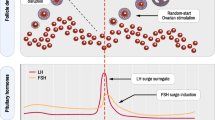
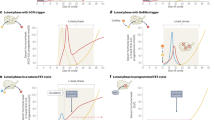
Abstract
Assisted reproduction technologies have substantially advanced since the birth of the first in vitro fertilization baby, and innovations within in vitro fertilization laboratories have been of paramount importance for the overall assisted reproduction technology success rates. However, one of the milestones in the history of in vitro fertilization is irrefutably the introduction of conventional ovarian stimulation. The objective of the present review is to provide an update on conventional ovarian stimulation, by giving an overview of treatment milestones, together with the latest innovations currently being investigated. The realization of an assisted reproduction technology treatment depends on many steps that can be medically manipulated and must be harmoniously combined, starting from the follicular phase and ending with luteal phase support. New technologies in the pharmaceutical sector are fundamental to optimize efficiency and tailor treatment approaches to individual needs. The present review aims to offer physicians a useful summary of the more recent publications and to facilitate the translation of research findings into daily clinical practice.







Similar content being viewed by others
References
Polyzos NP, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, Tournaye H, et al. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including ∼15,000 women. Fertil Steril. 2018;110(661–70):e1.
Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod. 2015;30(12):2703–7.
Castelló D, Motato Y, Basile N, Remohí J, Espejo-Catena M, Meseguer M. How much have we learned from time-lapse in clinical IVF? Mol Hum Reprod. 2016;22(10):719–27.
Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366.
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8.
Trounson AO, Leeton JF, Wood C, Webb J, Wood J. Pregnancies in humans by fertilization in vitro and embryo transfer in the controlled ovulatory cycle. Science. 1981;212(4495):681–2.
Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980;87(9):737–56.
Macklon NS, Stouffer RL, Giudice LC, Fauser BCJM. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27(2):170–207.
Groenewoud ER, Macklon NS, Cohlen BJ. Cryo-thawed embryo transfer: natural versus artificial cycle: a non-inferiority trial (ANTARCTICA trial). BMC Womens Health. 2012;12:27.
Groenewoud ER, Cantineau AEP, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update. 2013;19(5):458–70.
Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102(1):19–26.
Drakopoulos P, Blockeel C, Stoop D, Camus M, De Vos M, Tournaye H, et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod. 2016;31(2):370–6.
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406.
Maddock WO, Leach RB, Tokuyama I, Paulsen CA, Roy WR. Effects of hog pituitary follicle-stimulating hormone in women: antihormone formation and inhibition of ovarian function. J Clin Endocrinol Metab. 1956;16(4):433–48.
Gemzell CA. Induction of ovulation with human pituitary gonadotrophins. Fertil Steril. 1962;13:153–68.
Santi D, Casarini L, Alviggi C, Simoni M. Efficacy of follicle-stimulating hormone (FSH) alone, FSH + luteinizing hormone, human menopausal gonadotropin or FSH + human chorionic gonadotropin on assisted reproductive technology outcomes in the “personalized” medicine era: a meta-analysis. Front Endocrinol. 2017;8:114.
Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2006;21(12):3217–27.
Devroey P, Pellicer A, Nyboe Andersen A, Arce JC. A randomized assessor-blind trial comparing highly purified hMG and recombinant FSH in a GnRH antagonist cycle with compulsory single-blastocyst transfer. Fertil Steril. 2012;97(3):561–71.
Al-Inany HG, Abou-Setta AM, Aboulghar MA, Mansour RT, Serour GI. Efficacy and safety of human menopausal gonadotrophins versus recombinant FSH: a meta-analysis. Reprod Biomed Online. 2008;16(1):81–8.
van Wely M, Kwan I, Burt AL, Thomas J, Vail A, Van der Veen F, et al. Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles: a Cochrane review. Hum Reprod Update. 2012;18(2):111.
ESHRE Reproductive Endocrinology Guidelines Group. Ovarian stimulation for IVF/ICSI: guideline of the European Society of Human Reproduction and Embryology. Belgium: ESHRE; 2019.
Lunenfeld B. Historical perspectives in gonadotrophin therapy. Hum Reprod Update. 2004;10(6):453–67.
Howles CM. Genetic engineering of human FSH (Gonal-F). Hum Reprod Update. 1996;2(2):172–91.
Martinez G, Sanguineti F, Sepulveda J, Dorey J, Arici A, Patrizio P. A comparison between follitropin α filled by mass and follitropin a filled by bioassay in the same egg donors. Reprod Biomed Online. 2011;22(S1):S20–S2222.
Lunenfeld B, Bilger W, Longobardi S, Alam V, D’Hooghe T, Sunkara S. The development of gonadotropins for clinical use in the treatment of infertility. Front Endocrinol. 2019;10:429.
Tulppala M, Milla A, Tuuri T, Vilska S, Foudila T, Hakala-Ala-Pietilä T, et al. Comparison of two recombinant follicle-stimulating hormone preparations in in-vitro fertilization: a randomized clinical study. Hum Reprod. 1999;14(11):2709–15.
Bordewijk EM, Mol F, van der Veen F, Van Wely M. Required amount of rFSH, HP-hMG and HP-FSH to reach a live birth: a systematic review and meta-analysis. Hum Reprod Open. 2019;2019(3):1–12.
The European Recombinant Human LH Study Group. Recombinant human luteinizing hormone (LH) to hormone (FSH)-induced follicular development in LH- and FSH-deficient anovulatory women: a dose-finding study. J Clin Endocrinol Metab. 1998;83(5):1507–14.
Lehert P, Kolibianakis EM, Venetis CA, Schertz J, Saunders H, Arriagada P, et al. Recombinant human follicle-stimulating hormone (r-hFSH) plus recombinant luteinizing hormone versus r-hFSH alone for ovarian stimulation during assisted reproductive technology: systematic review and meta-analysis. Reprod Biol Endocrinol. 2014;12:17.
Humaidan P, Chin W, Rogoff D, D’Hooghe T, Longobardi S, Hubbard J, et al. Efficacy and safety of follitropin alfa/lutropin alfa in ART: a randomized controlled trial in poor ovarian responders. Hum Reprod. 2017;32(7):1537–8.
Polyzos NP, Sunkara SK. Sub-optimal responders following controlled ovarian stimulation: an overlooked group? Hum Reprod. 2015;30(9):2005–8.
Polyzos NP, Drakopoulos P. Management strategies for POSEIDON’s Group 1. Front Endocrinol. 2019;10:679.
Alviggi C, Conforti A, Esteves SC, Andersen CY, Bosch E, Buhler K, et al. Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril. 2018;109(4):644–64.
Fares FA, Suganuma N, Nishimori K, LaPolt PS, Hsueh AJ, Boime I. Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin beta subunit to the follitropin beta subunit. Proc Natl Acad Sci USA. 1992;89(10):4304–8.
Boostanfar R, Shapiro B, Levy M, Rosenwaks Z, Witjes H, Stegmann BJ, et al. Large, comparative, randomized double-blind trial confirming noninferiority of pregnancy rates for corifollitropin alfa compared with recombinant follicle-stimulating hormone in a gonadotropin-releasing hormone antagonist controlled ovarian stimulation pr. Fertil Steril. 2015;104(1):94–103.e1.
Devroey P, Boostanfar R, Koper NP, Mannaerts BMJL, Ijzerman-Boon PC, Fauser BCJM. A double-blind, non-inferiority RCT comparing corifollitropin alfa and recombinant FSH during the first seven days of ovarian stimulation using a GnRH antagonist protocol. Hum Reprod. 2009;24(12):3063–72.
Griesinger G, Boostanfar R, Gordon K, Gates D, McCrary Sisk C, Stegmann BJ. Corifollitropin alfa versus recombinant follicle-stimulating hormone: an individual patient data meta-analysis. Reprod Biomed Online. 2016;33(1):56–60.
Corifollitropin alfa Ensure Study Group. Corifollitropin alfa for ovarian stimulation in IVF: a randomized trial in lower-body-weight women. Reprod Biomed Online. 2010;21(1):66–76.
Drakopoulos P, Vuong TNL, Ho NAV, Vaiarelli A, Ho MT, Blockeel C, et al. Corifollitropin alfa followed by highly purified HMG versus recombinant FSH in young poor ovarian responders: a multicentre randomized controlled clinical trial. Hum Reprod. 2017;32(11):2225–33.
Polyzos NP, Corona R, Van De Vijver A, Blockeel C, Drakopoulos P, Vloeberghs V, et al. Corifollitropin alfa followed by hpHMG in GnRH agonist protocols: two prospective feasibility studies in poor ovarian responders. Gynecol Endocrinol. 2015;31(11):885–90.
Errazuriz J, Romito A, Drakopoulos P, Frederix B, Racca A, De Munck N, et al. Cumulative live birth rates following stimulation with corifollitropin alfa compared with hp-hMG in a GnRH antagonist protocol in poor ovarian responders. Front Endocrinol. 2019;10:175.
La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. 2014;20(1):124–40.
Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Müllerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698–710.
Nyboe Andersen A, Nelson SM, Fauser BCJM, García-Velasco JA, Klein BM, Arce JC, et al. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril. 2017;107(2):387–96.e4.
Bosch E, Havelock J, Martin FS, Rasmussen BB, Klein BM, Mannaerts B, et al. Follitropin delta in repeated ovarian stimulation for IVF: a controlled, assessor-blind phase 3 safety trial. Reprod Biomed Online. 2019;38(2):195–205.
Barbieri RL. The endocrinology of the menstrual cycle. Methods Mol Biol. 2014;1154:145–69.
Fleming R, Adam AH, Barlow DH, Black WP, MacNaughton MC, Coutts JR. A new systematic treatment for infertile women with abnormal hormone profiles. Br J Obstet Gynaecol. 1982;89(1):80–3.
Huirne JAF, Lambalk CB. New drug classes gonadotropin-releasing-hormone-receptor antagonists. Lancet. 2001;358(9295):1793–803.
Porter RN, Smith W, Craft IL, Abdulwahid NA, Jacobs HS. Induction of ovulation for in-vitro fertilisation using buserelin and gonadotropins. Lancet. 1984;324(8414):1284–5.
Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23(5):560–79.
Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:CD001750.
Griesinger G, Diedrich K, Devroey P, Kolibianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta-analysis. Hum Reprod Update. 2006;12(2):159–68.
Youssef M, Van Der Veen F, Van Wely M. GnRHa to trigger final oocyte maturation: a time to reconsider. Hum Reprod. 2010;25(2):559.
Kol S, Humaidan P, Alsbjerg B, Engmann L, Benadiva C, Garcia-Velasco JA, et al. The updated Cochrane review 2014 on GnRH agonist trigger: repeating the same errors. Reprod Biomed Online. 2015;30(6):563–5.
Casper RF. Introduction: gonadotropin-releasing hormone agonist triggering of final follicular maturation for in vitro fertilization. Fertil Steril. 2015;103(4):865–6.
Kolibianakis EM, Schultze-Mosgau A, Schroer A, van Steirteghem A, Devroey P, Diedrich K, et al. A lower ongoing pregnancy rate can be expected when GnRH agonist is used for triggering final oocyte maturation instead of HCG in patients undergoing IVF with GnRH antagonists. Hum Reprod. 2005;20(10):2887–922.
Humaidan P, Polyzos NP, Alsbjerg B, Erb K, Mikkelsen AL, Elbaek HO, et al. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi-centre studies in IVF patients. Hum Reprod. 2013;28(9):2511–21.
Devroey P, Polyzos NP, Blockeel C. An OHSS-free clinic by segmentation of IVF treatment. Hum Reprod. 2011;26(10):2593–7.
Toftager M, Bogstad J, Løssl K, Prætorius L, Zedeler A, Bryndorf T, et al. Cumulative live birth rates after one ART cycle including all subsequent frozen-thaw cycles in 1050 women: secondary outcome of an RCT comparing GnRH-antagonist and GnRH-agonist protocols. Hum Reprod. 2017;32(3):556–67.
Wildt L, Hutchison S, Marshall G, Pohl R, Knobil E. On the site of action of progesterone in the blockade of the estradiol-induced gonadotropin discharge in the rhesus monkey. Endocrinology. 1981;109(4):1293–4.
Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update. 2017;23(2):211–20.
Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, et al. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril. 2016;105(6):1488–95.e1.
Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu Q, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 2015;104(1):62–70.e3.
Chen Q, Wang Y, Sun L, Zhang S, Chai W, Hong Q, et al. Controlled ovulation of the dominant follicle using progestin in minimal stimulation in poor responders. Reprod Biol Endocrinol. 2017;15(1):1–9.
Beguería R, García D, Vassena R, Rodríguez A. Medroxyprogesterone acetate versus ganirelix in oocyte donation: a randomized controlled trial. Hum Reprod. 2019;34(5):872–80.
Nargund G, Chian RC. ISMAAR: leading the global agenda for a more physiological, patient-centred, accessible and safer approaches in ART. J Assist Reprod Genet. 2013;30(2):155–6.
Nargund G, Datta AK, Fauser BCJM. Mild stimulation for in vitro fertilization. Fertil Steril. 2017;108(4):558–67.
Labarta E, MartÍnez-Conejero JA, Alamá P, Horcajadas JA, Pellicer A, Simón C, et al. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod. 2011;26:1813–25.
Simón C, Cano F, Valbuena D, Remohí J, Pellicer A. Implantation: clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10(9):2432–7.
Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23(19):4347–53.
Teramoto S, Kato O. Minimal ovarian stimulation with clomiphene citrate: a large-scale retrospective study. Reprod Biomed Online. 2007;15(2):134–48.
Yilmaz S, Yilmaz Sezer N, Gönenç IM, Ilhan SE, Yilmaz E. Safety of clomiphene citrate: a literature review. Cytotechnology. 2018;70(2):489–95.
Bechtejew TN, Nadai MN, Nastri CO, Martins WP. Clomiphene citrate and letrozole to reduce follicle-stimulating hormone consumption during ovarian stimulation: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50(3):315–23.
Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23(3):619–29.
Akhtar M, Njar VCO, Neville WJ. Mechanistic studies on aromatase and related C–C bond cleaving P-450 enzymes. J Steroid Biochem Mol Biol. 1993;44(4–6):375–87.
Mitwally MF, Casper RF. Aromatase inhibitors in ovulation induction. Semin Reprod Med. 2004;22(1):61–78.
Fauser B, Devroey P, Yen SSC, Gosden R, Crowley WF, Baird DT, et al. Minimal ovarian stimulation for IVF: appraisal of potential benefits and drawbacks. Hum Reprod. 1999;14(11):2681–6.
Revelli A, Chiadò A, Dalmasso P, Stabile V, Evangelista F, Basso G, et al. “Mild” vs. “long” protocol for controlled ovarian hyperstimulation in patients with expected poor ovarian responsiveness undergoing in vitro fertilization (IVF): a large prospective randomized trial. J Assist Reprod Genet. 2014;31(7):809–15.
Goswami SK, Das T, Chattopadhyay R, Sawhney V, Kumar J, Chaudhury K, et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. 2004;19(9):2031–5.
Bastu E, Buyru F, Ozsurmeli M, Demiral I, Dogan M, Yeh J. A randomized, single-blind, prospective trial comparing three different gonadotropin doses with or without addition of letrozole during ovulation stimulation in patients with poor ovarian response. Eur J Obstet Gynecol Reprod Biol. 2016;203:30–4.
Biljan M, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole and gonadotropins. Fertil Steril. 2005;84(Suppl. 1):S95.
Shao Y-H, Tulandi T. Letrozole and unexplained infertility: a contemporary meta-analysis. J Obstet Gynaecol Can. 2019;41(6):832–4.
Lainas TG, Sfontouris IA, Venetis CA, Lainas GT, Zorzovilis IZ, Tarlatzis BC, et al. Live birth rates after modified natural cycle compared with high-dose FSH stimulation using GnRH antagonists in poor responders. Hum Reprod. 2015;30(10):2321–30.
Ferraretti AP, La Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–24.
Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. 2016;105(6):1452–3.
Tarlatzis BC, Zepiridis L, Grimbizis G, Bontis J. Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum Reprod Update. 2003;9(1):61–766.
Morgia F, Sbracia M, Schimberni M, Giallonardo A, Piscitelli C, Giannini P, et al. A controlled trial of natural cycle versus microdose gonadotropin-releasing hormone analog flare cycles in poor responders undergoing in vitro fertilization. Fertil Steril. 2004;81(6):1542–7.
Polyzos NP, Blockeel C, Verpoest W, De Vos M, Stoop D, Vloeberghs V, et al. Live birth rates following natural cycle IVF in women with poor ovarian response according to the Bologna criteria. Hum Reprod. 2012;27(12):3481–6.
Busnelli A, Papaleo E, Del Prato D, La Vecchia I, Iachini E, Paffoni A, et al. A retrospective evaluation of prognosis and cost-effectiveness of IVF in poor responders according to the Bologna criteria. Hum Reprod. 2015;30(2):315–22.
Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol. 1999;181(5 Pt 1):1263–9.
Taylor H, Pal L, Seli E. Speroff’s clinical gynecologic endocrinology and infertility. 9th ed. Philadelphia: Wolters Kluwer Health; 2019.
Goldzieher JW, Stanczyk FZ. Oral contraceptives and individual variability of circulating levels of ethinyl estradiol and progestins. Contraception. 2008;78(1):4–9.
Huirne JAF, van Loenen ACD, Donnez J, Pirard C, Homburg R, Schats R, et al. Effect of an oral contraceptive pill on follicular development in IVF/ICSI patients receiving a GnRH antagonist: a randomized study. Reprod Biomed Online. 2006;13(2):235–45.
Cédrin-Durnerin I, Bständig B, Parneix I, Bied-Damon V, Avril C, Decanter C, et al. Effects of oral contraceptive, synthetic progestogen or natural estrogen pre-treatments on the hormonal profile and the antral follicle cohort before GnRH antagonist protocol. Hum Reprod. 2007;22(1):109–16.
Rombauts L, Healy D, Norman RJ, Speirs A, Watkins B, Yovich J, et al. A comparative randomized trial to assess the impact of oral contraceptive pretreatment on follicular growth and hormone profiles in GnRH antagonist-treated patients. Hum Reprod. 2006;21(1):95–103.
Barmat LI, Chantilis SJ, Hurst BS, Dickey RP. A randomized prospective trial comparing gonadotropin-releasing hormone (GnRH) antagonist/recombinant follicle-stimulating hormone (rFSH) versus GnRH-agonist/rFSH in women pretreated with oral contraceptives before in vitro fertilization. Fertil Steril. 2005;83(2):321–30.
Kolibianakis EM, Papanikolaou EG, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Effect of oral contraceptive pill pretreatment on ongoing pregnancy rates in patients stimulated with GnRH antagonists and recombinant FSH for IVF. A randomized controlled trial. Hum Reprod. 2006;21(2):352–7.
Biljan MM, Mahutte NG, Dean N, Hemmings R, Bissonnette F, Tan SL. Effects of pretreatment with an oral contraceptive on the time required to achieve pituitary suppression with gonadotropin-releasing hormone analogues and on subsequent implantation and pregnancy rates. Fertil Steril. 1998;70(6):1063–9.
Garcia-Velasco JA, Bermejo A, Ruiz F, Martinez-Salazar J, Requena A, Pellicer A. Cycle scheduling with oral contraceptive pills in the GnRH antagonist protocol vs the long protocol: a randomized, controlled trial. Fertil Steril. 2011;96(3):590–3.
Huirne JA, Hugues JN, Pirard C, Fischl F, Sage JC, Pouly JL, et al. Cetrorelix in an oral contraceptive-pretreated stimulation cycle compared with buserelin in IVF/ICSI patients treated with r-hFSH: a randomized, multicentre, phase IIIb study. Hum Reprod. 2006;21(6):1408–15.
Farquhar C, Rombauts L, Kremer JAM, Lethaby A, Ayeleke RO. Oral contraceptive pill, progestogen or oestrogen pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques. Cochrane Database Syst Rev. 2017;5:CD006109.
Kim CH, You RM, Kang HJ, Ahn JW, Jeon I, Lee JW, et al. GnRH antagonist multiple dose protocol with oral contraceptive pill pretreatment in poor responders undergoing IVF/ICSI. Clin Exp Reprod Med. 2011;38(4):228–33.
Bakas P, Hassiakos D, Grigoriadis C, Vlahos NF, Liapis A, Creatsas G. Effect of a low dose combined oral contraceptive pill on the hormonal profile and cycle outcome following COS with a GnRH antagonist protocol in women over 35 years old. Gynecol Endocrinol. 2014;30(11):825–9.
Pan JX, Liu Y, Ke ZH, Zhou CL, Meng Q, Ding GL, et al. Successive and cyclic oral contraceptive pill pretreatment improves IVF/ICSI outcomes of PCOS patients and ameliorates hyperandrogenism and antral follicle excess. Gynecol Endocrinol. 2015;31(4):332–6.
Özmen B, Şükür YE, Seval MM, Ateş C, Atabekoʇlu CS, Sönmezer M, et al. Dual suppression with oral contraceptive pills in GnRH antagonist cycles for patients with polycystic ovary syndrome undergoing intracytoplasmic sperm injection. Eur J Obstet Gynecol Reprod Biol. 2014;183:137–40.
Hwang JL, Seow KM, Lin YH, Huang LW, Hsieh BC, Tsai YL, et al. Ovarian stimulation by concomitant administration of cetrorelix acetate and HMG following Diane-35 pre-treatment for patients with polycystic ovary syndrome: a prospective randomized study. Hum Reprod. 2004;19(9):1993–2000.
Decanter C, Robin G, Thomas P, Leroy M, Lefebvre C, Soudan B, et al. First intention IVF protocol for polycystic ovaries: does oral contraceptive pill pretreatment influence COH outcome? Reprod Biol Endocrinol. 2013;11(1):1–9.
Wei D, Shi Y, Li J, Wang Z, Zhang L, Sun Y, et al. Effect of pretreatment with oral contraceptives and progestins on IVF outcomes in women with polycystic ovary syndrome. Hum Reprod. 2017;32(2):354–61.
Bellver J, Albert C, Labarta E, Pellicer A. Early pregnancy loss in women stimulated with gonadotropin-releasing hormone antagonist protocols according to oral contraceptive pill pretreatment. Fertil Steril. 2007;87(5):1098–101.
Andersen AN, Witjes H, Gordon K, Mannaerts B. Predictive factors of ovarian response and clinical outcome after IVF/ICSI following a rFSH/GnRH antagonist protocol with or without oral contraceptive pre-treatment. Hum Reprod. 2011;26(12):3413–23.
Montoya-Botero P, Martinez F, Rodríguez-Purata J, Rodríguez I, Coroleu B, Polyzos N. The effect of type of oral contraceptive pill and duration of use on fresh and cumulative live birth rates in IVF/ICSI cycles. Hum Reprod. 2020;35(4):826–36.
Guivarc’h-Levêque A, Homer L, Arvis P, Broux PL, Moy L, Priou G, et al. Programming in vitro fertilization retrievals during working days after a gonadotropin-releasing hormone antagonist protocol with estrogen pretreatment: does the length of exposure to estradiol impact on controlled ovarian hyperstimulation outcomes? Fertil Steril. 2011;96(4):872–6.
Fanchin R, Salomon L, Castelo-Branco A, Olivennes F, Frydman N, Frydman R. Luteal estradiol pre-treatment coordinates follicular growth during controlled ovarian hyperstimulation with GnRH antagonists. Hum Reprod. 2003;18(12):2698–703.
Fanchin R, Cunha-Filho JS, Schonäuer LM, Kadoch IJ, Cohen-Bacri P, Frydman R. Coordination of early antral follicles by luteal estradiol administration provides a basis for alternative controlled ovarian hyperstimulation regimens. Fertil Steril. 2003;79(2):316–21.
Cédrin-Durnerin I, Guivarc’h-Levêque A, Hugues JN. Pretreatment with estrogen does not affect IVF-ICSI cycle outcome compared with no pretreatment in GnRH antagonist protocol: a prospective randomized trial. Fertil Steril. 2012;97(6):1359–64.e1.
Blockeel C, Engels S, De Vos M, Haentjens P, Polyzos NP, Stoop D, et al. Oestradiol valerate pretreatment in GnRH-antagonist cycles: a randomized controlled trial. Reprod Biomed Online. 2012;24(3):272–80.
Dragisic KG, Davis OK, Fasouliotis SJ, Rosenwaks Z. Use of a luteal estradiol patch and a gonadotropin-releasing hormone antagonist suppression protocol before gonadotropin stimulation for in vitro fertilization in poor responders. Fertil Steril. 2005;84(4):1023–6.
Chang EM, Han JE, Won HJ, Kim YS, Yoon TK, Lee WS. Effect of estrogen priming through luteal phase and stimulation phase in poor responders in in-vitro fertilization. J Assist Reprod Genet. 2012;29(3):225–30.
Shastri SM, Barbieri E, Kligman I, Schoyer KD, Davis OK, Rosenwaks Z. Stimulation of the young poor responder: comparison of the luteal estradiol/gonadotropin-releasing hormone antagonist priming protocol versus oral contraceptive microdose leuprolide. Fertil Steril. 2011;95(2):592–5.
Griesinger G, Felberbaum R, Diedrich K. GnRH-antagonists in reproductive medicine. Arch Gynecol Obstet. 2005;273(2):71–8.
Fanchin R, Branco AC, Kadoch IJ, Hosny G, Bagirova M, Frydman R. Premenstrual administration of gonadotropin-releasing hormone antagonist coordinates early antral follicle sizes and sets up the basis for an innovative concept of controlled ovarian hyperstimulation. Fertil Steril. 2004;81(6):1554–9.
Blockeel C, Riva A, De Vos M, Haentjens P, Devroey P. Administration of a gonadotropin-releasing hormone antagonist during the 3 days before the initiation of the in vitro fertilization/intracytoplasmic sperm injection treatment cycle: impact on ovarian stimulation: a pilot study. Fertil Steril. 2011;95(5):1714–9.e2.
Viardot-Foucault V, Nadarajah S, Lye WK, Tan HH. GnRH antagonist pre-treatment: one centre’s experience for IVF-ICSI cycle scheduling. Reprod Biomed Online. 2015;30(4):366–72.
Castillo JC, Humaidan P, Bernabeu R. Pharmaceutical options for triggering of final oocyte maturation in ART. Biomed Res Int. 2014;2014:580171.
Humaidan P, Nelson SM, Devroey P, Coddington CC, Schwartz LB, Gordon K, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod. 2016;31(9):1997–2004.
Casarini L, Lispi M, Longobardi S, Milosa F, La Marca A, Tagliasacchi D, et al. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signalling. PLoS One. 2012;7(10):e46682.
Abbara A, Clarke SA, Dhillo WS. Novel concepts for inducing final oocyte maturation in in vitro fertilization treatment. Endocr Rev. 2018;39(5):593–628.
Induction of final follicular maturation and early luteinization in women undergoing ovulation induction for assisted reproduction treatment: recombinant HCG versus urinary HCG. The European Recombinant Human Chorionic Gonadotrophin Study Group. Hum Reprod. 2000;15(7):1446–51.
Chang P, Kenley S, Burns T, Denton G, Currie K, DeVane G, et al. Recombinant human chorionic gonadotropin (rhCG) in assisted reproductive technology: results of a clinical trial comparing two doses of rhCG (Ovidrel) to urinary hCG (Profasi) for induction of final follicular maturation in in vitro fertilization-embryo t. Fertil Steril. 2001;76(1):67–74.
Youssef MA, Abou-Setta AM, Lam WS. Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles. Cochrane Database Syst Rev. 2016;4:CD003719.
Nakano R, Mizuno T, Kotsuji F, Katayama K, Wshio M, Tojo S. “Triggering” of ovulation after infusion of synthetic luteinizing hormone releasing factor (LRF). Acta Obstet Gynecol Scand. 1973;52(3):269–72.
Gonen Y, Balakier H, Powell W, Casper RF. Use of gonadotropin-releasing hormone agonist to trigger follicular maturation for in vitro fertilization. J Clin Endocrinol Metab. 1990;71(4):918–22.
Itskovitz J, Boldes R, Levron J, Erlik Y, Kahana L, Brandes JM. Induction of preovulatory luteinizing hormone surge and prevention of ovarian hyperstimulation syndrome by gonadotropin-releasing hormone agonist. Fertil Steril. 1991;56(2):213–20.
Atef A, Francois P, Christian V, Marc-Andre S. The potential role of gap junction communication between cumulus cells and bovine oocytes during in vitro maturation. Mol Reprod Dev. 2005;71(3):358–67.
Kol S. Luteolysis induced by a gonadotropin-releasing hormone agonist is the key to prevention of ovarian hyperstimulation syndrome. Fertil Steril. 2004;81(1):1–5.
Blockeel C, Drakopoulos P, Santos-Ribeiro S, Polyzos NP, Tournaye H. A fresh look at the freeze-all protocol: a SWOT analysis. Hum Reprod. 2016;31(3):491–7.
Oktay K, Turkcuoglu I, Rodriguez-Wallberg KA. GnRH agonist trigger for women with breast cancer undergoing fertility preservation by aromatase inhibitor/FSH stimulation. Reprod Biomed Online. 2010;20(6):783–8.
Fauser BC, de Jong D, Olivennes F, Wramsby H, Tay C, Itskovitz-Eldor J, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87(2):709–15.
Griffin D, Feinn R, Engmann L, Nulsen J, Budinetz T, Benadiva C. Dual trigger with gonadotropin-releasing hormone agonist and standard dose human chorionic gonadotropin to improve oocyte maturity rates. Fertil Steril. 2014;102(2):405–9.
Son WY, Chung JT, Chian RC, Herrero B, Demirtas E, Elizur S, et al. A 38 h interval between hCG priming and oocyte retrieval increases in vivo and in vitro oocyte maturation rate in programmed IVM cycles. Hum Reprod. 2008;23(9):2010–6.
Fabris AM, Cruz M, Legidos V, Iglesias C, Muñoz M, García-Velasco JA. Dual triggering with gonadotropin-releasing hormone agonist and standard dose human chorionic gonadotropin in patients with a high immature oocyte rate. Reprod Sci. 2017;24(8):1221–5.
Ben-Haroush A, Sapir O, Salman L, Altman E, Garor R, Margalit T, et al. Does “dual trigger” increase oocyte maturation rate? J Obstet Gynaecol. 2019;71:1–3.
Kasum M, Kurdija K, Orešković S, Čehić E, Pavičić-Baldani D, Škrgatić L. Combined ovulation triggering with GnRH agonist and hCG in IVF patients. Gynecol Endocrinol. 2016;32(11):861–5.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Thomas S. Gonadotropin-releasing hormone agonist combined with a reduced dose of human chorionic gonadotropin for final oocyte maturation in fresh autologous cycles of in vitro fertilization. Fertil Steril. 2008;90(1):231–3.
Humaidan P, Bungum L, Bungum M, Andersen CY. Rescue of corpus luteum function with peri-ovulatory HCG supplementation in IVF/ICSI GnRH antagonist cycles in which ovulation was triggered with a GnRH agonist: a pilot study. Reprod Biomed Online. 2006;13(2):173–8.
Santos-Ribeiro S, Mackens S, Popovic B, Polyzos N, Racca A, Drakopoulos P, et al. Implantation enhancement by elective cryopreservation of all viable embryos (ICE): randomized controlled trial (RCT) comparing the two safest approaches in IVF/ICSI for high-responders. Hum Reprod. 2018;33:110.
Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92(3):1235–316.
Jayasena CN, Comninos AN, Narayanaswamy S, Bhalla S, Abbara A, Ganiyu-Dada Z, et al. Acute and chronic effects of kisspeptin-54 administration on GH, prolactin and TSH secretion in healthy women. Clin Endocrinol (Oxf). 2014;81(6):891–8.
Jayasena CN, Abbara A, Comninos AN, Nijher GMK, Christopoulos G, Narayanaswamy S, et al. Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization. J Clin Investig. 2014;124(8):3667–77.
Dosouto C, Haahr T, Humaidan P. Advances in ovulation trigger strategies. Panminerva Med. 2019;61(1):42–51.
Fatemi HM, Popovic-Todorovic B, Papanikolaou E, Donoso P, Devroey P. An update of luteal phase support in stimulated IVF cycles. Hum Reprod Update. 2007;13(6):581–90.
Csapo AI, Pulkkinen MO, Ruttner B, Sauvage J, Wiest W. The significance of the human corpus luteum in pregnancy maintenance: I. Preliminary studies. Am J Obs Gynecol. 1972;112(8):1061–7.
Smitz J, Devroey P, Camus M, Deschacht J, Khan I, Staessen C, et al. The luteal phase and early pregnancy after combined GnRH-agonist/HMG treatment for superovulation in IVF or GIFT. Hum Reprod. 1988;3(5):585–90.
Beckers NG, Laven JS, Eijkemans MJ, Fauser BC. Follicular and luteal phase characteristics following early cessation of gonadotrophin-releasing hormone agonist during ovarian stimulation for in-vitro fertilization. Hum Reprod. 2000;15(1):43–9.
Delcour C, Robin G, Delesalle A-S, Drumez E, Plouvier P, Dewailly D, et al. Weekly intramuscular progesterone for luteal phase support in women receiving oocyte donation is associated with a decreased miscarriage rate. Reprod Biomed Online. 2019;39(3):446–51.
Miles R, Paulson R, Lobo R, Press M, Dahmoush L, Sauer M. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study. Fertil Steril. 1994;62(3):485–90.
Bulletti C, De Ziegler D, Flamigni C, Giacomucci E, Polli V, Bolelli G, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12(5):1073–9.
Cicinelli E, De Ziegler D. New hypotheses. Transvaginal progesterone: evidence for a new functional 'portal system' flowing from the vagina to the uterus. Hum Reprod Update. 1999;5(4):365–72.
van der Linden M, Buckingham K, Farquhar C, Kremer JAM, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015;7:CD009154.
Shapiro DB, Pappadakis JA, Ellsworth NM, Hait HI, Nagy ZP. Progesterone replacement with vaginal gel versus i.m. injection: cycle and pregnancy outcomes in IVF patients receiving vitrified blastocysts. Hum Reprod. 2014;29(8):1706–11.
Schindler AE. Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas. 2009;65(Suppl. 1):S3–11.
Tournaye H, Sukhikh GT, Kahler E, Griesinger G. A Phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum Reprod. 2017;32(5):1019–27.
Griesinger G, Blockeel C, Sukhikh G, Patki A, Dhorepatil B, Yang D-Z, et al. Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial. Hum Reprod. 2018;123:10–87.
Yang D-Z, Griesinger G, Wang W, Gong F, Liang X, Zhang H, et al. A phase III randomized controlled trial of oral dydrogesterone versus intravaginal progesterone gel for luteal phase support in in vitro fertilization (Lotus II): results from the Chinese mainland subpopulation. Gynecol Endocrinol. 2019;3:1–9.
Watters M, Noble M, Child T, Nelson S. Short versus extended progesterone supplementation for luteal phase support in fresh IVF cycles: a systematic review and meta-analysis. Reprod Biomed Online. 2019;40(1):143–50.
Soliman S, Daya M, Collins J, Hughes E. The role of luteal phase support in infertility treatment: a meta-analysis of randomized trials. Fertil Steril. 1994;61(6):1068–76.
Mochtar MH, Hogerzeil HV, Mol BW. Progesterone alone versus progesterone combined with HCG as luteal support in GnRHa/HMG induced IVF cycles: a randomized clinical trial. Hum Reprod. 1996;11(8):1602–5.
Pritts EA, Atwood AK. Luteal phase support in infertility treatment: a meta-analysis of the randomized trials. Hum Reprod. 2002;17(9):2287–99.
Daya S, Gunby J. Luteal phase support in assisted reproduction cycles. Cochrane Database Syst Rev. 2004;3:CD004830.
Fatemi HM, Popovic-Todorovic B, Humaidan P, Kol S, Banker M, Devroey P, et al. Severe ovarian hyperstimulation syndrome after gonadotropin-releasing hormone (GnRH) agonist trigger and “freeze-all” approach in GnRH antagonist protocol. Fertil Steril. 2014;101(4):1008–111.
Haahr T, Roque M, Esteves SC, Humaidan P. GnRH agonist trigger and LH activity luteal phase support versus hCG trigger and conventional luteal phase support in fresh embryo transfer IVF/ICSI cycles-a systematic PRISMA review and meta-analysis. Front Endocrinol (Lausanne). 2017;8:116.
Pirard C, Donnez J, Loumaye E. GnRH agonist as luteal phase support in assisted reproduction technique cycles: results of a pilot study. Hum Reprod. 2006;21(7):1894–900.
Tesarik J, Hazout A, Mendoza C. Luteinizing hormone affects uterine receptivity independently of ovarian function. Reprod Biomed Online. 2003;7(1):59–64.
Bar-Hava I, Mizrachi Y, Karfunkel-Doron D, Omer Y, Sheena L, Carmon N, et al. Intranasal gonadotropin-releasing hormone agonist (GnRHa) for luteal-phase support following GnRHa triggering, a novel approach to avoid ovarian hyperstimulation syndrome in high responders. Fertil Steril. 2016;106(2):330–3.
Wiser A, Klement AH, Shavit T, Berkovitz A, Koren RR, Gonen O, et al. Repeated GnRH agonist doses for luteal support: a proof of concept. Reprod Biomed Online. 2019;39(5):770–6.
Çakar E, Tasan HA, Kumru P, Cogendez E, Usal NT, Kutlu HT, et al. Combined use of oestradiol and progesterone to support luteal phase in antagonist intracytoplasmic sperm injection cycles of normoresponder women: a case-control study. J Obstet Gynaecol. 2019;104:1–6.
Farhi J, Weissman A, Steinfeld Z, Shorer M, Nahum H, Levran D. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization-embryo transfer cycles. Fertil Steril. 2000;73(4):761–6.
Drakakis P, Loutradis D, Vomvolaki E, Stefanidis K, Kiapekou E, Anagnostou E, et al. Luteal estrogen supplementation in stimulated cycles may improve the pregnancy rate in patients undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer. Gynecol Endocrinol. 2007;23(11):645–52.
Ghanem ME, Sadek EE, Elboghdady LA, Helal AS, Gamal A, Eldiasty A, et al. The effect of luteal phase support protocol on cycle outcome and luteal phase hormone profile in long agonist protocol intracytoplasmic sperm injection cycles: a randomized clinical trial. Fertil Steril. 2009;92(2):486–93.
Engmann L, DiLuigi A, Schmidt D, Benadiva C, Maier D, Nulsen J. The effect of luteal phase vaginal estradiol supplementation on the success of in vitro fertilization treatment: a prospective randomized study. Fertil Steril. 2008;89(3):554–61.
Fatemi H, Kolibianakis E, Camus M, Tournaye H, Donoso P, Papanikolaou E, et al. Addition of estradiol to progesterone for luteal supplementation in patients stimulated with GnRH antagonist/rFSH for IVF: a randomized controlled trial. Hum Reprod. 2006;21(10):2628–32.
Kwon S-K, Kim C-H, Lee K-H, Jeon IK, Ahn J-W, Kim S-H, et al. Luteal estradiol supplementation in gonadotropin-releasing hormone antagonist cycles for infertile patients in vitro fertilization. Clin Exp Reprod Med. 2013;40(3):131–4.
Huang N, Situ B, Chen X, Liu J, Yan P, Kang X, et al. Meta-analysis of estradiol for luteal phase support in in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2015;103(2):367–73.e5.
Ismail Madkour WA, Noah B, Abdel Hamid AMS, Zaheer H, Al-Bahr A, Shaeer M, et al. Luteal phase support with estradiol and progesterone versus progesterone alone in GnRH antagonist ICSI cycles: a randomized controlled study. Hum Fertil (Camb). 2016;19(2):142–9.
Leão Rogériode Barros F, Esteves Sandro C. Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech. Clinics. https://doi.org/10.6061/clinics/2014(04)10. Available from: https://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322014000400279&lng=en
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No specific funding was used in the preparation of this review article.
Conflict of interest
Racca Annalisa, Drakopoulos Panagiotis, Neves Ana Raquel, and Polyzos P. Nikolaos have no conflicts of interest that are directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Racca, A., Drakopoulos, P., Neves, A.R. et al. Current Therapeutic Options for Controlled Ovarian Stimulation in Assisted Reproductive Technology. Drugs 80, 973–994 (2020). https://doi.org/10.1007/s40265-020-01324-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01324-w