Abstract
The transient receptor potential vanilloid-1 (TRPV1) is a non-specific cation channel known for its sensitivity to pungent vanilloid compound (i.e. capsaicin) and noxious stimuli, including heat, low pH or inflammatory mediators. TRPV1 is found in the somatosensory system, particularly primary afferent neurons that respond to damaging or potentially damaging stimuli (nociceptors). Stimulation of TRPV1 evokes a burning sensation, reflecting a central role of the channel in pain. Pharmacological and genetic studies have validated TRPV1 as a therapeutic target in several preclinical models of chronic pain, including cancer, neuropathic, postoperative and musculoskeletal pain. While antagonists of TRPV1 were found to be a valuable addition to the pain therapeutic toolbox, their clinical use has been limited by detrimental side effects, such as hyperthermia. In contrast, capsaicin induces a prolonged defunctionalisation of nociceptors and thus opened the door to the development of a new class of therapeutics with long-lasting pain-relieving effects. Here we review the list of TRPV1 agonists undergoing clinical trials for chronic pain management, and discuss new indications, formulations or combination therapies being explored for capsaicin. While the analgesic pharmacopeia for chronic pain patients is ancient and poorly effective, modern TRPV1-targeted drugs could rapidly become available as the next generation of analgesics for a broad spectrum of pain conditions.

Similar content being viewed by others
References
Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. Calcium-permeable ion channels in pain signaling. Physiol Rev. 2014;94(1):81–140 (2014/01/03).
Lapointe TK, Basso L, Iftinca MC, Flynn R, Chapman K, Dietrich G, et al. TRPV1 sensitization mediates postinflammatory visceral pain following acute colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309(2):G87-99 (2015/05/30).
Domenichiello AF, Ramsden CE. The silent epidemic of chronic pain in older adults. Prog Neuro-psychopharmacol Biol Psychiatry. 2019;93:284–90 (6538291Epub 2019/04/21).
Basso L, Altier C. Transient receptor potential channels in neuropathic pain. Curr Opin Pharmacol. 2017;32:9–15 (2016/11/12).
Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32 (2768555Epub 2009/04/30).
Offiah I, McMahon SB, O’Reilly BA. Interstitial cystitis/bladder pain syndrome: diagnosis and management. Int Urogynecol J. 2013;24(8):1243–56 (2013/02/23).
Harbaugh CM, Suwanabol PA. Optimizing pain control during the opioid epidemic. Surg Clin N Am. 2019;99(5):867–83 (2019/08/27).
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24 (1997/12/31 23:16).
Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107(4):490–502 (3169333Epub 2011/08/20).
Baranidharan G, Das S, Bhaskar A. A review of the high-concentration capsaicin patch and experience in its use in the management of neuropathic pain. Ther Adv Neurol Disord. 2013;6(5):287–97 (3755533Epub 2013/09/03).
Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224(5216):285–7 (1969/10/18).
Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13(12):1511–8 (3058928Epub 2010/11/16).
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–43 (1998/10/13).
Cuypers E, Yanagihara A, Karlsson E, Tytgat J. Jellyfish and other cnidarian envenomations cause pain by affecting TRPV1 channels. FEBS Lett. 2006;580(24):5728–32 (1800888Epub 2006/10/03).
Hakim MA, Jiang W, Luo L, Li B, Yang S, Song Y, et al. Scorpion toxin, BmP01, induces pain by targeting TRPV1 channel. Toxins. 2015;7(9):3671–87 (4591660Epub 2015/09/22).
Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, et al. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444(7116):208–12 (2006/11/10).
Yang S, Yang F, Wei N, Hong J, Li B, Luo L, et al. A pain-inducing centipede toxin targets the heat activation machinery of nociceptor TRPV1. Nat Commun. 2015;6:8297 (4589873Epub 2015/10/01).
Boillat A, Alijevic O, Kellenberger S. Calcium entry via TRPV1 but not ASICs induces neuropeptide release from sensory neurons. Mol Cell Neurosci. 2014;61:13–22 (2014/05/06).
Gouin O, L’Herondelle K, Lebonvallet N, Le Gall-Ianotto C, Sakka M, Buhe V, et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell. 2017;8(9):644–61 (5563280Epub 2017/04/02).
Barnes PJ. Neurogenic inflammation and asthma. J Asthma. 1992;29(3):165–80 (1992/01/01).
Butler CA, Heaney LG. Neurogenic inflammation and asthma. Inflamm Allergy Drug Targets. 2007;6(2):127–32 (2007/08/19).
Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15(8):1063–7 (3520068Epub 2012/07/28).
Levine JD, Moskowitz MA, Basbaum AI. The contribution of neurogenic inflammation in experimental arthritis. J Immunol. 1985;135(2 Suppl):843s-s847 (1985/08/01).
Pabbidi MR, Premkumar LS. Role of transient receptor potential channels Trpv1 and Trpm8 in diabetic peripheral neuropathy. J Diabetes Treat. 2017;2017(4):29 (6317870Epub 2017/01/01).
Ramachandran R. Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40(3):301–14 (2018/03/24).
Schaper NC, Huijberts M, Pickwell K. Neurovascular control and neurogenic inflammation in diabetes. Diabetes Metab Res Rev. 2008;24(Suppl 1):S40–4 (2008/04/30).
Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575(Pt 2):555–71 (1819458Epub 2006/06/24).
Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24(18):4300–12 (6729438Epub 2004/05/07).
Asfaha S, Cenac N, Houle S, Altier C, Papez MD, Nguyen C, et al. Protease-activated receptor-4: a novel mechanism of inflammatory pain modulation. Br J Pharmacol. 2007;150(2):176–85 (2042908Epub 2006/12/21).
Cernit V, Senecal J, Othman R, Couture R. Reciprocal regulatory interaction between TRPV1 and Kinin B1 receptor in a rat neuropathic pain model. Int J Mol Sci. 2020;21(3):821 (7037982Epub 2020/02/06).
Devesa I, Ferrandiz-Huertas C, Mathivanan S, Wolf C, Lujan R, Changeux JP, et al. AlphaCGRP is essential for algesic exocytotic mobilization of TRPV1 channels in peptidergic nociceptors. Proc Natl Acad Sci USA. 2014;111(51):18345–50 (4280602Epub 2014/12/10).
Efendiev R, Bavencoffe A, Hu H, Zhu MX, Dessauer CW. Scaffolding by A-kinase anchoring protein enhances functional coupling between adenylyl cyclase and TRPV1 channel. J Biol Chem. 2013;288(6):3929–37 (3567646Epub 2012/12/25).
Eskander MA, Ruparel S, Green DP, Chen PB, Por ED, Jeske NA, et al. Persistent nociception triggered by nerve growth factor (NGF) is mediated by TRPV1 and oxidative mechanisms. J Neurosci. 2015;35(22):8593–603 (4452557Epub 2015/06/05).
Fu Q, Cheng J, Gao Y, Zhang Y, Chen X, Xie J. Protease-activated receptor 4: a critical participator in inflammatory response. Inflammation. 2015;38(2):886–95 (2014/08/15).
Shim WS, Tak MH, Lee MH, Kim M, Koo JY, Lee CH, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27(9):2331–7 (6673467Epub 2007/03/03).
Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol. 2002;88(1):544–8 (2002/07/02).
Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98(12):6951–6 (34459Epub 2001/05/24).
Wang Y, Feng C, He H, He J, Wang J, Li X, et al. Sensitization of TRPV1 receptors by TNF-alpha orchestrates the development of vincristine-induced pain. Oncol Lett. 2018;15(4):5013–9 (5840530Epub 2018/03/20).
Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24(24):4211–23 (1356334Epub 2005/12/02).
Basso L, Lapointe TK, Iftinca M, Marsters C, Hollenberg MD, Kurrasch DM, et al. Granulocyte-colony-stimulating factor (G-CSF) signaling in spinal microglia drives visceral sensitization following colitis. Proc Natl Acad Sci USA. 2017;114(42):11235–40 (5651747Epub 2017/10/05).
Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6(5):357–72 (2007/04/28).
Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol. 2012;165(4):787–801 (3312478Epub 2011/07/30).
Flynn R, Chapman K, Iftinca M, Aboushousha R, Varela D, Altier C. Targeting the transient receptor potential vanilloid type 1 (TRPV1) assembly domain attenuates inflammation-induced hypersensitivity. J Biol Chem. 2014;289(24):16675–87 (4059113Epub 2014/05/09).
Bolcskei K, Helyes Z, Szabo A, Sandor K, Elekes K, Nemeth J, et al. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117(3):368–76 (2005/09/10).
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–13 (2000/04/15).
Christoph T, Bahrenberg G, De Vry J, Englberger W, Erdmann VA, Frech M, et al. Investigation of TRPV1 loss-of-function phenotypes in transgenic shRNA expressing and knockout mice. Mol Cell Neurosci. 2008;37(3):579–89 (2008/02/06).
Kasama S, Kawakubo M, Suzuki T, Nishizawa T, Ishida A, Nakayama J. RNA interference-mediated knock-down of transient receptor potential vanilloid 1 prevents forepaw inflammatory hyperalgesia in rat. Eur J Neurosci. 2007;25(10):2956–63 (2007/05/19).
Lee JY, Shin TJ, Choi JM, Seo KS, Kim HJ, Yoon TG, et al. Antinociceptive curcuminoid, KMS4034, effects on inflammatory and neuropathic pain likely via modulating TRPV1 in mice. Br J Anaesth. 2013;111(4):667–72 (2013/05/31).
Salat K, Filipek B. Antinociceptive activity of transient receptor potential channel TRPV1, TRPA1, and TRPM8 antagonists in neurogenic and neuropathic pain models in mice. J Zhejiang Univ Sci B. 2015;16(3):167–78 (4357366Epub 2015/03/07).
Yamamoto W, Sugiura A, Nakazato-Imasato E, Kita Y. Characterization of primary sensory neurons mediating static and dynamic allodynia in rat chronic constriction injury model. J Pharm Pharmacol. 2008;60(6):717–22 (2008/05/24).
Pabbidi RM, Yu SQ, Peng S, Khardori R, Pauza ME, Premkumar LS. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;4:9 (2275252Epub 2008/03/04).
Cui YY, Xu H, Wu HH, Qi J, Shi J, Li YQ. Spatio-temporal expression and functional involvement of transient receptor potential vanilloid 1 in diabetic mechanical allodynia in rats. PLoS ONE. 2014;9(7):e102052 (4096595Epub 2014/07/16).
Ristoiu V, Shibasaki K, Uchida K, Zhou Y, Ton BH, Flonta ML, et al. Hypoxia-induced sensitization of transient receptor potential vanilloid 1 involves activation of hypoxia-inducible factor-1 alpha and PKC. Pain. 2011;152(4):936–45 (2011/03/08).
Kahya MC, Naziroglu M, Ovey IS. Modulation of diabetes-induced oxidative stress, apoptosis, and Ca(2+) entry through TRPM2 and TRPV1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol Neurobiol. 2017;54(3):2345–60 (2016/03/10).
Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17 (1142339Epub 2005/04/29).
Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11 (1892784Epub 2007/05/25).
Khomula EV, Viatchenko-Karpinski VY, Borisyuk AL, Duzhyy DE, Belan PV, Voitenko NV. Specific functioning of Cav3.2 T-type calcium and TRPV1 channels under different types of STZ-diabetic neuropathy. Biochim Biophys Acta. 2013;1832(5):636–49 (2013/02/05).
Narayanaswamy H, Facer P, Misra VP, Timmers M, Byttebier G, Meert T, et al. A longitudinal study of sensory biomarkers of progression in patients with diabetic peripheral neuropathy using skin biopsies. J Clin Neurosci. 2012;19(11):1490–6 (2012/06/19).
Chen Y, Yang C, Wang ZJ. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience. 2011;193:440–51 (2011/07/19).
Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25(12):3126–31 (6725088Epub 2005/03/25).
Menendez L, Juarez L, Garcia E, Garcia-Suarez O, Hidalgo A, Baamonde A. Analgesic effects of capsazepine and resiniferatoxin on bone cancer pain in mice. Neurosci Lett. 2006;393(1):70–3 (2005/10/26).
Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth. 2009;102(2):251–8 (2008/11/29).
Shinoda M, Ogino A, Ozaki N, Urano H, Hironaka K, Yasui M, et al. Involvement of TRPV1 in nociceptive behavior in a rat model of cancer pain. J Pain. 2008;9(8):687–99 (2008/05/06).
Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient receptor potential vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain. 2010;6:15 (2848188Epub 2010/03/09).
Gao Y, Cao E, Julius D, Cheng Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature. 2016;534(7607):347–51 (4911334Epub 2016/06/10).
Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504(7478):107–12 (4078027Epub 2013/12/07).
Moiseenkova-Bell VY, Stanciu LA, Serysheva II, Tobe BJ, Wensel TG. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc Natl Acad Sci USA. 2008;105(21):7451–5 (2396679Epub 2008/05/21).
Roberts JC, Davis JB, Benham CD. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995(2):176–83 (2003/12/16).
Palkar R, Lippoldt EK, McKemy DD. The molecular and cellular basis of thermosensation in mammals. Curr Opin Neurobiol. 2015;34:14–9 (4512934Epub 2015/01/27).
Yarmolinsky DA, Peng Y, Pogorzala LA, Rutlin M, Hoon MA, Zuker CS. Coding and plasticity in the mammalian thermosensory system. Neuron. 2016;92(5):1079–92 (5145739Epub 2016/11/15).
Christie S, Wittert GA, Li H, Page AJ. Involvement of TRPV1 channels in energy homeostasis. Front Endocrinol. 2018;9:420 (6079260Epub 2018/08/16).
Senaris R, Ordas P, Reimundez A, Viana F. Mammalian cold TRP channels: impact on thermoregulation and energy homeostasis. Pflugers Arch Eur J Physiol. 2018;470(5):761–77 (2018/04/28).
Amantini C, Ballarini P, Caprodossi S, Nabissi M, Morelli MB, Lucciarini R, et al. Triggering of transient receptor potential vanilloid type 1 (TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of urothelial cancer cells in an ATM-dependent manner. Carcinogenesis. 2009;30(8):1320–9 (2009/06/09).
Friedman JR, Richbart SD, Merritt JC, Brown KC, Denning KL, Tirona MT, et al. Capsaicinoids: multiple effects on angiogenesis, invasion and metastasis in human cancers. Biomed Pharmacother. 2019;118:109317 (6759410Epub 2019/08/14).
Sterle I, Zupancic D, Romih R. Correlation between urothelial differentiation and sensory proteins P2X3, P2X5, TRPV1, and TRPV4 in normal urothelium and papillary carcinoma of human bladder. BioMed Res Int. 2014;2014:805236 (4020497Epub 2014/05/29).
Zhang S, Wang D, Huang J, Hu Y, Xu Y. Application of capsaicin as a potential new therapeutic drug in human cancers. J Clin Pharm Ther. 2020;45(1):16–28 (2019/09/24).
Mistretta F, Buffi NM, Lughezzani G, Lista G, Larcher A, Fossati N, et al. Bladder cancer and urothelial impairment: the role of TRPV1 as potential drug target. BioMed Res Int. 2014;2014:987149 (4034493Epub 2014/06/06).
Baskaran P, Markert L, Bennis J, Zimmerman L, Fox J, Thyagarajan B. Assessment of pharmacology, safety, and metabolic activity of capsaicin feeding in mice. Sci Rep. 2019;9(1):8588 (6565628Epub 2019/06/15).
Baskaran P, Krishnan V, Fettel K, Gao P, Zhu Z, Ren J, et al. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int J Obes (Lond). 2017;41(5):739–49 (5413365Epub 2017/01/21).
Lee E, Jung DY, Kim JH, Patel PR, Hu X, Lee Y, et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015;29(8):3182–92 (4511197Epub 2015/04/19).
Choowanthanapakorn M, Lu KW, Yang J, Hsieh CL, Lin YW. Targeting TRPV1 for body weight control using TRPV1(−/−) mice and electroacupuncture. Sci Rep. 2015;5:17366 (4664894Epub 2015/12/02).
Baker K, Raemdonck K, Dekkak B, Snelgrove RJ, Ford J, Shala F, et al. Role of the ion channel, transient receptor potential cation channel subfamily V member 1 (TRPV1), in allergic asthma. Respir Res. 2016;17(1):67 (4890475Epub 2016/06/04).
Cantero-Recasens G, Gonzalez JR, Fandos C, Duran-Tauleria E, Smit LA, Kauffmann F, et al. Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J Biol Chem. 2010;285(36):27532–5 (2934619Epub 2010/07/20).
Choi JY, Lee HY, Hur J, Kim KH, Kang JY, Rhee CK, et al. TRPV1 blocking alleviates airway inflammation and remodeling in a chronic asthma murine model. Allergy Asthma Immunol Res. 2018;10(3):216–24 (5911440Epub 2018/04/21).
McGarvey LP, Butler CA, Stokesberry S, Polley L, McQuaid S, Abdullah H, et al. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J Allergy Clin Immunol. 2014;133(3):704 e4-712 e4 (2013/11/12).
Li YR, Gupta P. Immune aspects of the bi-directional neuroimmune facilitator TRPV1. Mol Biol Rep. 2019;46(1):1499–510 (2018/12/17).
Alenmyr L, Hogestatt ED, Zygmunt PM, Greiff L. TRPV1-mediated itch in seasonal allergic rhinitis. Allergy. 2009;64(5):807–10 (2009/02/18).
Basith S, Cui M, Hong S, Choi S. Harnessing the therapeutic potential of capsaicin and its analogues in pain and other diseases. Molecules. 2016;21(8):966 (6272969Epub 2016/07/28).
Fattori V, Hohmann MS, Rossaneis AC, Pinho-Ribeiro FA, Verri WA. Capsaicin: current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules. 2016;21(7):844 (6273101Epub 2016/07/02).
Lu M, Chen C, Lan Y, Xiao J, Li R, Huang J, et al. Capsaicin-the major bioactive ingredient of chili peppers: bio-efficacy and delivery systems. Food Funct. 2020;11(4):2848–60 (2020/04/05).
Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol. 2013;716(1–3):61–76 (2013/03/19).
Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020;72(2):149–62 (2020/01/08).
Bonezzi C, Costantini A, Cruccu G, Fornasari DMM, Guardamagna V, Palmieri V, et al. Capsaicin 8% dermal patch in clinical practice: an expert opinion. Expert Opin Pharmacother 2020:1-11 (2020/06/09).
Mayo Clinic—Drugs and Supplements. https://www.mayoclinic.org/drugs-supplements/capsaicin-topical-route/description/drg-20062561(2020/06/10).
Services USDoHaH. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. 2019. https://www.hhs.gov/ash/advisory-committees/pain/reports/index.html(2020/06/10).
Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17(10):3525–37.
Wang S, Asgar J, Joseph J, Ro JY, Wei F, Campbell JN, et al. Ca(2+) and calpain mediate capsaicin-induced ablation of axonal terminals expressing transient receptor potential vanilloid 1. J Biol Chem. 2017;292(20):8291–303 (5437236Epub 2017/04/01).
Touska F, Marsakova L, Teisinger J, Vlachova V. A “cute” desensitization of TRPV1. Curr Pharm Biotechnol. 2011;12(1):122–9 (2010/10/12).
Joseph J, Wang S, Lee J, Ro JY, Chung MK. Carboxyl-terminal domain of transient receptor potential vanilloid 1 contains distinct segments differentially involved in capsaicin- and heat-induced desensitization. J Biol Chem. 2013;288(50):35690–702 (3861621Epub 2013/11/01).
Yang F, Xiao X, Cheng W, Yang W, Yu P, Song Z, et al. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat Chem Biol. 2015;11(7):518–24 (4472570Epub 2015/06/09).
Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108(3):421–30 (2002/02/21).
Yuan P. Structural biology of thermoTRPV channels. Cell Calcium. 2019;84:102106 (6893863Epub 2019/11/15).
Lau SY, Procko E, Gaudet R. Distinct properties of Ca2+-calmodulin binding to N- and C-terminal regulatory regions of the TRPV1 channel. J Gen Physiol. 2012;140(5):541–55 (3483115Epub 2012/10/31).
Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA. 2003;100(13):8002–6 (164702Epub 2003/06/17).
Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123(1):53–62 (2217413Epub 2003/12/31).
Grycova L, Lansky Z, Friedlova E, Obsilova V, Janouskova H, Obsil T, et al. Ionic interactions are essential for TRPV1 C-terminus binding to calmodulin. Biochem Biophys Res Commun. 2008;375(4):680–3 (2008/08/30).
Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54(6):905–18 (2007/06/22).
Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch Eur J Physiol. 1996;431(6):828–37 (1996/04/01).
Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280(14):13424–32 (2005/02/05).
Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWT. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35(4):721–31 (2002/08/27).
Mandadi S, Numazaki M, Tominaga M, Bhat MB, Armati PJ, Roufogalis BD. Activation of protein kinase C reverses capsaicin-induced calcium-dependent desensitization of TRPV1 ion channels. Cell Calcium. 2004;35(5):471–8 (2004/03/09).
Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278(50):50080–90 (2003/09/25).
Brauchi S, Orta G, Mascayano C, Salazar M, Raddatz N, Urbina H, et al. Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc Natl Acad Sci USA. 2007;104(24):10246–51 (1891241Epub 2007/06/06).
Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27(26):7070–80 (6672228Epub 2007/06/29).
Lukacs V, Yudin Y, Hammond GR, Sharma E, Fukami K, Rohacs T. Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons. J Neurosci. 2013;33(28):11451–63 (3724548Epub 2013/07/12).
Senning EN, Collins MD, Stratiievska A, Ufret-Vincenty CA, Gordon SE. Regulation of TRPV1 ion channel by phosphoinositide (4,5)-bisphosphate: the role of membrane asymmetry. J Biol Chem. 2014;289(16):10999–1006 (4036241Epub 2014/03/07).
Ufret-Vincenty CA, Klein RM, Hua L, Angueyra J, Gordon SE. Localization of the PIP2 sensor of TRPV1 ion channels. J Biol Chem. 2011;286(11):9688–98 (3058964Epub 2011/01/13).
Gamse R, Lackner D, Gamse G, Leeman SE. Effect of capsaicin pretreatment on capsaicin-evoked release of immunoreactive somatostatin and substance P from primary sensory neurons. Naunyn–Schmiedeberg's Arch Pharmacol. 1981;316(1):38–41
Gamse R, Petsche U, Lembeck F, Jancso G. Capsaicin applied to peripheral nerve inhibits axoplasmic transport of substance P and somatostatin. Brain Res. 1982;239(2):447–-62 (1982/05/13).
Jessell TM, Iversen LL, Cuello AC. Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res. 1978;152(1):183–8 (1978/08/18).
Liu Z, Li Z. Regulation of galanin and galanin receptor 2 expression by capsaicin in primary cultured dorsal root ganglion neurons. In vitro Cell Dev Biol Anim. 2008;44(8-9):379-84 (2008/06/17).
Yaksh TL, Farb DH, Leeman SE, Jessell TM. Intrathecal capsaicin depletes substance P in the rat spinal cord and produces prolonged thermal analgesia. Science. 1979;206(4417):481–3 (1979/10/26).
Smutzer G, Jacob JC, Tran JT, Shah DI, Gambhir S, Devassy RK, et al. Detection and modulation of capsaicin perception in the human oral cavity. Physiol Behav. 2018;194:120-31 (2018/05/12).
Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136(1–2):202–10 (2008/03/14).
Jeong KY. Changes in TRPV1-Mediated physiological function in rats systemically treated with capsaicin on the neonate. Int J Mol Sci. 2020;21(9) (7247669Epub 2020/05/06).
Huang YB, Lin YH, Lu TM, Wang RJ, Tsai YH, Wu PC. Transdermal delivery of capsaicin derivative-sodium nonivamide acetate using microemulsions as vehicles. Int J Pharm. 2008;349(1–2):206–11 (2007/09/04).
Muzzalupo R, Tavano L, Cassano R, Trombino S, Ferrarelli T, Picci N. A new approach for the evaluation of niosomes as effective transdermal drug delivery systems. Eur J Pharm Biopharm. 2011;79(1):28–35 (2011/02/10).
Tavano L, Alfano P, Muzzalupo R, de Cindio B. Niosomes vs microemulsions: new carriers for topical delivery of Capsaicin. Colloids Surf B, Biointerfaces. 2011;87(2):333–9 (2011/06/21).
Teixeira MJ, Menezes LM, Silva V, Galhardoni R, Sasson J, Okada M, et al. Liposomal topical capsaicin in post-herpetic neuralgia: a safety pilot study. Arq Neuropsiquiatr. 2015;73(3):237–40 (2015/03/26).
Predel HG, Ebel-Bitoun C, Peil B, Weiser TW, Lange R. Efficacy and Safety of diclofenac + capsaicin gel in patients with acute back/neck pain: a multicenter randomized controlled study. Pain Ther. 2020;9(1):279–96 (7203310Epub 2020/03/30).
Capsaicin + diclofenac gel in acute back pain or neck pain. 2016. https://www.clinicaltrials.gov/ct2/show/results/NCT02700815(2020/06/20).
Galvez R, Navez ML, Moyle G, Maihofner C, Stoker M, Ernault E, et al. Capsaicin 8% patch repeat treatment in nondiabetic peripheral neuropathic pain: a 52-week, open-label, single-arm, safety study. Clin J Pain. 2017;33(10):921–31 (2017/09/06).
Safety and effectiveness of repeated administration of QUTENZA patches for treatment of pain caused by nerve damage (STRIDE). 2010. https://www.clinicaltrials.gov/ct2/show/NCT01252160(2020/06/23).
Localized neuropathic pain: topical treatment versus systemic treatment (PELICAN). 2017. https://www.clinicaltrials.gov/ct2/show/study/NCT03348735(2020/05/08).
Clinical trial assessing the efficacy of capsaicin patch (Qutenza®) in cancer patients with neuropathic pain (CAPSONCO). 2017. https://www.clinicaltrials.gov/ct2/show/NCT03317613(2020/05/08).
Evaluation in the treatment of neuropathic pain post breast surgery (CAPTRANE). 2018. https://www.clinicaltrials.gov/ct2/show/NCT03794388(2020/05/08).
Capsaicin 8% patch for spinal cord injury neuropathic pain (capsaicin). 2015. https://www.clinicaltrials.gov/ct2/show/NCT02441660(2020/05/09).
0.075% capsaicin lotion for the treatment of painful diabetic neuropathy. 2017. https://www.clinicaltrials.gov/ct2/show/study/NCT03113448(2020/06/11).
Capsaicin in treatment of rhinogenic headache. 2020. https://www.clinicaltrials.gov/ct2/show/NCT03330639(2020/06/11).
Billard M TJ, Fleming M, Warneke T, Qiu Y, Ly N, Aronstein W, Moore W. A Phase 2 double-blind clinical trial to examine the comparative effects on osteoarthritic knee pain of CGS-200-1 (1% capsaicin topical liquid), CGS-200-5 (5% capsaicin topical liquid), and CGS-200-0 (vehicle, no capsaicin) Arthritis Rheumatol 2019;71(suppl 10); https://www.acrabstracts.org/abstract/a-phase-2-double-blind-clinical-trial-to-examine-the-comparative-effects-on-osteoarthritic-knee-pain-of-cgs-200-1-1-capsaicin-topical-liquid-cgs-200-5-5-capsaicin-topical-liquid-and-cgs-200-0-v/(2020/06/11).
A phase 2 clinical trial examining the effects on osteoarthritic knee pain of CGS-200-1, CGS-200-5 and vehicle control. 2019. https://www.clinicaltrials.gov/ct2/show/NCT03528369(2020/06/11).
Osteoarthritis knee pain relief study of 0.25% 920-CGS-200. 2020. https://www.clinicaltrials.gov/ct2/show/results/NCT03124407(2020/06/11).
Capsaicin patches in knee osteoarthritis in obese patients (CHILI-OB). 2017. https://www.clinicaltrials.gov/ct2/show/NCT03153813(2020/06/11).
Bourne N, Bernstein DI, Stanberry LR. Civamide (cis-capsaicin) for treatment of primary or recurrent experimental genital herpes. Antimicrob Agents Chemother. 1999;43(11):2685–8 (89543Epub 1999/10/30).
Salat K, Jakubowska A, Kulig K. Zucapsaicin for the treatment of neuropathic pain. Expert Opin Investig Drugs. 2014;23(10):1433–40 (2014/08/30).
Saper JR, Klapper J, Mathew NT, Rapoport A, Phillips SB, Bernstein JE. Intranasal civamide for the treatment of episodic cluster headaches. Arch Neurol. 2002;59(6):990–4 (2002/06/18).
Civamide nasal solution for postherpetic neuralgia of the trigeminal nerve. 2017. https://www.clinicaltrials.gov/ct2/show/NCT01886313(2020/06/15).
Evaluation of civamide patch in treatment of postherpetic neuralgia and post-incisional neuralgia. 2010. https://www.clinicaltrials.gov/ct2/show/NCT00845923(2020/06/15).
Civamide Nasal solution for cluster headache (ECH). 2011. https://www.clinicaltrials.gov/ct2/show/NCT01341548(2020/06/15).
The effect of injection site cooling on pain experienced after the administration of CNTX-4975-05 into the knee. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03472677(2020/07/01).
A study to compare levels of capsaicin after intra-articular injection and topical application in patients with painful knee osteoarthritis. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03576508(2020/06/25).
A clinical study to test efficacy and safety of CNTX-4975-05 in patients with osteoarthritis knee pain. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03429049(2020/07/01).
Stevens RM, Ervin J, Nezzer J, Nieves Y, Guedes K, Burges R, et al. Randomized, double-blind, placebo-controlled trial of intraarticular trans-capsaicin for pain associated with osteoarthritis of the knee. Arthritis Rheumatol. 2019;71(9):1524–33 (6772016Epub 2019/03/20).
A clinical study to test efficacy and safety of repeat doses of CNTX-4975-05 in patients with osteoarthritis knee pain. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03660943(2020/07/01).
Yu Y, Lu ST. Trans-capsaicin for pain reduction and improvement in function in patients with knee osteoarthritis: comment on the article by Stevens et al. Arthritis Rheumatol. 2020. https://www.ncbi.nlm.nih.gov/pubmed/32293119(2020/07/08).
Concentric Analgesics-Press releases. https://www.concentricanalgesics.com/press-releases(2020/07/08).
Concentric Analgesics—Press release. 2018. https://www.concentricanalgesics.com/fda-grants-breakthrough-therapy-designation-for-concentric-analgesics-ca-008-in-post-surgical-pain(2020/07/08).
Gottlieb IJB, A.; Solanki, D.; Singla, N.; Shafer, S.; Brennan, T.; Hodge, C.; Donovan, J.; Royal, M. A Randomized Placebo-Controlled Trial of Intraoperative Administration of CA-008 for Postoperative Analgesia after Bunionectomy 44th annual regional anesthesiology and acute pain medicine meeting. 2019. https://www.epostersonline.com/ASRASPRING19/node/1194(2020/07/15).
Pain study in total knee arthroplasty. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03731364(2020/06/10).
Safety, tolerability, pharmacokinetics, and preliminary efficacy of intraoperative administration of CA-008 for the correction of hallux valgus deformity. 2017. https://www.clinicaltrials.gov/ct2/show/results/NCT03307837(2020/06/10).
Study Evaluating the Safety, Efficacy and Pharmacokinetics of CA-008. 2019. https://www.clinicaltrials.gov/ct2/show/NCT04203537(2020/06/10).
Study in Subjects Undergoing Complete Abdominoplasty. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03789318(2020/06/10).
Open-Label CA-008 in Bunionectomy. 2019. https://www.clinicaltrials.gov/ct2/show/NCT03885596(2020/06/14).
Study of CA-008 in Bunionectomy Patients. 2018. https://www.clinicaltrials.gov/ct2/show/record/NCT03599089(2020/06/14).
Acs G, Biro T, Acs P, Modarres S, Blumberg PM. Differential activation and desensitization of sensory neurons by resiniferatoxin. J Neurosci. 1997;17(14):5622–8 (6793835Epub 1997/07/15).
Karai LJ, Russell JT, Iadarola MJ, Olah Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J Biol Chem. 2004;279(16):16377–87 (2004/02/14).
Szallasi A, Szabo T, Biro T, Modarres S, Blumberg PM, Krause JE, et al. Resiniferatoxin-type phorboid vanilloids display capsaicin-like selectivity at native vanilloid receptors on rat DRG neurons and at the cloned vanilloid receptor VR1. Br J Pharmacol. 1999;128(2):428–34 (1571651Epub 1999/10/08).
Caudle RM, Karai L, Mena N, Cooper BY, Mannes AJ, Perez FM, et al. Resiniferatoxin-induced loss of plasma membrane in vanilloid receptor expressing cells. Neurotoxicology. 2003;24(6):895–908 (2003/11/26).
Neubert JK, Karai L, Jun JH, Kim HS, Olah Z, Iadarola MJ. Peripherally induced resiniferatoxin analgesia. Pain. 2003;104(1–2):219–28 (2003/07/12).
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–41 (1999/04/14).
Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103(5):1052–9 (2005/10/27).
Brown JD, Saeed M, Do L, Braz J, Basbaum AI, Iadarola MJ, et al. CT-guided injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Science Transl Med. 2015;7(305):305ra145 (4854290Epub 2015/09/18).
Neubert JK, Mannes AJ, Karai LJ, Jenkins AC, Zawatski L, Abu-Asab M, et al. Perineural resiniferatoxin selectively inhibits inflammatory hyperalgesia. Mol Pain. 2008;4:3 (2242785Epub 2008/01/18).
Pecze L, Viskolcz B, Olah Z. Molecular surgery concept from bench to bedside: a focus on TRPV1+ pain-sensing neurons. Front Physiol. 2017;8:378 (5455100Epub 2017/06/20).
Brown DC. Resiniferatoxin: the evolution of the “molecular scalpel” for chronic pain relief. Pharmaceuticals (Basel). 2016;9(3):47 (5039500Epub 2016/08/17).
Brown DC, Agnello K, Iadarola MJ. Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain. 2015;156(6):1018–24 (4431903Epub 2015/02/07).
Iadarola MJ, Gonnella GL. Resiniferatoxin for pain treatment: an interventional approach to personalized pain medicine. Open Pain J. 2013;6:95–107 (4711370Epub 2013/01/01).
Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Investig. 2004;113(9):1344–52 (398431Epub 2004/05/05).
Neubert JK, Mannes AJ, Keller J, Wexel M, Iadarola MJ, Caudle RM. Peripheral targeting of the trigeminal ganglion via the infraorbital foramen as a therapeutic strategy. Brain Res Protoc. 2005;15(3):119–26 (2005/07/19).
Goso C, Piovacari G, Szallasi A. Resiniferatoxin-induced loss of vanilloid receptors is reversible in the urinary bladder but not in the spinal cord of the rat. Neurosci Lett. 1993;162(1–2):197–200 (1993/11/12).
Zhang N, Zhang P, Zhang X, Yang Y. The efficacy of resiniferatoxin in prevention of catheter related bladder discomfort in patients after TURP— a pilot, randomized, open study. Transl Androl Urol. 2012;1(1):14–8 (4713214Epub 2012/03/01).
Periganglionic resiniferatoxin for the treatment of intractable pain due to cancer-induced bone pain. 2015. https://www.clinicaltrials.gov/ct2/show/NCT02522611(2020/05/17).
Mannes AH, Quezado Z, Berger A, Fojo T, Smith R, Butman J, Lonser R, Iadarola M. Resiniferatoxin, a potent TRPV1 agonist: intrathecal administration to treat severe pain associated with advanced cancer—case report. J Pain. 2010;11(4):S43.
Heiss JI, Cantor F, Oughourli A, Smith R, Mannes A. A phase I study of the intrathecal administration of resiniferatoxin for treating severe refractory pain associated with advanced cancer. J Pain. 2014;15(Suppl):S67.
Resiniferatoxin to treat severe pain associated with advanced cancer. 2008. https://www.clinicaltrials.gov/ct2/show/NCT00804154(2020/05/17).
Study to evaluate safety and MTD of epidural resiniferatoxin injection for treatment of intractable cancer pain. 2017. https://www.clinicaltrials.gov/ct2/show/NCT03226574(2020/05/17).
Sorrento Therapeutics— Press release. 2020. https://www.globenewswire.com/news-release/2020/02/27/1992194/0/en/Sorrento-Therapeutics-Presents-Interim-Positive-Results-of-Phase-1b-Resiniferatoxin-RTX-in-Cancer-Pain-Trial.html(2020/05/17).
Nedeljkovic SS NS, Rickerson E, Levitt RC, Horn DB, Patin DJ, Albores-Ibarra N, Nahama A, Zhao T, Bharathi P, Luchi M, Minkowitz H, Solanki D, Leiman D. A multicenter, open-label, Phase 1b study to assess the safety and define the maximally tolerated dose of epidural Resiniferatoxin (RTX) injection for treatment of intractable pain associated with cancer. 2020. https://www.sorrentotherapeutics.com/research/product-candidates/rtx-cancer-pain/(2020/05/17).
Bert J, Mahowald ML, Frizelle S, Dorman CW, Funkenbusch SC, Krug HE. The effect of treatment with resiniferatoxin and capsaicin on dynamic weight bearing measures and evoked pain responses in a chronic inflammatory arthritis murine model. Intern Med Rev (Wash D C). 2016;2016(6):89 (5070479Epub 2016/10/25).
Iadarola MJ, Sapio MR, Raithel SJ, Mannes AJ, Brown DC. Long-term pain relief in canine osteoarthritis by a single intra-articular injection of resiniferatoxin, a potent TRPV1 agonist. Pain. 2018;159(10):2105–14 (2018/07/18).
Kissin EY, Freitas CF, Kissin I. The effects of intra-articular resiniferatoxin in experimental knee-joint arthritis. Anesth Analg. 2005;101(5):1433–9 (1409708Epub 2005/10/26).
Intra-articular lopain (MTX-071) phase I/IIa study in chronic osteoarthritic knee joint pain. 2015. https://www.clinicaltrials.gov/ct2/show/NCT02566564(2020/05/21).
Lopain (MTX-071 / resiniferatoxin) An open label, single dose phase Ib/IIa study to determine the safety and clinical effects of intra-articular injections of low doses of Lopain (MTX-071). 2017. https://www.clinicaltrialsregister.eu/ctr-search/search?query=Lopain(2020/05/21).
A randomized, double-blind, placebo-controlled, single dose phase IIb exploratory study to document the clinical effects and safety of intra-articular injections of Lopain (MTX-071) in patients witth knee osteoarthritis. 2019. https://www.clinicaltrialsregister.eu/ctr-search/search?query=Lopain(2020/05/09).
Study of resiniferatoxin for knee pain in moderate to severe osteoarthritis. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03542838(2020/05/09).
A phase 3 study to evaluate the efficacy and safety of resiniferatoxin for pain due to osteoarthritis of the knee. 2019. https://www.clinicaltrials.gov/ct2/show/NCT04044742(2020/05/10).
Study to evaluate resiniferatoxin in patients with knee osteoarthritis whose total knee replacement surgery is delayed. 2020. https://www.clinicaltrials.gov/ct2/show/NCT04386980(2020/06/28).
Sorrento Therapeutics—Press release. 2019. https://www.globenewswire.com/news-release/2019/06/19/1871054/0/en/Sorrento-Therapeutics-Updates-Positive-Results-of-Phase-1b-Resiniferatoxin-RTX-in-Knee-Osteoarthritis-Pain-Trial.html(2020/06/28).
Salas MM, Clifford JL, Hayden JR, Iadarola MJ, Averitt DL. Local resiniferatoxin induces long-lasting analgesia in a rat model of full thickness thermal injury. Pain Med. 2017;18(12):2453–65 (6279302Epub 2016/10/31).
Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–7 (2000/05/23).
Lee Y, Hong S, Cui M, Sharma PK, Lee J, Choi S. Transient receptor potential vanilloid type 1 antagonists: a patent review (2011–2014). Expert Opin Ther Patents. 2015;25(3):291–318 (2015/02/11).
Trevisani M, Gatti R. TRPV1 antagonists as analgesic agents. Open Pain J. 2013;6(Suppl 1: M11):108–18.
Voight EA, Kort ME. Transient receptor potential vanilloid-1 antagonists: a survey of recent patent literature. Expert Opin Ther Patents. 2010;20(9):1107–22 (2010/07/01).
Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, et al. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107(2):544–52 (1907893Epub 1992/10/01).
Weil A, Moore SE, Waite NJ, Randall A, Gunthorpe MJ. Conservation of functional and pharmacological properties in the distantly related temperature sensors TRVP1 and TRPM8. Mol Pharmacol. 2005;68(2):518–27 (2005/05/25).
Brown BS, Keddy R, Perner RJ, DiDomenico S, Koenig JR, Jinkerson TK, et al. Discovery of TRPV1 antagonist ABT-116. Bioorg Med Chem Lett. 2010;20(11):3291–4 (2010/05/12).
Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther. 2005;314(1):410–21 (2005/04/20).
Maher MP, Bhattacharya A, Ao H, Swanson N, Wu NT, Freedman J, et al. Characterization of 2-(2,6-dichloro-benzyl)-thiazolo[5,4-d]pyrimidin-7-yl]-(4-trifluoromethyl-phenyl) -amine (JNJ-39729209) as a novel TRPV1 antagonist. Eur J Pharmacol. 2011;663(1–3):40–50 (2011/05/18).
Puttfarcken PS, Han P, Joshi SK, Neelands TR, Gauvin DM, Baker SJ, et al. A-995662 [(R)-8-(4-methyl-5-(4-(trifluoromethyl)phenyl)oxazol-2-ylamino)-1,2,3,4-tetrahydr onaphthalen-2-ol], a novel, selective TRPV1 receptor antagonist, reduces spinal release of glutamate and CGRP in a rat knee joint pain model. Pain. 2010;150(2):319–26 (2010/07/14).
Surowy CS, Neelands TR, Bianchi BR, McGaraughty S, El Kouhen R, Han P, et al. (R)-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(1H-indazol-4-yl)-urea (ABT-102) blocks polymodal activation of transient receptor potential vanilloid 1 receptors in vitro and heat-evoked firing of spinal dorsal horn neurons in vivo. J Pharmacol Exp Ther. 2008;326(3):879–88 (2008/06/03).
Watabiki T, Kiso T, Kuramochi T, Yonezawa K, Tsuji N, Kohara A, et al. Amelioration of neuropathic pain by novel transient receptor potential vanilloid 1 antagonist AS1928370 in rats without hyperthermic effect. J Pharmacol Exp Ther. 2011;336(3):743–50 (2010/11/26).
Garami A, Shimansky YP, Rumbus Z, Vizin RCL, Farkas N, Hegyi J, et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis. Pharmacol Ther. 2020;208:107474 (2020/01/14).
Krarup AL, Ny L, Astrand M, Bajor A, Hvid-Jensen F, Hansen MB, et al. Randomised clinical trial: the efficacy of a transient receptor potential vanilloid 1 antagonist AZD1386 in human oesophageal pain. Aliment Pharmacol Ther. 2011;33(10):1113–22 (2011/03/18).
Garami A, Pakai E, McDonald HA, Reilly RM, Gomtsyan A, Corrigan JJ, et al. TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiol (Oxf). 2018;223(3):e13038 (6032921Epub 2018/01/21).
Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29(11):550–7 (2008/09/23).
Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, et al. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61(3):228–61 (2763780Epub 2009/09/15).
Gavva NR, Bannon AW, Surapaneni S, Hovland DN Jr, Lehto SG, Gore A, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27(13):3366–74 (6672109Epub 2007/03/30).
Szolcsanyi J. Effect of capsaicin on thermoregulation: an update with new aspects. Temperature (Austin). 2015;2(2):277–96 (4843897Epub 2016/05/27).
Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26(37):9385–93 (6674601Epub 2006/09/15).
Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27(28):7459–68 (6672610Epub 2007/07/13).
Szelenyi Z, Hummel Z, Szolcsanyi J, Davis JB. Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur J Neurosci. 2004;19(5):1421–4 (2004/03/16).
Miller F, Bjornsson M, Svensson O, Karlsten R. Experiences with an adaptive design for a dose-finding study in patients with osteoarthritis. Contemp Clin Trials. 2014;37(2):189–99 (2014/01/08).
A study to evaluate the safety and efficacy of an investigational drug in the treatment of postoperative dental pain (MK-2295-005). 2006. https://www.clinicaltrials.gov/ct2/show/NCT00387140(2020/07/12).
Serafini M, Griglio A, Aprile S, Seiti F, Travelli C, Pattarino F, et al. Targeting transient receptor potential vanilloid 1 (TRPV1) channel softly: the discovery of passerini adducts as a topical treatment for inflammatory skin disorders. J Med Chem. 2018;61(10):4436–55 (2018/05/04).
Lehto SG, Tamir R, Deng H, Klionsky L, Kuang R, Le A, et al. Antihyperalgesic effects of (R, E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluorom ethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther. 2008;326(1):218–29 (2008/04/19).
Jakab B, Helyes Z, Varga A, Bolcskei K, Szabo A, Sandor K, et al. Pharmacological characterization of the TRPV1 receptor antagonist JYL1421 (SC0030) in vitro and in vivo in the rat. Eur J Pharmacol. 2005;517(1–2):35–44 (2005/06/28).
Chizh BA, O’Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132(1–2):132–41 (2007/07/31).
SB-705498 dental pain study after tooth extraction. 2006. https://www.clinicaltrials.gov/ct2/show/NCT00281684(2020/07/15).
Sb-705498 rectal pain study. 2007. https://www.clinicaltrials.gov/ct2/show/NCT00461682(2020/07/15).
Arsenault P, Chiche D, Brown W, Miller J, Treister R, Leff R, et al. NEO6860, modality-selective TRPV1 antagonist: a randomized, controlled, proof-of-concept trial in patients with osteoarthritis knee pain. Pain Rep. 2018;3(6):e696 (6344137Epub 2019/02/02).
Brown W, Leff RL, Griffin A, Hossack S, Aubray R, Walker P, et al. Safety, pharmacokinetics, and pharmacodynamics study in healthy subjects of oral NEO6860, a modality selective transient receptor potential vanilloid subtype 1 antagonist. J Pain. 2017;18(6):726–38 (2017/02/12).
Chiche DB, Walker P. NEO6860, a novel modality selective TRPV1 antagonist: results from a phase I, double-blind, placebo-controlled study in healthy subjects. J Pain. 2016;17:S79.
NEO6860, a TRPV1 antagonist, first in human study. 2015. https://www.clinicaltrials.gov/ct2/show/NCT02337543(2020/07/16).
A proof-of-concept study assessing NEO6860 in osteoarthritis pain. 2016. https://www.clinicaltrials.gov/ct2/show/NCT02712957(2020/07/16).
A clinical study to investigate the effect on pain relief of a single dose of JNJ-39439335 in patients with chronic osteoarthritis pain of the knee. 2009. https://www.clinicaltrials.gov/ct2/show/NCT00933582(2020/05/17).
A study to evaluate the bioavailability and food effect of JNJ-39439335 in healthy adult male volunteers. 2011. https://www.clinicaltrials.gov/ct2/show/NCT01454245(2020/05/17).
A multiple dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of JNJ-39439335 in patients with osteoarthritis. 2011. https://www.clinicaltrials.gov/ct2/show/NCT01343303(2020/05/17).
A study to investigate the safety, tolerability, and pharmacokinetics of JNJ-39439335 in healthy Japanese and Caucasian adult male participants. 2012. https://www.clinicaltrials.gov/ct2/show/NCT01631487(2020/08/18).
An exploratory study to assess the effects of JNJ-39439335 on the relief of pain using a thermal-grill experimental model. 2009. https://www.clinicaltrials.gov/ct2/show/NCT01006304(2020/05/18).
Manitpisitkul P, Shalayda K, Russell L, Sanga P, Williams Y, Solanki B, et al. Bioavailability and pharmacokinetics of TRPV1 antagonist mavatrep (JNJ-39439335) tablet and capsule formulations in healthy men: two open-label, crossover, single-dose phase 1 studies. Clin Pharmacol Drug Dev. 2018;7(7):699–711 (2017/11/11).
Mayorga AJ, Flores CM, Trudeau JJ, Moyer JA, Shalayda K, Dale M, et al. A randomized study to evaluate the analgesic efficacy of a single dose of the TRPV1 antagonist mavatrep in patients with osteoarthritis. Scand J Pain. 2017;17:134–43 (2017/08/30).
Manitpisitkul P, Mayorga A, Shalayda K, De Meulder M, Romano G, Jun C, et al. Safety, tolerability and pharmacokinetic and pharmacodynamic learnings from a double-blind, randomized, placebo-controlled, sequential group first-in-human study of the TRPV1 antagonist, JNJ-38893777 in healthy men. Clin Drug Investig. 2015;35(6):353–63 (2015/04/22).
Manitpisitkul P, Shalayda K, Russell L, Sanga P, Solanki B, Caruso J, et al. Pharmacokinetics and safety of mavatrep (JNJ-39439335), a TRPV1 antagonist in healthy japanese and caucasian men: a double-blind, randomized, placebo-controlled, sequential-group phase 1 study. Clin Pharmacol Drug Dev. 2018;7(7):712–26 (2017/11/11).
DWP05195 in healthy adult male volunteers. 2009. https://www.clinicaltrials.gov/ct2/show/NCT00969787(2020/07/18).
A multiple dose study of DWP05195 in healthy adult subjects. 2010. https://www.clinicaltrials.gov/ct2/show/NCT01094834(2020/07/18).
Lee J, Kim BH, Yu KS, Kim HS, Kim JD, Cho JY, et al. A first-in-human, double-blind, placebo-controlled, randomized, dose escalation study of DWP05195, a novel TRPV1 antagonist, in healthy volunteers. Drug Des Dev Ther. 2017;11:1301–13 (5411174Epub 2017/05/10).
Evaluate the efficacy and safety of DWP05195 in subjects with post-herpetic neuralgia. 2012. https://www.clinicaltrials.gov/ct2/show/NCT01557010(2020/07/18).
Experimental biomarker study for pain thresholds. 2016. https://www.clinicaltrials.gov/ct2/show/NCT02695745(2020/07/18).
Arendt-Nielsen L, Harris S, Whiteside GT, Hummel M, Knappenberger T, O’Keefe S, et al. A randomized, double-blind, positive-controlled, 3-way cross-over human experimental pain study of a TRPV1 antagonist (V116517) in healthy volunteers and comparison with preclinical profile. Pain. 2016;157(9):2057–67 (2016/05/12).
Tafesse L, Kanemasa T, Kurose N, Yu J, Asaki T, Wu G, et al. Structure–activity relationship studies and discovery of a potent transient receptor potential vanilloid (TRPV1) antagonist 4-[3-chloro-5-[(1S)-1,2-dihydroxyethyl]-2-pyridyl]-N-[5-(trifluoromethyl)-2-pyrid yl]-3,6-dihydro-2H-pyridine-1-carboxamide (V116517) as a clinical candidate for pain management. J Med Chem. 2014;57(15):6781–94 (2014/07/25).
Analgesic efficacy and safety of V116517 in subjects with moderate to severe chronic pain due to postherpetic neuralgia (PHN). 2012. https://www.clinicaltrials.gov/ct2/show/NCT01688947(2020/07/18).
Analgesic efficacy and safety of V116517 in subjects with moderate to severe chronic pain due to osteoarthritis (OA) of the knee. 2012. https://www.clinicaltrials.gov/ct2/show/NCT01688934(2020/07/18).
To evaluate the safety, tolerability and analgesic efficacy of SAF312 in postoperative dental pain patients. 2009. https://www.clinicaltrials.gov/ct2/show/NCT00986882(2020/05/22).
Study of SAF312 as an eye drop for treatment of eye pain following photorefractive keratectomy (PRK) surgery. 2016. https://www.clinicaltrials.gov/ct2/show/NCT02961062(2020/05/22).
Novartis—trial results. 2019. https://www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=6323(2020/05/22).
Novartis—trial results. 2019. https://www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=17355(2020/05/22).
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54 (1993/12/03).
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–11 (1998/03/05).
Pilot study to evaluate SYL1001 safety and effect in patients with ocular Pain. 2013. https://www.clinicaltrials.gov/ct2/show/NCT01776658(2020/07/28).
Benitez-Del-Castillo JM, Moreno-Montanes J, Jimenez-Alfaro I, Munoz-Negrete FJ, Turman K, Palumaa K, et al. Safety and efficacy clinical trials for SYL1001, a novel short interfering RNA for the treatment of dry eye disease. Investig Ophthalmol Vis Sci. 2016;57(14):6447–54 (2016/11/29).
Moreno-Montanes J, Bleau AM, Jimenez AI. Tivanisiran, a novel siRNA for the treatment of dry eye disease. Expert Opin Investig Drugs. 2018;27(4):421–6 (2018/03/24).
Dose-finding study to assess the safety and effect of SYL1001 in patients with ocular pain. 2015. https://www.clinicaltrials.gov/ct2/show/NCT02455999(2020/07/28).
HELIX, a double-masked study of SYL1001 in patients with moderate to severe dry eye disease (DED). 2017. https://www.clinicaltrials.gov/ct2/show/NCT03108664(2020/07/28).
Sylentis—Press release. 2019. https://www.sylentis.com/index.php/en/news/general-news/145-sylentis-announces-results-of-phase-3-helix-trial-with-tivanisiran-for-the-treatment-of-dry-eye-disease(2020/07/28).
Ann J, Kim HS, Thorat SA, Kim H, Ha HJ, Choi K, et al. Discovery of nonpungent transient receptor potential vanilloid 1 (TRPV1) agonist as strong topical analgesic. J Med Chem. 2020;63(1):418–24 (2019/11/09).
Mostinski Y, Noy G, Kumar R, Tsvelikhovsky D, Priel A. Tricyclic spirolactones as modular TRPV1 synthetic agonists. ACS Chem Neurosci. 2017;8(8):1688–96 (2017/05/19).
Wang S, Joseph J, Ro JY, Chung MK. Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain. 2015;156(5):931–41 (4402251Epub 2015/03/04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by operating grants from the Canadian Institutes of Health Research (CIHR) and Natural Sciences and Engineering Research Council of Canada (NSERC) [CA]. CA holds a Canada Research Chair in Inflammatory Pain. MD has an ACHRI (Alberta Children's Hospital Research Institute) and CSM (Cumming School of Medicine) postdoctoral fellowship. No sponsor funding was received for the open access fee.
Conflicts of interest
No conflicts of interest.
Ethics approval
Not applicable.
Consent
Not applicable.
Author contributions
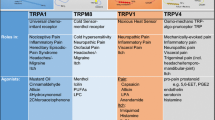
MI and CA wrote the paper. MD designed the figure.
Data availability statement
Not applicable.
Rights and permissions
About this article
Cite this article
Iftinca, M., Defaye, M. & Altier, C. TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs 81, 7–27 (2021). https://doi.org/10.1007/s40265-020-01429-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01429-2