Abstract
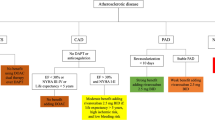
Secondary cardiovascular (CV) prevention in patients with vascular disease [e.g. coronary (CAD) and peripheral (PAD) artery disease] is crucial and typically involves antiplatelet therapy with aspirin; however, managing residual ischaemic and bleeding risks in CV disease (CVD) remains a challenge. Combining the oral anticoagulant rivaroxaban (Xarelto®) with aspirin targets both the platelet and thrombotic processes of atherosclerosis, a common pathophysiological process associated with CVD. In the global COMPASS trial (n > 27,000), rivaroxaban 2.5 mg twice daily plus aspirin 100 mg once daily (vs aspirin alone) significantly reduced the risk of the primary composite major adverse CV event (MACE) outcome (i.e. myocardial infarction, stroke or CV death) in adults with stable CAD and/or PAD and, in those with PAD, significantly reduced the risk of the composite major adverse limb event (MALE) outcome. Rivaroxaban + aspirin treatment was generally well tolerated; however, the risk of the composite major bleeding outcome, but not intracranial or fatal bleeding, was significantly higher with rivaroxaban + aspirin than aspirin. The increased risk for the composite major bleeding outcome did not negate the composite net clinical benefits of rivaroxaban + aspirin for secondary CV prevention, with rivaroxaban + aspirin especially beneficial in those with a greater CV risk at baseline. Ongoing clinical experience is required to fully define the role of rivaroxaban + aspirin in secondary CV prevention. In the meantime, dual therapy with rivaroxaban + aspirin is an important emerging option for secondary CV prevention of atherothrombotic events in adults with CAD or symptomatic PAD who are at high risk of ischaemic events.

Similar content being viewed by others
References
World Health Organization. Cardiovascular diseases (CVDs): key facts. 2017. https://www.who.int/news-room/fact-sheets. Accessed 18 Jun 2020.
Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol. 2015;2021(Suppl 1):S1–7.
Gurbel PA, Fox KAA, Tantry US, et al. Combination antiplatelet and oral anticoagulant therapy in patients with coronary and peripheral artery disease: focus on the COMPASS trial. Circulation. 2019;139(18):2170–85.
Nicholls SJ, Nelson AJ. Rivaroxaban with or without aspirin for the secondary prevention of cardiovascular disease: clinical implications of the COMPASS trial. Am J Cardiovasc Drugs. 2019;19(4):343–8.
Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969–74.
Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with recent acute coronary syndrome. N Engl J Med. 2012;366(1):9–19.
Weitz JI, Angiolillo DJ, Geisler T, et al. Dual pathway inhibition for vascular protection in patients with atherosclerotic disease: rationale and review of evidence. Thromg Haemost. 2019. https://doi.org/10.1055/s-0040-1713376.
Duggan ST, Scott LJ, Plosker GL. Rivaroxaban: a review of its use for the prevention of venous thromboembolism after total hip or knee replacement surgery. Drugs. 2009;69:1829–51.
Cater NJ, Plosker GL. Rivaroxaban: a review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Drugs. 2013;73:715–39.
Burness CB, Perry CM. Rivaroxaban: a review in its use in the treatment of deep vein thrombosis or pulmonary embolism and the prevention of recurrent venous thromboembolism. Drugs. 2014;74:243–62.
Plosker GL. Rivaroxaban: a review of its use in acute coronary syndromes. Drugs. 2014;74:451–64.
European Medicines Agency. Xarelto 2.5 mg film-coated tablets: EU summary of product characteristics. 2019. https://www.ema.europa.eu/. Accessed 12 May 2020.
Bayer AG. Xarelto® (rivaroxaban): US prescribing information. 2019. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf. Accessed 12 May 2020.
Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319–30.
Bosch J, Eikelboom JW, Connolly SJ, et al. Rationale, design and baseline characteristics of participants in the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial. Can J Cardiol. 2017;33(8):1027–35.
Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157(3):682–91.e2.
Moayyedi P, Eikelboom JW, Bosch J, et al. Pantoprazole to prevent gastroduodenal events in patients receiving rivaroxaban and/or aspirin in a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2019;157(2):403–12.e5.
Sharma M, Hart RG, Connolly SJ, et al. Stroke outcomes in the COMPASS trial. Circulation. 2019;139(9):1134–45.
Connolly SJ, Eikelboom JW, Bosch J, et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):205–18.
Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):219–29.
Fox KAA, Eikelboom JW, Shestakovska O, et al. Rivaroxaban plus aspirin in patients with vascular disease and renal dysfunction: from the COMPASS trial. J Am Coll Cardiol. 2019;73(18):2243–50.
Liang Y, Zhu J, Liu L, et al. Efficacy and safety of rivaroxaban plus aspirin in women and men with chronic coronary or peripheral artery disease. Cardiovasc Res. 2020. https://doi.org/10.1093/cvr/cvaa100.
Bhatt DL, Eikelboom JW, Connolly SJ, et al. Role of combination antiplatelet and anticoagulation therapy in diabetes mellitus and cardiovascular disease: insights from the COMPASS trial. Circulation. 2020;141:1841–54.
Anand SS, Caron F, Eikelboom JW, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 2018;71(20):2306–15.
Lamy A, Eikelboom J, Sheth T, et al. Rivaroxaban, aspirin, or both to prevent early coronary bypass graft occlusion: the COMPASS-CABG study. J Am Coll Cardiol. 2019;73(2):121–30.
Bainey KR, Welsh RC, Connolly SJ, et al. Rivaroxaban plus aspirin versus aspirin alone in patients with prior percutaneous coronary intervention (COMPASS-PCI). Circulation. 2020;141(14):1141–51.
Branch KR, Probstfield JL, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with heart failure and chronic coronary or peripheral artery disease: the COMPASS trial. Circulation. 2019;140(7):529–37.
Perera KS, Ng KKH, Nayar S, et al. Association between low-dose rivaroxaban with or without aspirin and ischemic stroke subtypes: a secondary analysis of the COMPASS trial. JAMA Neurol. 2019;77(1):43–8.
Vanassche T, Verhamme P, Anand SS, et al. Risk factors and clinical outcomes in chronic coronary and peripheral artery disease: an analysis of the randomized, double-blind COMPASS trial. Eur J Prevent Cardiol. 2020;27(3):296–307.
Anand SS, Eikelboom JW, Dyal L, et al. Rivaroxaban plus aspirin versus aspirin in relation to vascular risk in the COMPASS trial. J Am Coll Cardiol. 2019;73(25):3271–80.
Eikelboom JW, Bosch JJ, Connolly SJ, et al. Major bleeding in patients with coronary or peripheral artery disease treated with rivaroxaban plus aspirin. J Am Coll Cardiol. 2019;74(12):1519–28.
Moore KT, Wong P, Zhang L, et al. Influence of age on the pharmacokinetics, pharmacodynamics, efficacy, and safety of Rivaroxaban. Curr Med Res Opin. 2018;34(12):2053–61.
Eikelboom JW, Connolly SJ, Bosch J, et al. Bleeding and new cancer diagnosis in patients with atherosclerosis. Circulation. 2019;140(18):1451–9.
Piepoli MF, Hoes AW, Agewall S, et al. European guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives from 10 societies and by individual experts). Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–81.
Aboyans V, Ricco J-B, Bartelink M-LEL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral artery diseases, in collaboration with the European Society for Vascular Surgery (ESVS): endorsed by the European Stroke Organization (ESO). The Task Force for the diagnosis of peripheral arterial diseases of the European Cardiology Society (ESC) and European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39(9):763–821.
Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58(19):2020–45.
Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update A guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–73.
Sharma M, Hart RG, Smith EE, et al. Rationale, design, and baseline participant characteristics in the MRI and cognitive substudy of the cardiovascular outcomes for people using anticoagulation strategies trial. Int J Stroke. 2019;14(3):270–81.
Darmon A, Bhatt DL, Elbez Y, et al. External applicability of the COMPASS trial: an analysis of the reduction of atherothrombosis for continued health (REACH) registry. Eur Heart J. 2018;39(9):750–7.
Desperak P, Hudzik B, Gasior M. Assessment of patients with coronary artery disease who may benefit from the use of rivaroxaban in the real world: implementation of the COMPASS trial criteria in the TERCET registry population. Pol Arch Intern Med. 2019;129(7–8):460–8.
Darmon A, Ducrocq G, Jasliek A, et al. Use of risk score to identify lower and higher risk subsets among COMPASS-eligible patients with stable CAD: insights from the CLARIFY Registry [abstract no. P5010]. Eur Heart J. 2019;40(Suppl 1):3070.
Darmon A, Ducrocq G, Jasilek A, et al. Frequency, management and outcomes of patients with stable coronary artery disease eligible for COMPASS: an analysis of the CLARIFY registry [abstract no. P3294]. Eur Heart J. 2019;40(Suppl 1):1934.
Wurtz M, Olesen KKW, Thim T, et al. Applicability of the COMPASS trial in a Danish all-comers coronary angiography cohort: an analysis of the Western Denmark heart registry [abstract no. P4204]. Eur Heart J. 2018;39(Suppl 1):851.
De Luca L, Formigli D, Meessen J, et al. COMPASS criteria applied to a contemporary cohort of unselected patients with stable coronary artery diseases: insights from the START registry. Eur Heart J Qual Care Clin Outcomes. 2020. https://doi.org/10.1093/ehjqcco/qcaa054.
Schiele F, Puymirat E, Ferrieres J, et al. The FAST-MI 2005–2010-2015 registries in the light of the COMPASS trial: the COMPASS criteria applied to a post-MI population. Int J Cardiol. 2019;278:7–13.
Fox KA, Anand SS, Aboyans V, et al. Xarelto plus Acetylsalicylic acid: Treatment patterns and Outcomes in patients with Atherosclerosis (XATOA). Rationale and design of a prospective registry study to access rivaroxaban 2.5 mg twice daily plus aspirin for prevention of atherosclerotic events in coronaray artery disease, peripheral artery disease, or both. Am Heart J. 2020;222:166–73.
Lim GB. Prevention: rivaroxaban plus aspirin in CAD or PAD. Nat Rev Cardiol. 2018;15(1):3.
Darmon A, Sorbets E, Ducrocq G, et al. Association of multiple enrichment criteria with ischemic and bleeding risks among COMPASS-eligible patients. J Am Coll Cardiol. 2019;73(25):3281–91.
de Vries TI, Eikelboom JW, Bosch J, et al. Estimating individual lifetime benefit and bleeding risk of adding rivaroxaban to aspirin for patients with stable cardiovascular disease: results from the COMPASS trial. Eur Heart J. 2019;40(46):3771–8.
Acknowledgements
During the peer review process, the manufacturer of rivaroxaban was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflicts of interest
Lesley Scott is a salaried employee of Adis International Ltd/Springer Nature and declares no conflict of interest. All authors contributed to the review and are responsible for the article content.
Ethics Approval, Consent to Participate, Consent to Publish, Availability of Data and Material, Code Availability
Not applicable.
Additional information
Enhanced material for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.12580745.
The manuscript was reviewed by: S. Frol, Department of Vascular Neurology, University Clinical Centre Ljubljana, Ljubljana, Slovenia; R. B. Grobben, Department of Cardiology, University Medical Center Utrecht, Utrecht, Netherlands; D. Morrone, Department of Cardiology Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy; A. J. Nelson, Department of Cardiology, South Australian Health and Medical Research Institute, Royal Adelaide Hospital, Adelaide, South Australia, Australia.
Rights and permissions
About this article
Cite this article
Scott, L.J. Rivaroxaban: A Review for Secondary CV Prevention in CAD and PAD. Drugs 80, 1465–1475 (2020). https://doi.org/10.1007/s40265-020-01397-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01397-7