Abstract
For many years, obesity was believed to be a condition of overeating that could be resolved through counseling and short-term drug treatment. Obesity was not recognized as a chronic disease until 1985 by the scientific community, and 2013 by the medical community. Pharmacotherapy for obesity has advanced remarkably since the first class of drugs, amphetamines, were approved for short-term use. Most amphetamines were removed from the obesity market due to adverse events and potential for addiction, and it became apparent that obesity pharmacotherapies were needed that could safely be administered over the long term. This review of central nervous system (CNS) acting anti-obesity drugs evaluates current therapies such as phentermine/topiramate, which act through multiple neurotransmitter pathways to reduce appetite. In the synergistic mechanism of bupropion/naltrexone, naltrexone blocks the feed-back inhibitory circuit of bupropion to give greater weight loss. Lorcaserin, a selective agonist of a serotonin receptor that regulates food intake, and the glucagon-like-peptide-1 (GLP-1) receptor agonist liraglutide are reviewed. Future drugs include tesofensine, a potent triple reuptake inhibitor in Phase III trials for obesity, and semaglutide, an oral GLP-1 analog approved for diabetes and currently in trials for obesity. Another potential new pharmacotherapy, setmelanotide, is a melanocortin-4 receptor agonist, which is still in an early stage of development. As our understanding of the communication between the CNS, gut, adipose tissue, and other organs evolves, it is anticipated that obesity drug development will move toward new centrally acting combinations and then to drugs acting on peripheral target tissues.

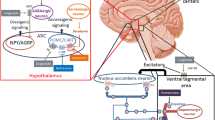
Reproduced with permission from Elsevier Ltd. The Lancet, 2008;372:1906–1913 [117]
Similar content being viewed by others
References
Health implications of obesity. National institutes of health consensus development conference statement. Ann Intern Med. 1985;103(1):147–51.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32.
Williamson DA, Ravussin E, Wong ML, Wagner A, Dipaoli A, Caglayan S, et al. Microanalysis of eating behavior of three leptin deficient adults treated with leptin therapy. Appetite. 2005;45(1):75–80.
Nathanson M. The Central Action of beta-aminopropylbenzene (Benzedrine) clinical observations. JAMA. 1937;108:528–31.
Lesses MF, Myerson A. Human autonomic pharmacology. XVI. Benzedrine sulfate as an aid in the treatment of obesity. 1938. Obes Res. 1994;2(3):286–92.
Harris SC, Ivy AC, Searle LM. The mechanism of amphetamine-induced loss of weight; a consideration of the theory of hunger and appetite. J Am Med Assoc. 1947;134(17):1468–75.
Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–98.
Scoville BA. Review of amphetamine-like drugs by the Food and Drug Administration. Washington: DC US Government Printing Office; 1976.
Byrne-Quinn E, Grover RF. Aminorex (Menocil) and amphetamine: acute and chronic effects on pulmonary and systemic haemodynamics in the calf. Thorax. 1972;27(1):127–31.
Michelakis ED, Weir EK. Anorectic drugs and pulmonary hypertension from the bedside to the bench. Am J Med Sci. 2001;321(4):292–9.
Gurtner HP. Pulmonary hypertension, “plexogenic pulmonary arteriopathy” and the appetite depressant drug aminorex: post or propter? Bull Eur Physiopathol Respir. 1979;15(5):897–923.
Weintraub M. Long-term weight control: the National Heart, Lung, and Blood Institute funded multimodal intervention study. Clin Pharmacol Ther. 1992;51(5):581–5.
Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335(9):609–16.
Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–8.
Rothman RB, Baumann MH. Serotonergic drugs and valvular heart disease. Expert Opin Drug Saf. 2009;8(3):317–29.
Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, et al. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374(6522):542–6.
Greenway F, Herber D, Raum W, Herber D, Morales S. Double-blind, randomized, placebo-controlled clinical trials with non-prescription medications for the treatment of obesity. Obes Res. 1999;7(4):370–8.
Greenway FL. Clinical studies with phenylpropanolamine: a metaanalysis. Am J Clin Nutr. 1992;55(1 Suppl):203S–5S.
Kernan WN, Viscoli CM, Brass LM, Broderick JP, Brott T, Feldmann E, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343(25):1826–32.
Domingue JN. Phenylpropanolamine contained in cold remedies and risk of hemorrhagic stroke. Neurology. 2007;69(3):320 (author reply -1).
Astrup A, Greenway FL, Ling W, Pedicone L, Lachowicz J, Strader CD, et al. Randomized controlled trials of the D1/D5 antagonist ecopipam for weight loss in obese subjects. Obesity (Silver Spring). 2007;15(7):1717–31.
Simiand J, Keane M, Keane PE, Soubrie P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9(2):179–81.
Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S, Group RI-ES. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365(9468):1389–97.
Tam J, Szanda G, Drori A, Liu Z, Cinar R, Kashiwaya Y, et al. Peripheral cannabinoid-1 receptor blockade restores hypothalamic leptin signaling. Mol Metab. 2017;6(10):1113–25.
Tam J, Hinden L, Drori A, Udi S, Azar S, Baraghithy S. The therapeutic potential of targeting the peripheral endocannabinoid/CB1 receptor system. Eur J Intern Med. 2018;49:23–9.
Kim SH, Lee YM, Jee SH, Nam CM. Effect of sibutramine on weight loss and blood pressure: a meta-analysis of controlled trials. Obes Res. 2003;11(9):1116–23.
James WP, Astrup A, Finer N, Hilsted J, Kopelman P, Rossner S, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM study group. Sibutramine trial of obesity reduction and maintenance. Lancet. 2000;356(9248):2119-25.
James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–17.
The amphetamine appetite suppressant saga. Prescrire Int. 2004;13(69):26–9.
Chen K. Ephedrine and related substances. Medicine. 1930;9:1–119.
Greenway FL. The safety and efficacy of pharmaceutical and herbal caffeine and ephedrine use as a weight loss agent. Obes Rev. 2001;2(3):199–211.
Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med. 2000;343(25):1833–8.
Shekelle PG, Hardy ML, Morton SC, Maglione M, Mojica WA, Suttorp MJ, et al. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA. 2003;289(12):1537–45.
Dong Z, Xu L, Liu H, Lv Y, Zheng Q, Li L. Comparative efficacy of five long-term weight loss drugs: quantitative information for medication guidelines. Obes Rev. 2017;18(12):1377–85.
Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. Br Med J. 1968;1(5588):352–4.
Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8(5):571–8.
Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring). 2009;17(1):30–9.
Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab. 2009;94(12):4898–906.
Smith SR, Fujioka K, Gupta AK, Billes SK, Burns C, Kim D, et al. Combination therapy with naltrexone and bupropion for obesity reduces total and visceral adiposity. Diabetes Obes Metab. 2013;15(9):863–6.
Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled. Phase III trial. Lancet. 2010;376(9741):595–605.
Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, et al. A randomized, Phase III trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21(5):935–43.
Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O’Neil PM, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19(1):110–20.
Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–9.
McElroy SL, Guerdjikova AI, Kim DD, Burns C, Harris-Collazo R, Landbloom R, et al. Naltrexone/Bupropion combination therapy in overweight or obese patients with major depressive disorder: results of a pilot study. Prim Care Companion CNS Disord. 2013. https://doi.org/10.4088/PCC.12m01494.
Kolotkin RL, Chen S, Klassen P, Gilder K, Greenway FL. Patient-reported quality of life in a randomized placebo-controlled trial of naltrexone/bupropion for obesity. Clin Obes. 2015;5(5):237–44.
Hong K, Herrmann K, Dybala C, Halseth AE, Lam H, Foreyt JP. Naltrexone/Bupropion extended release-induced weight loss is independent of nausea in subjects without diabetes. Clin Obes. 2016;6(5):305–12.
Dalton M, Finlayson G, Walsh B, Halseth AE, Duarte C, Blundell JE. Early improvement in food cravings are associated with long-term weight loss success in a large clinical sample. Int J Obes (Lond). 2017;41(8):1232–6.
2017 [cited; Contrave Prescribing Instructions]. https://contrave.com/wp-content/uploads/2017/05/Contrave_PI.pdf. Accessed 16 Feb 2018.
Nissen SE, Wolski KE, Prcela L, Wadden T, Buse JB, Bakris G, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315(10):990–1004.
Fujioka K, Plodkowski R, O’Neil PM, Gilder K, Walsh B, Greenway FL. The relationship between early weight loss and weight loss at 1 year with naltrexone ER/bupropion ER combination therapy. Int J Obes (Lond). 2016;40(9):1369–75.
Coomans CP, Geerling JJ, van den Berg SA, van Diepen HC, Garcia-Tardon N, Thomas A, et al. The insulin sensitizing effect of topiramate involves KATP channel activation in the central nervous system. Br J Pharmacol. 2013;170(4):908–18.
White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia. 2000;41(Suppl 1):S17–20.
Gibbs JW 3rd, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41(Suppl 1):S10–6.
Picard F, Deshaies Y, Lalonde J, Samson P, Richard D. Topiramate reduces energy and fat gains in lean (Fa/?) and obese (fa/fa) Zucker rats. Obes Res. 2000;8(9):656–63.
Richard D, Picard F, Lemieux C, Lalonde J, Samson P, Deshaies Y. The effects of topiramate and sex hormones on energy balance of male and female rats. Int J Obes Relat Metab Disord. 2002;26(3):344–53.
Ben-Menachem E, Axelsen M, Johanson EH, Stagge A, Smith U. Predictors of weight loss in adults with topiramate-treated epilepsy. Obes Res. 2003;11(4):556–62.
Bray GA, Hollander P, Klein S, Kushner R, Levy B, Fitchet M, et al. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11(6):722–33.
Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring). 2013;21(11):2163–71.
Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled. Phase III trial. Lancet. 2011;377(9774):1341–52.
Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, Phase III extension study. Am J Clin Nutr. 2012;95(2):297–308.
Garvey WT, Ryan DH, Henry R, Bohannon NJ, Toplak H, Schwiers M, et al. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care. 2014;37(4):912–21.
Garvey WT, Ryan DH, Bohannon NJ, Kushner RF, Rueger M, Dvorak RV, et al. Weight-loss therapy in type 2 diabetes: effects of phentermine and topiramate extended release. Diabetes Care. 2014;37(12):3309–16.
Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330–42.
Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep. 2012;35(11):1529–39.
Mines D, Tennis P, Curkendall SM, Li DK, Peterson C, Andrews EB, et al. Topiramate use in pregnancy and the birth prevalence of oral clefts. Pharmacoepidemiol Drug Saf. 2014;23(10):1017–25.
Kolotkin RL, Gadde KM, Peterson CA, Crosby RD. Health-related quality of life in two randomized controlled trials of phentermine/topiramate for obesity: What mediates improvement? Qual Life Res. 2016;25(5):1237–44.
Li J, Reaven NL, Funk SE, McGaughey K, Neovius M. 4-year cost trajectories in real-world patients matched to the metabolic profiles of trial subjects before/after treatment with phentermine-topiramate. Drugs Real World Outcomes. 2015;2(2):143–51.
Finkelstein EA, Kruger E, Karnawat S. Cost-effectiveness analysis of qsymia for weight loss. Pharmacoeconomics. 2015;33(7):699–706.
Roth BL, Willins DL, Kristiansen K, Kroeze WK. 5-Hydroxytryptamine2-family receptors (5-hydroxytryptamine2A, 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): where structure meets function. Pharmacol Ther. 1998;79(3):231–57.
[cited; http://www.diabetesincontrol.com/fda-approves-lorcaserin-belviq-for-treatment-of-obesity/. Accessed 16 Feb 2018.
Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WR, et al. Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring). 2009;17(3):494–503.
Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245–56.
Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96(10):3067–77.
O’Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20(7):1426–36.
Smith SR, O’Neil PM, Astrup A, Finer N, Sanchez-Kam M, Fraher K, et al. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22(10):2137–46.
Shram MJ, Schoedel KA, Bartlett C, Shazer RL, Anderson CM, Sellers EM. Evaluation of the abuse potential of lorcaserin, a serotonin 2C (5-HT2C) receptor agonist, in recreational polydrug users. Clin Pharmacol Ther. 2011;89(5):683–92.
Hurt RT, Croghan IT, Schroeder DR, Hays JT, Choi DS, Ebbert JO. Combination varenicline and lorcaserin for tobacco dependence treatment and weight gain prevention in overweight and obese smokers: a pilot study. Nicotine Tob Res. 2017;19(8):994–8.
Smith SR, Garvey WT, Greenway FL, Zhou S, Fain R, Pilson R, et al. Coadministration of lorcaserin and phentermine for weight management: a 12-week, randomized, pilot safety study. Obesity (Silver Spring). 2017;25(5):857–65.
Rebello CJ, Nikonova EV, Zhou S, Aronne LJ, Fujioka K, Garvey WT, et al. Effect of lorcaserin alone and in combination with phentermine on food cravings after 12-week treatment: a randomized substudy. Obesity (Silver Spring). 2018;26(2):332–9.
Farr OM, Upadhyay J, Gavrieli A, Camp M, Spyrou N, Kaye H, et al. Lorcaserin administration decreases activation of brain centers in response to food cues and these emotion- and salience-related changes correlate with weight loss effects: a 4-week-long randomized, placebo-controlled. Double-blind clinical trial. Diabetes. 2016;65(10):2943–53.
Martin CK, Redman LM, Zhang J, Sanchez M, Anderson CM, Smith SR, et al. Lorcaserin, a 5-HT(2C) receptor agonist, reduces body weight by decreasing energy intake without influencing energy expenditure. J Clin Endocrinol Metab. 2011;96(3):837–45.
Nesto R, Fain R, Li Y, Shanahan W. Evaluation of lorcaserin on progression of prediabetes to type 2 diabetes and reversion to euglycemia. Postgrad Med. 2016;128(4):364–70.
Kolotkin RL, Crosby RD, Wang Z. Health-related quality of life in randomized controlled trials of lorcaserin for obesity management: what mediates improvement? Clin Obes. 2017;7(6):347–53.
Weissman NJ, Smith SR, Fain R, Hall N, Shanahan WR. Effects of lorcaserin on pre-existing valvulopathy: a pooled analysis of Phase III trials. Obesity (Silver Spring). 2017;25(1):39–44.
Lord CC, Wyler SC, Wan R, Castorena CM, Ahmed N, Mathew D, et al. The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J Clin Invest. 2017;127(9):3402–6.
Hurren KM, Dunham MW. Pharmacokinetic drug evaluation of extended release lorcaserin for the treatment of obesity. Expert Opin Drug Metab Toxicol. 2017;13(8):891–6.
Madsbad S. Exenatide and liraglutide: different approaches to develop GLP-1 receptor agonists (incretin mimetics)–preclinical and clinical results. Best Pract Res Clin Endocrinol Metab. 2009;23(4):463–77.
van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221(1):T1–16.
Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–16.
Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36(6):843–54.
Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22.
le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–409.
Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37(11):1443–51.
Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–99.
Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016;40(8):1310–9.
Davies MJ, Aronne LJ, Caterson ID, Thomsen AB, Jacobsen PB, Marso SP, et al. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: a post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab. 2018;20(3):734–39.
Steinberg WM, Buse JB, Ghorbani MLM, Orsted DD, Nauck MA, Committee LS, et al. Amylase, lipase, and acute pancreatitis in people with type 2 diabetes treated with liraglutide: results from the LEADER randomized trial. Diabetes Care. 2017;40(7):966–72.
Kolotkin RL, Gabriel Smolarz B, Meincke HH, Fujioka K. Improvements in health-related quality of life over 3 years with liraglutide 3.0 mg compared with placebo in participants with overweight or obesity. Clin Obes. 2018;8(1):1–10.
le Roux C, Aroda V, Hemmingsson J, Cancino AP, Christensen R, Pi-Sunyer X. Comparison of efficacy and safety of liraglutide 3.0 mg in individuals with BMI above and below 35 kg/m2: a post hoc analysis. Obes Facts. 2017;10(6):531–44.
Ard J, Cannon A, Lewis CE, Lofton H, Vang Skjoth T, Stevenin B, et al. Efficacy and safety of liraglutide 3.0 mg for weight management are similar across races: subgroup analysis across the SCALE and Phase II randomized trials. Diabetes Obes Metab. 2016;18(4):430–5.
O’Neil PM, Aroda VR, Astrup A, Kushner R, Lau DCW, Wadden TA, et al. Neuropsychiatric safety with liraglutide 3.0 mg for weight management: results from randomized controlled phase 2 and 3a trials. Diabetes Obes Metab. 2017;19(11):1529–36.
Fujioka K, O’Neil PM, Davies M, Greenway F, D CWL, Claudius B, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24(11):2278-88.
Halawi H, Khemani D, Eckert D, O’Neill J, Kadouh H, Grothe K, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2(12):890–9.
Jensterle M, Pirs B, Goricar K, Dolzan V, Janez A. Genetic variability in GLP-1 receptor is associated with inter-individual differences in weight lowering potential of liraglutide in obese women with PCOS: a pilot study. Eur J Clin Pharmacol. 2015;71(7):817–24.
Robert SA, Rohana AG, Shah SA, Chinna K, Wan Mohamud WN, Kamaruddin NA. Improvement in binge eating in non-diabetic obese individuals after 3 months of treatment with liraglutide—a pilot study. Obes Res Clin Pract. 2015;9(3):301-4.
Inoue K, Maeda N, Kashine S, Fujishima Y, Kozawa J, Hiuge-Shimizu A, et al. Short-term effects of liraglutide on visceral fat adiposity, appetite, and food preference: a pilot study of obese Japanese patients with type 2 diabetes. Cardiovasc Diabetol. 2011;1(10):109.
Iepsen EW, Lundgren JR, Hartmann B, Pedersen O, Hansen T, Jorgensen NR, et al. GLP-1 receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women. J Clin Endocrinol Metab. 2015;100(8):2909–17.
Lambadiari V, Pavlidis G, Kousathana F, Varoudi M, Vlastos D, Maratou E, et al. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):8.
Larsen JR, Vedtofte L, Jakobsen MSL, Jespersen HR, Jakobsen MI, Svensson CK, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719–28.
FDA. 2014 [cited; Liraglutide label]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206321Orig1s000lbl.pdf. Accessed 16 Feb 2018.
Tran KL, Park YI, Pandya S, Muliyil NJ, Jensen BD, Huynh K, et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits. 2017;10(4):178–88.
Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6(3):237–48.
Lehr T, Staab A, Tillmann C, Nielsen EO, Trommeshauser D, Schaefer HG, et al. Contribution of the active metabolite M1 to the pharmacological activity of tesofensine in vivo: a pharmacokinetic-pharmacodynamic modelling approach. Br J Pharmacol. 2008;153(1):164–74.
Thatte U. NS-2330 (Neurosearch). Curr Opin Investig Drugs. 2001;2(11):1592–4.
Hauser RA, Salin L, Juhel N, Konyago VL. Randomized trial of the triple monoamine reuptake inhibitor NS 2330 (tesofensine) in early Parkinson’s disease. Mov Disord. 2007;22(3):359–65.
Astrup A, Meier DH, Mikkelsen BO, Villumsen JS, Larsen TM. Weight loss produced by tesofensine in patients with Parkinson’s or Alzheimer’s disease. Obesity (Silver Spring). 2008;16(6):1363–9.
Astrup A, Madsbad S, Breum L, Jensen TJ, Kroustrup JP, Larsen TM. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9653):1906–13.
Axel AM, Mikkelsen JD, Hansen HH. Tesofensine, a novel triple monoamine reuptake inhibitor, induces appetite suppression by indirect stimulation of alpha1 adrenoceptor and dopamine D1 receptor pathways in the diet-induced obese rat. Neuropsychopharmacology. 2010;35(7):1464–76.
Hansen HH, Jensen MM, Overgaard A, Weikop P, Mikkelsen JD. Tesofensine induces appetite suppression and weight loss with reversal of low forebrain dopamine levels in the diet-induced obese rat. Pharmacol Biochem Behav. 2013;110:265–71.
Appel L, Bergstrom M, Buus Lassen J, Langstrom B. Tesofensine, a novel triple monoamine re-uptake inhibitor with anti-obesity effects: dopamine transporter occupancy as measured by PET. Eur Neuropsychopharmacol. 2014;24(2):251–61.
Sjodin A, Gasteyger C, Nielsen AL, Raben A, Mikkelsen JD, Jensen JK, et al. The effect of the triple monoamine reuptake inhibitor tesofensine on energy metabolism and appetite in overweight and moderately obese men. Int J Obes (Lond). 2010;34(11):1634–43.
Gilbert JA, Gasteyger C, Raben A, Meier DH, Astrup A, Sjodin A. The effect of tesofensine on appetite sensations. Obesity (Silver Spring). 2012;20(3):553–61.
Schoedel KA, Meier D, Chakraborty B, Manniche PM, Sellers EM. Subjective and objective effects of the novel triple reuptake inhibitor tesofensine in recreational stimulant users. Clin Pharmacol Ther. 2010;88(1):69–78.
Bentzen BH, Grunnet M, Hyveled-Nielsen L, Sundgreen C, Lassen JB, Hansen HH. Anti-hypertensive treatment preserves appetite suppression while preventing cardiovascular adverse effects of tesofensine in rats. Obesity (Silver Spring). 2013;21(5):985–92.
Marso SP, Holst AG, Vilsboll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2017;376(9):891–2.
Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318(15):1460–70.
Kuhnen P, Clement K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016;375(3):240–6.
Banting W. Letter on corpulence, addressed to the public. London: Harris and Sons; 1863.
Sjostrom L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167–72.
Sari R, Balci MK, Cakir M, Altunbas H, Karayalcin U. Comparison of efficacy of sibutramine or orlistat versus their combination in obese women. Endocr Res. 2004;30(2):159–67.
Hollander P, Bays HE, Rosenstock J, Frustaci ME, Fung A, Vercruysse F, et al. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: a randomized clinical trial. Diabetes Care. 2017;40(5):632–9.
Acknowledgements
This work is supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center and in part on work that was supported by the National Institutes of Health under an award (T32 A T004094) from the National Center for Complementary and Integrative Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Frank Greenway has served on the advisory boards for BAROnova, Eisai Inc., Curves, Novo Nordisk, Microbiome Therapeutics, Orexigen, Pamlab, PlenSat, Zaluvida and Zafgen. He has consulted for Basic Research, General Nutrition Corporation, Neothetics, Takeda, and Tech Enterprises.
Rights and permissions
About this article
Cite this article
Coulter, A.A., Rebello, C.J. & Greenway, F.L. Centrally Acting Agents for Obesity: Past, Present, and Future. Drugs 78, 1113–1132 (2018). https://doi.org/10.1007/s40265-018-0946-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0946-y