonosetron (used in combination with dexamethasone) aligns well with guideline-recommended prophylaxis against CINV for patients receiving HEC (with the further option of including olanzapine) or for select patients receiving MEC. Furthermore, there is evidence that netupitant and palonosetron act synergistically (Sect. 2). A range of clinical trials has shown that netupitant/palonosetron (plus dexamethasone) has good efficacy in the prevention of CINV (Sect. 4.1). In the key trials, CR rates in the overall phase post chemotherapy cycle 1 were 74–90%, with efficacy maintained with repeat netupitant/palonosetron doses over multiple cycles of chemotherapy. The netupitant/palonosetron combination was superior to palonosetron in preventing CINV (Sects. 4.1.1 and 4.1.2), and a single oral dose of netupitant/palonosetron was found to be non-inferior to a 3-day aprepitant plus granisetron regimen in patients receiving cisplatin-based HEC (Sect. 4.1.4). Further, a post hoc pooled analysis of the phase II dose-finding trial (Sect. 4.1.1), the phase III trial NCT01376297 (Sect. 4.1.3) and the phase III non-inferiority trial (Sect. 4.1.4) suggested that while netupitant/palonosetron has a similar response rate to a 3-day aprepitant regimen during the acute (day 1) and overall (days 1–5) phases post cisplatin-based chemotherapy, it may be associated with a higher CR rate during the delayed phase (days 2–5) [35]. Overall, the efficacy of netupitant/palonosetron was demonstrated in patients receiving HEC and MEC, including cisplatin-based, AC-based and non-AC regimens. In post hoc pooled subgroup analyses of data from key trials, netupitant/palonosetron was shown to be efficacious in patients with breast [36], lung [37] and gynaecological cancers [38], and in older patients [39]. Findings from a study of patients in the real-world setting were consistent with data from the key clinical trials (Sect. 4.1.5). Although based on trials that were primarily designed to assess safety, available evidence shows that the more recently developed IV fosnetupitant/palonosetron fixed-dose combination has similar efficacy to the oral netupitant/palonosetron formulation (Sect. 4.2).
Both the oral and IV formulations of (fos)netupitant/palonosetron are well tolerated, and no increase in the incidence, severity or general range of TEAEs was observed with use of the agent over multiple cycles (Sect. 5). The most commonly reported TRAEs included headache, constipation and fatigue. With the IV formulation, no treatment-related infusion-site reactions were observed in the pivotal trials (Sect. 5). The apparent low risk of infusion-site reactions with fosnetupitant/palonosetron is possibly due to the simplified IV formulation, with fosnetupitant requiring no surfactant, emulsifier or solubility enhancer [17]. Further, it suggests a potential advantage over fosaprepitant, which has been associated with a risk of infusion-site reactions (including some severe reactions) [40].
(Fos)netupitant and palonosetron exhibit complementary pharmacokinetic profiles, with both drugs having long half-lives and being widely distributed in the body (Sect. 3) [41]. Furthermore, there appear to be no significant drug–drug interactions between the two components in the fixed-drug combination. There are some other potential drug–drug interactions to be aware of during use of (fos)netupitant/palonosetron, most notably involving substrates, inducers or inhibitors of CYP3A4 (Sect. 3).
Besides efficacy, safety, tolerability and potential drug interaction considerations, pharmacoeconomic considerations may be important for decisions regarding choice of CINV prophylaxis. In this regard, several pharmacoeconomic analyses have been performed, based on healthcare settings in a variety of countries (including the USA, Germany, Greece, Italy, Mexico and Singapore) comparing netupitant/palonosetron with other combinations of an NK1- and a 5-HT3 receptor antagonist (all combinations used with dexamethasone) [42,43,44,45,46,47]. Although the analyses were subject to several limitations, netupitant/palonosetron performed favourably relative to the comparators across the analyses, with pharmacoeconomic benefits of netupitant/palonosetron primarily arising from a reduction in costs associated with CINV events.
In summary, (fos)netupitant/palonosetron (administered with dexamethasone) is well-tolerated and efficacious in the prevention of acute and delayed CINV in patients receiving HEC or MEC, with tolerability and efficacy maintained over multiple cycles. The fixed-dose combination is concordant with guideline recommendations, and provides a simple and convenient option for CINV prophylaxis, targeting two pathways central to the development of nausea and vomiting, both acute and delayed. The availability of the drug combination in oral and IV formulations provides additional convenience, particularly for patients unable to tolerate one or the other route of administration. Administered as a single dose once per chemotherapy cycle, (fos)netupitant/palonosetron minimises any risk of patient non-compliance to CINV prophylaxis, which in turn has the potential to improve adherence to chemotherapy by reducing the impact of CINV. While further head-to-head trials comparing (fos)netupitant/palonosetron with other CINV prophylaxis combinations would be of interest, in conclusion, (fos)netupitant/palonosetron oral and IV formulations provide effective, simple, convenient, guideline-concordant options to consider for prophylaxis against acute and delayed CINV in patients receiving HEC or MEC.
Data Selection—Netupitant/Palonosetron: 286 records identified
Duplicates removed | 60 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 160 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 19 |
Cited efficacy/tolerability articles | 11 |
Cited articles not efficacy/tolerability | 36 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were: Akynzeo, netupitant-palonosetron, fosnetupitant-palonosetron, pro-netupitant-palonosetron, palonosetron-netupitant, palonosetron-fosnetupitant, palonosetron-pro-netupitant. Records were limited to those in English language. Searches last updated 07 June 2021. | |
Change history
24 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40265-021-01607-w
References
Adel N. Overview of chemotherapy-induced nausea and vomiting and evidence-based therapies. Am J Manag Care. 2017;23(14 Suppl):S259–65.
Herrstedt J, Roila F, Warr D, et al. 2016 Updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following high emetic risk chemotherapy. Support Care Cancer. 2017;25(1):277–88.
Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: ASCO guideline update. J Clin Oncol. 2020;38(24):2782–97.
National Comprehensive Cancer Network. NCCN Clinical Practice Guildelines in Oncology (NCCN Guidelines®): antiemesis (version 1.2021). 2020. https://www.nccn.org. Accessed 24 May 2021.
Roila F, Warr D, Hesketh PJ, et al. 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following moderately emetogenic chemotherapy. Support Care Cancer. 2017;25(1):289–94.
US FDA. Akynzeo® (netupitant and palonosetron capsules; fosnetupitant and palonosetron for injection; fosnetupitant and palonosetron injection): US prescribing information. 2020. https://www.fda.gov. Accessed 24 May 2021.
European Medicines Agency. Akynzeo: summary of product characteristics 2021. https://www.ema.europa.eu. Accessed 24 May 2021.
Keating GM. Netupitant/palonosetron: a review in the prevention of chemotherapy-induced nausea and vomiting. Drugs. 2015;75(18):2131–41.
European Medicines Agency. Akynzeo: EPAR—public assessment report. 2015. https://www.ema.europa.eu. Accessed 24 May 2021.
Wong EH, Clark R, Leung E, et al. The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol. 1995;114(4):851–9.
Yang LP, Scott LJ. Palonosetron: in the prevention of nausea and vomiting. Drugs. 2009;69(16):2257–78.
Rojas C, Stathis M, Thomas AG, et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008;107(2):469–78.
Rojas C, Li Y, Zhang J, et al. The antiemetic 5-HT3 receptor antagonist palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther. 2010;335(2):362–8.
Stathis M, Pietra C, Rojas C, et al. Inhibition of substance P-mediated responses in NG108-15 cells by netupitant and palonosetron exhibit synergistic effects. Eur J Pharmacol. 2012;689(1–3):25–30.
Thomas AG, Stathis M, Rojas C, et al. Netupitant and palonosetron trigger NK1 receptor internalization in NG108-15 cells. Exp Brain Res. 2014;232(8):2637–44.
Rojas C, Thomas AG, Alt J, et al. Palonosetron triggers 5-HT3 receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol. 2010;626(2–3):193–9.
Schwartzberg L, Roeland E, Andric Z, et al. Phase III safety study of intravenous NEPA: a novel fixed antiemetic combination of fosnetupitant and palonosetron in patients receiving highly emetogenic chemotherapy. Ann Oncol. 2018;29(7):1535–40.
Calcagnile S, Lanzarotti C, Gutacker M, et al. Evaluation of the effect of food and age on the pharmacokinetics of oral netupitant and palonosetron in healthy subjects: a randomized, open-label, crossover phase 1 study. Clin Pharmacol Drug Dev. 2015;4(5):377–86.
Calcagnile S, Lanzarotti C, Rossi G, et al. Effect of netupitant, a highly selective NK1 receptor antagonist, on the pharmacokinetics of palonosetron and impact of the fixed dose combination of netupitant and palonosetron when coadministered with ketoconazole, rifampicin, and oral contraceptives. Support Care Cancer. 2013;21(10):2879–87.
Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25(7):1340–6.
Aapro M, Karthaus M, Schwartzberg L, et al. NEPA, a fixed oral combination of netupitant and palonosetron, improves control of chemotherapy-induced nausea and vomiting (CINV) over multiple cycles of chemotherapy: results of a randomized, double-blind, phase 3 trial versus oral palonosetron. Support Care Cancer. 2017;25(4):1127–35.
Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25(7):1328–33.
Gralla RJ, Bosnjak SM, Hontsa A, et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol. 2014;25(7):1333–9.
Zhang L, Lu S, Feng J, et al. A randomized phase III study evaluating the efficacy of single-dose NEPA, a fixed antiemetic combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Ann Oncol. 2018;29(2):452–8.
Schwartzberg L, Navari R, Clark-Snow R, et al. Phase IIIb safety and efficacy of intravenous NEPA for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients with breast cancer receiving initial and repeat cycles of anthracycline and cyclophosphamide (AC) chemotherapy. Oncologist. 2020;25(3):e589–97.
Schwartzberg L, Karthaus M, Rossi G, et al. Fixed combination of oral NEPA (netupitant-palonosetron) for the prevention of acute and delayed chemotherapy-induced nausea and vomiting in patients receiving multiple cycles of chemotherapy: efficacy data from 2 randomized, double-blind phase III studies. Cancer Med. 2019;8(5):2064–73.
Karthaus M, Oskay-Özcelik G, Wülfing P, et al. Real-world evidence of NEPA, netupitant-palonosetron, in chemotherapy-induced nausea and vomiting prevention: effects on quality of life. Future Oncol. 2020;16(14):939–53.
European Medicines Agency. Akynzeo: EPAR—assessment report variation. 2020. https://www.ema.europa.eu. Accessed 24 May 2021.
US FDA. Akynzeo (fosnetupitant and palonosetron) for injection, for intravenous use: multidiscipline review. 2017. https://www.fda.gov. Accessed 24 May 2021.
US FDA. Palonosetron hydrochloride injection, for intravenous use: US prescribing information. 2016. https://www.fda.gov. Accessed 24 May 2021.
European Medicines Agency. Aloxi: summary of product characteristics. 2018. https://www.ema.europa.eu. Accessed 24 May 2021.
Karthaus M, Szabò P, Voisin D, et al. Phase III study of palonosetron (PALO) given as 30-min IV infusion (IV inf) versus 30-sec IV bolus (IV bol) for prevention of chemotherapy-induced nausea and vomiting (CINV) associated with highly emetogenic chemotherapy (HEC). J Clin Oncol. 2017;35(31 Suppl 1):227.
Aapro M, Hesketh PJ, Jordan K, et al. Safety of an oral fixed combination of netupitant and palonosetron (NEPA): pooled data from the phase II/III clinical program. Oncologist. 2016;21(4):494–502.
Spinelli T, Moresino C, Baumann S, et al. Effects of combined netupitant and palonosetron (NEPA), a cancer supportive care antiemetic, on the ECG of healthy subjects: an ICH E14 thorough QT trial. Springerplus. 2014;3(389):1–11.
Navari RM, Binder G, Bonizzoni E, et al. Single-dose netupitant/palonosetron versus 3-day aprepitant for preventing chemotherapy-induced nausea and vomiting: a pooled analysis. Future Oncol. 2021. https://doi.org/10.2217/fon-2021-0023.
Rugo HS, Rossi G, Rizzi G, et al. Efficacy of NEPA (netupitant/palonosetron) across multiple cycles of chemotherapy in breast cancer patients: a subanalysis from two phase III trials. Breast. 2017;33:76–82.
Hesketh PJ, Palmas M, Nicolas P. Preventing chemotherapy-induced nausea and vomiting in patients with lung cancer: efficacy of NEPA (netupitant-palonosetron), the first combination antiemetic. Support Care Cancer. 2018;26(4):1151–9.
Bošnjak SM, Stamatovic L, Borroni ME, et al. Efficacy and safety of oral NEPA (netupitant/palonosetron), the first fixed-combination antiemetic, in patients with gynecological cancers receiving platinum-based chemotherapy. Int J Gynecol Cancer. 2018;28(6):1153–61.
Aapro M, Jordan K, Gralla RJ, et al. Safety and efficacy of NEPA, an oral fixed combination of netupitant and palonosetron, in older patients. J Geriatr Oncol. 2017;8(1):56–63.
US FDA. Emend (fosaprepitant for injection): US prescribing information. 2019. https://www.fda.gov. Accessed 24 May 2021.
Gilmore J, Bernareggi A. Complementary pharmacokinetic profiles of netupitant and palonosetron support the rationale for their oral fixed combination for the prevention of chemotherapy-induced nausea and vomiting. J Clin Pharmacol. 2019;59(4):472–87.
Botteman M, Nickel K, Corman S, et al. Cost-effectiveness of a fixed combination of netupitant and palonosetron (NEPA) relative to aprepitant plus granisetron (APR + GRAN) for prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a trial-based analysis. Support Care Cancer. 2020;28(2):857–66.
Bourhis F, Eriksson J, Ruffo P, et al. NEPA, an oral fixed combination of netupitant and palonosetron, is a cost-effective intervention for the prevention of chemotherapy-induced nausea and vomiting (CINV) in Germany and Greece [abstract no. PCN174]. Value Health. 2018;21(Suppl 3):S43–4.
Lim SW, Loh KWJ, Boisseau S, et al. Netupitant and palonosetron (NEPA), is a cost-effective intervention for the prevention of chemotherapy-induced nausea and vomiting (CINV) in Singapore [abstract no. PCN206]. Value Health. 2019;22(Suppl 3):S475–6.
Park SH, Binder G, Corman S, et al. Budget impact of netupitant/palonosetron for the prevention of chemotherapy-induced nausea and vomiting. J Med Econ. 2019;22(8):840–7.
Restelli U, Saibene G, Nardulli P, et al. Cost-utility and budget impact analyses of the use of NEPA for chemotherapy-induced nausea and vomiting prophylaxis in Italy. BMJ Open. 2017;7(e015645):1–9.
Rubio Ponce R, Díaz O, Sinta Cortes G, et al. Economic evaluation of palonosetron/netupitant for the treatment of chemotherapy-related nausea and vomiting [abstract no. PCN54]. Value Health. 2019;22(Suppl 2):S66.
Acknowledgements
During the peer review process, the manufacturer of netupitant/palonosetron was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
M. Shirley is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: R. A. Clark-Snow, Oncology Supportive Care Consultant, Kansas, USA; J. M. du Plessis, Faculty of Health Sciences, North-West University, Potchefstroom, South Africa; A. Maeda, Department of Pharmacy, Aichi Cancer Center Hospital, Nagoya, Aichi, Japan.
The original version of this article was revised due to a retrospective Open Access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shirley, M. Netupitant/Palonosetron: A Review in Chemotherapy-Induced Nausea and Vomiting. Drugs 81, 1331–1342 (2021). https://doi.org/10.1007/s40265-021-01558-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01558-2