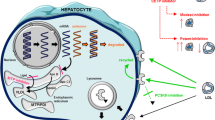
Abstract
Since their introduction, statin (HMG-CoA reductase inhibitor) drugs have advanced the practice of cardiology to unparalleled levels. Even so, coronary heart disease (CHD) still remains the leading cause of death in developed countries, and is predicted to soon dominate the causes of global mortality and disability as well. The currently available non-statin drugs have had limited success in reversing the burden of heart disease, but new information suggests they have roles in sizeable subpopulations of those affected. In this review, the status of approved non-statin drugs and the significant potential of newer drugs are discussed. Several different ways to raise plasma high-density lipoprotein (HDL) cholesterol (HDL-C) levels have been proposed, but disappointments are now in large part attributed to a preoccupation with HDL quantity, rather than quality, which is more important in cardiovascular (CV) protection. Niacin, an old drug with many antiatherogenic properties, was re-evaluated in two imperfect randomized controlled trials (RCTs), and failed to demonstrate clear effectiveness or safety. Fibrates, also with an attractive antiatherosclerotic profile and classically used for hypertriglyceridemia, lacks evidence-based proof of efficacy, save for a subgroup of diabetic patients with atherogenic dyslipidemia. Omega-3 fatty acids fall into this category as well, even with an impressive epidemiological evidence base. Omega-3 research has been plagued with methodological difficulties yielding tepid, uncertain, and conflicting results; well-designed studies over longer periods of time are needed. Addition of ezetimibe to statin therapy has now been shown to decrease levels of low-density lipoprotein (LDL) cholesterol (LDL-C), accompanied by a modest decrease in the number of CV events, though without any improvement in CV mortality. Importantly, the latest data provide crucial evidence that LDL lowering is central to the management of CV disease. Of drugs that inhibit cholesteryl ester transfer protein (CETP) tested thus far, two have failed and two remain under investigation and may yet prove to be valuable therapeutic agents. Monoclonal antibodies to proprotein convertase subtilisin/kexin type 9, now in phase III trials, lower LDL-C by over 50 % and are most promising. These drugs offer new ability to lower LDL-C in patients in whom statin drug use is, for one reason or another, limited or insufficient. Mipomersen and lomitapide have been approved for use in patients with familial hypercholesterolemia, a more common disease than appreciated. Anti-inflammatory drugs are finally receiving due attention in trials to elucidate potential clinical usefulness. All told, even though statins remain the standard of care, non-statin drugs are poised to assume a new, vital role in managing dyslipidemia.
Similar content being viewed by others
References
Kones R, Rumana U. Current therapy of dyslipidemia. A new paradigm for statin drug use and the need for additional therapies. Drugs. 2015. doi:10.1007/s40265-015-0428-4.
Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(Suppl 2):S49–S73. App. http://www.cvriskcalculator.com/.
Ray KK, Kastelein JJ, Boekholdt SM, Nicholls SJ, Khaw KT, Ballantyne CM, et al. The ACC/AHA 2013 guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: the good the bad and the uncertain: a comparison with ESC/EAS guidelines for the management of dyslipidaemias 2011. Eur Heart J. 2014;35:960–8.
Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, et al. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:484–525.
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–14.
Sacks FM, Tonkin AM, Shepherd J, Braunwald E, Cobbe S, Hawkins CM, et al. Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: the Prospective Pravastatin Pooling Project. Circulation. 2000;102:1893–900.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22.
Nicholls SJ, Tuzcu E, Sipahi I, Grasso AW, Schoenhagen P, Hu T, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007;297:499–508.
Rosenson RS, Brewer HB Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, et al. Cholesterol efflux and atheroprotection. Advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–19.
Rader DJ, deGoma EM. Approach to the patient with extremely low HDL-cholesterol. J Clin Endocrinol Metab. 2012;97:3399–407.
Oldoni F, Sinke RJ, Kuivenhoven JA. Mendelian disorders of high density lipoprotein metabolism. Circ Res. 2014;114:124–42.
Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289:24020–9.
Kamanna VS, Kashyap ML. Mechanism of action of niacin on lipoprotein metabolism. Curr Atheroscler Rep. 2000;2:36–46.
Kamanna VS, Ganji SH, Kashyap ML. Recent advances in niacin and lipid metabolism. Curr Opin Lipidol. 2013;24:239–45.
Holzhäuser E, Albrecht C, Zhou Q, Buttler A, Preusch MR, Blessing E, et al. Nicotinic acid has anti-atherogenic and anti-inflammatory properties on advanced atherosclerotic lesions independent of its lipid-modifying capabilities. J Cardiovasc Pharmacol. 2011;57:447–54.
Wu BJ, Yan L, Charlton F, Witting P, Barter PJ, Rye K-A. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler Thromb Vasc Biol. 2010;30:968–75.
Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–55.
Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, the AIM-HIGH Investigators, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67.
HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25,673 high-risk patients of ER niacin/laropiprant: trial design, prespecified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–91.
The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–312.
Landmesser U. The difficult search for a ‘partner’ of statins in lipid-targeted prevention of vascular events: the re-emergence and fall of niacin. Eur Heart J. 2013;34:1254–7.
Lloyd-Jones D. Niacin and HDL cholesterol—time to face facts. N Engl J Med. 2014;371:271–3.
Olbetam (acipimox). http://www.netdoctor.co.uk/heart-and-blood/medicines/olbetam.html. Accessed 24 Sep 2014.
European Medicines Agency, EMA Pharmacovigilance unit. Acipimox only to be used as additional or alternative treatment to reduce high triglyceride levels. Press release 20/12/2013. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/12/news_detail_002000.jsp&mid=WC0b01ac058004d5c1. Accessed 20 Sep 2014.
Masson D, Jiang XC, Lagrost L, Tall AR. The role of plasma lipid transfer proteins in lipoprotein metabolism and atherogenesis. J Lipid Res. 2009;50(suppl):S201–6.
Vasan RS, Pencina MJ, Robins SJ, Zachariah JP, Kaur G, D’Agostino RB, et al. Association of circulating cholesteryl ester transfer protein with incidence of cardiovascular disease in the community. Circulation. 2009;120:14–20.
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22.
Barter P, Rye KA. Cholesteryl ester transfer protein inhibition to reduce cardiovascular risk: where are we now? Trends Pharmacol Sci. 2011;32:694–9.
de Haan J, de Vries-van der Weij J, van der Hoorn J. Torcetrapib does not reduce atherosclerosis beyond atorvastatin and induces more proinflammatory lesions than atorvastatin. Circulation. 2008;117:2515–22.
Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, for the dal-PLAQUE Investigators, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–59.
Lüscher TF, Taddei S, Kaski JC, Jukema JW, Kallend D, Münzel T, dal-VESSEL Investigators, et al. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur Heart J. 2012;33:857–65.
Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, Gotto AM, Determining the Efficacy and Tolerability Investigators, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–15.
University of Oxford. Randomized Evaluation of the Effects of Anacetrapib Through Lipid-modification (REVEAL) study [Clinicaltrials.gov identifier NCT01252953]. Bethesda: US National Institutes of Health. 2011. http://clinicaltrials.gov/show/NCT01252953. Accessed 20 Sep 2014.
Nicholls SJ, Brewer HB, Kastelein JJP, Krueger KA, Wang MD, Shao M, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol. A randomized controlled trial. JAMA. 2011;306:2099–109.
University of Oxford. A study of evacetrapib in high-risk vascular disease (ACCELERATE) [Clinicaltrials.gov identifier NCT01252953]. Bethesda: US National Institutes of Health, 2012 [updated December 19, 2013]. http://clinicaltrials.gov/show/NCT01687998. Accessed 22 Sep 2014.
Rosenson RS, Brewer HB Jr, Ansell B, Barter P, Chapman MJ, Heinecke JW, et al. Translation of high-density lipoprotein function into clinical practice: current prospects and future challenges. Circulation. 2013;128:1256–67.
Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, et al. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114:1733–42.
Turner S, Voogt J, Davidson M, et al. Measurement of reverse cholesterol transport pathways in humans: in vivo rates of free cholesterol efflux, esterification, and excretion. J Am Heart Assoc. 2012;1:e001826.
Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. HDL function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32:2813–20.
Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35.
Spotlight issue on: HDL biology: new insights in metabolism, function, and translation. Table of Contents. Cardiovas Res. 2013;103(3). http://cardiovascres.oxfordjournals.org/content/103/3.toc. Accessed 20 Sep 2014.
Nicholls SJ, Gordon A, Johannson J, Ballantyne CM, Barter PJ, Brewer HB, et al. ApoA-I induction as a potential cardioprotective strategy: rationale for the SUSTAIN and ASSURE studies. Cardiovasc Drugs Ther. 2012;26:181–7.
Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lespérance J, Heinonen T, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–82.
Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–300.
Waksman R, Torguson R, Kent K, Pichard A, Suddath W, Satler L, et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol. 2010;55:2727–35.
Gille A, Easton R, D’Andrea D, Wright SD, Shear C. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 2014;34:2106–14.
Wood S. HDL mimetic fails to regress plaque in CHI-SQUARE ACS trial, January 3, 2014. Available at http://www.medscape.com/viewarticle/818629. Accessed 21 June 2015.
Neels JG, Grimaldi PA. Physiological functions of peroxisome proliferator-activated receptor β. Physiol Rev. 2014;94:795–858.
Bishop-Bailey D, Wray J. Peroxisome proliferator-activated receptors: a critical review on endogenous pathways for ligand generation. Prostaglandins Other Lipid Mediat. 2003;71:1–22.
Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications—a review. Nutrition J. 2014;13:17.
Tenenbaum A, Fisman EZ. Balanced pan-PPAR activator bezafibrate in combination with statin: comprehensive lipids control and diabetes prevention? Cardiovasc Diabetol. 2012;11:140.
Torra IP, Chinetti G, Duval C, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors: from transcriptional control to clinical practice. Curr Opin Lipidol. 2001;12:245–54.
Plutzky J. The PPAR-RXR transcriptional complex in the vasculature: energy in the balance. Circ Res. 2011;108:1002–16.
Watts GF, Staels B. Regulation of endothelial nitric oxide synthase by PPAR agonists: molecular and clinical perspectives. Arterioscler Thromb Vasc Biol. 2004;24:619–21.
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–45.
Tenkanen L, Mänttäri M, Kovanen PT, Virkkunen H, Manninen V. Gemfibrozil in the treatment of dyslipidemia: an 18-year mortality follow-up of the Helsinki Heart Study. Arch Intern Med. 2006;166:743–8.
Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–8.
The Bezafibrate Infarction Prevention Study Group. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102:21–7.
Vakkilainen J, Steiner G, Ansquer JC, Perttunen-Nio H, Taskinen MR. Fenofibrate lowers plasma triglycerides and increases LDL particle diameter in subjects with type 2 diabetes. Diabetes Care. 2002;25:627–8.
Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Kim JA, et al. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of combined hyperlipidemia. J Am Coll Cardiol. 2005;45:1649–53.
Lim S, Park YM, Sakuma I, Koh KK. How to control residual cardiovascular risk despite statin treatment: focusing on HDL-cholesterol. Int J Cardiol. 2013;166:8–14.
Grundy SM, Vega GL, Yuan Z, Battisti WP, Brady WE, Palmisano J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial). Am J Cardiol. 2005;95:462–8.
Athyros VG, Papageorgiou AA, Athyrou VV, Demitriadis DS, Kontopoulos AG. Atorvastatin and micronized fenofibrate alone and in combination in type 2 diabetes with combined hyperlipidemia. Diabetes Care. 2002;25:1198–202.
Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, FIELD Study Investigators, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–61.
Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Effects of combination lipid therapy in type 2 diabetes mellitus, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–74.
Sirimarco G, Labreuche J, Bruckert E, Goldstein LB, Fox KM, Rothwell PM, on behalf of the PERFORM and SPARCL Investigators and Committees, et al. Stroke. 2014;45:1429–36.
Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375:1875–84.
Sacks FM, Carey VJ, Fruchart JC. Combination lipid therapy in type 2 diabetes. N Engl J Med. 2010;363:692-4; author reply 694-5.
Lee M, Saver JL, Towfighi A, Chow J, Ovbiagele B. Efficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: a meta-analysis. Atherosclerosis. 2011;217:492–8.
Ballantyne CM, Jones PH, Kelly MT, Setze CM, Lele A, Thakker KM, et al. Long-term efficacy of adding fenofibric acid to moderate-dose statin therapy in patients with persistent elevated triglycerides. Cardiovas Drugs Ther. 2011;25:s59–67.
Tenenbaum A, Fisman EZ. Fibrates are an essential part of modern anti-dyslipidemic arsenal: spotlight on atherogenic dyslipidemia and residual risk reduction. Cardiovasc Diabetol. 2012;11:125.
Lincoff A, Tardif J, Schwartz GG, Nicholls SJ, Rydén L, Neal B, AleCardio Investigators, et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio Randomized Clinical Trial. JAMA. 2014;311:1515–25.
Goldfine AB, Kaul S, Hiatt WR. Fibrates in the treatment of dyslipidemias—time for a reassessment. N Engl J Med. 2011;365:481–4.
Food and Drug Administration. FDA Drug Safety Communication: review update of Trilipix (fenofibric acid) and the ACCORD Lipid trial. 2011. http://www.fda.gov/Drugs/DrugSafety/ucm278837.htm. Accessed 20 Sep 2014.
Jackevicius CA, Tu JV, Ross JS, Ko DT, Carreon D, Krumholz HM. Use of fibrates in the United States and Canada. JAMA. 2011;305:1217–24.
Downing NS, Ross JS, Jackevicius CA, Krumholz HM. Avoidance of generic competition by Abbott Laboratories’ fenofibrate franchise. Arch Intern Med. 2012;172:724–30.
Brown WV, Ansell BJ, Mackey RH. JCL roundtable: HDL in the primary care setting. J Clin Lipidol. 2014;8:364–72.
Sprecher DL. Targeting triglycerides as prognostic indicators and determining lowest values for patient benefit. Curr Cardiol Rep. 2001;3:424–32.
Le NA, Walter MF. The role of hypertriglyceridemia in atherosclerosis. Curr Atheroscler Rep. 2007;9:110–5.
Havel RJ. Triglyceride-rich lipoproteins and plasma lipid transport. Arterioscler Thromb Vasc Biol. 2010;30:9–19.
Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13:544–52.
Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, for the European Atherosclerosis Society Consensus Panel, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32:1345–61.
Kholi P, Cannon CP. Triglycerides: how much credit do they deserve? Med Clin N Am. 2012;96:39–55.
Berglund L, Sacks F, Brunzell JD. Renewed interest in triglycerides. Clin Lipidol. 2013;8:1–4.
Libby P. Fat fuels the flame: triglyceride-rich lipoproteins and arterial inflammation. Circ Res. 2007;100:299–301.
Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation. 1998;97:1029–36.
Li XL, Hong LF, Luo SH, Guo YL, Zhu CG, Sun J, et al. Impact of admission triglyceride for early outcome in diabetic patients with stable coronary artery disease. Lipids Health Dis. 2014;13:73.
Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E, PROVE IT-TIMI 22 Investigators. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE-IT TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–30.
Faergeman O, Holme I, Fayyad R, Bhatia S, Grundy SM, Kastelein JJ, Steering Committees of IDEAL and TNT Trials, et al. Plasma triglycerides and cardiovascular events in the treating to new targets and incremental decrease in end-points through aggressive lipid lowering trials of statins in patients with coronary artery disease. Am J Cardiol. 2009;104:459–63.
Schwartz GG, Abt M, Bao W, DeMicco D, Kallend, M et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65:2267–75.
Stauffer ME, Weisenfluh L, Morrison A. Association between triglycerides and cardiovascular events in primary populations: a meta-regression analysis and synthesis of evidence. Vasc Health Risk Manag. 2013;9:671–80.
Rader DJ. Spotlight on HDL biology: new insights in metabolism, function, and translation. Cardiovasc Res. 2014;103(3):337–40.
Jørgensen A, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41.
Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–35.
ISIS Pharmaceuticals. ISIS-APOCIIIRx. http://isispharm.com/Pipeline/Therapeutic-Areas/SevereandRare.htm#ISIS-APOCIIIRx. Accessed 20 Sep 2014.
Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, Council on the Kidney in Cardiovascular Disease, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–333.
Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39.
Expert Dyslipidemia Panel of the International Atherosclerosis Society. An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia—full report. J Clin Lipid. 2014;8:29–60.
Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, for the European Atherosclerosis Society Consensus Panel, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32:1345–61.
Reiner Z, Catapano AL, DeBacker G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–818.
Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969–89.
Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1—executive summary. J Clin Lipidol. 2014;8:473–88.
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45.
Perk J, DeBacker G, Gohlke H, GrahamI Reiner Z, Verschuren M, et al. European guidelines on cardiovascular disease prevention in clinical practice (version, 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;2012(33):1635–701.
Rabinowitch IM. Clinical and other observations on Canadian Eskimos in the Eastern Arctic. Canadian Med Assoc J. 1936;35(5):487–501.
Rudkowska I, Guénard F, Julien P, Couture P, Lemieux S, Barbier O, et al. Genome-wide association study of the plasma triglyceride response to an n-3 polyunsaturated fatty acid (PUFA) supplementation. J Lipid Res. 2014;55:1245–53.
Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–9.
Lee JH, O’Keefe JH, Lavie CJ, Harris WS. Omega-3 fatty acids: cardiovascular benefits, sources and sustainability. Nat Rev Cardiol. 2009;6:753–8.
Kromhout D, de Goode J. Update on cardiometabolic health effects of ω-3 fatty acids. Curr Opin Lipidol. 2014;25:85–90.
Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–8.
Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease—effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–67.
von Schacky C. Omega-3 fatty acids: anti-arrythmic, proarrhythmic, or both? Front Cardiac Electrophysiol. 2012;3:98.
Kang JX, Leaf A. Antiarrhythmic effects of polyunsaturated fatty acids. Circulation. 1996;94:1774–80.
Mozaffarian D, Lemaitre RN, King IB, Song X, Spiegalman D, Sacks FM, et al. Circulating ω-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann Intern Med. 2011;155:160–70.
Scorletti E, Byrne CD. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr. 2013;33:231–48.
Poudyal H, Panchal SK, Diwan V, Brown L. Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Progr Lipid Res. 2011;50:372–87.
Delmastro-Greenwood M, Freeman BA, Wendell SG. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu Rev Physiol. 2014;76:79–105.
Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010;21:781–92.
Sala-Vila A, Cofan M, Mateo-Gallego R, Cenarro A, Civeira F, Ros E. Eicosapentaenoic acid in serum phospholipids relates to a less atherogenic lipoprotein profile in subjects with familial hypercholesterolemia. J Nutr Biochem. 2013;24:1604–8.
Rudkowska I. Fish oils for cardiovascular disease: impact on diabetes. Maturitas. 2010;67:25–8.
Merino J, Sala-Vila A, Kones R, Ferre R, Plana N, Girona J, et al. Increasing long-chain n-3 PUFA consumption improves small peripheral artery function in patients at intermediate-high cardiovascular risk. J Nutr Biochem. 2014;25:642–6.
Kones R. Inflammation, CRP, and cardiometabolic risk: how compelling is the potential therapeutic role of omega-3 PUFA in cardiovascular disease? Clin Lipidol. 2011;6:627–30.
Simopoulos AP. Omega-6/omega-3 essential fatty acid ratio and chronic diseases. Food Res Int. 2004;20:77–90.
Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008;233:674–88.
Schaebel LH, Vertergaard H, Laurberg P, Rathcke CN, Andersen S. Intake of traditional Inuit diet vary in parallel with inflammation as estimated from YKL-40 and hsCRP in Inuit and non-Inuit in Greenland. Atherosclerosis. 2013;228:496–501.
GISSI-Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455.
Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, GISSI-Prevenzione Investigators, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903.
Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Japan EPA lipid intervention study (JELIS) Investigators, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis [erratum. In: Lancet. 2007;370:220]. Lancet. 2007;369:1090–8.
Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival. Cardiovasc Drugs Ther. 1997;11:485–91.
Svensson M, Schmidt EB, Jørgensen KA, Christensen JH, OPACH Study Group. N-3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: a randomized, placebo-controlled intervention trial. Clin J Am Soc Nephrol. 2006;1:780–6.
von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;130:554–62.
Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–9.
Schiano V, Laurenzano E, Brevetti G, Schiano V, Laurenzano E, Brevetti G. Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clin Nutr. 2008;27:241–7.
Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989;2:757–61.
Marik PE, Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32:365–72.
León H, Shibata MC, Sivakumaran S, Dorgan M, Chatterley T, Tsuyuki RT. Effect of fish oil on arrhythmias and mortality: systematic review. BMJ. 2008;337:a2931.
Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304.
Bosch J, Gerstein HC, Dagenais GR, Díaz R, Dyal L, Jung H, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–18.
Kwak SM, Myung SK, Lee YJ, Seo HG. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med. 2012;172:686–94.
The Risk and Prevention Study Collaborative Group. N-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–8.
Sekikawa A, Miura K, Lee S, Fujiyoshi A, Edmundowicz D, Kadowaki T, ERA JUMP Study Group, et al. Long-chain n-3 polyunsaturated fatty acids and incidence rate of coronary artery calcification in Japanese in Japan and United states whites—population-based prospective cohort study. Heart. 2014;100:569–73.
Wu JHY, Mozaffarian D. ω-3 fatty acids, atherosclerosis progression and cardiovascular outcomes in recent trials: new pieces in a complex puzzle. Heart. 2014;100:530–533.
Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–33.
Teva launches first generic of GSK’s Lovaza in US, April 9, 2014. http://www.pmlive.com/pharma_news/teva_launches_first_generic_of_gsks_lovaza_in_us_559273. Accessed 15 Feb 2015.
Itakura H, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, et al. JELIS. The change in low-density lipoprotein cholesterol concentration is positively related to plasma docosahexaenoic acid but not eicosapentaenoic acid. J Atheroscler Thromb. 2012;19:673–9.
Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, placebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] Trial). Am J Cardiol. 2011;108:682–90.
Bays HE, Braeckman RA, Ballantyne CM, Kastelein JJ, Otvos JD, Stirtan WG. Icosapent ethyl, a pure EPA omega-3 fatty acid: effects on lipoprotein particle concentration and size in patients with very high triglyceride levels (the MARINE study). J Clin Lipidol. 2012;6:565–72.
Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Soni PN. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR Studies. Am J Cardiovasc Drugs. 2013;13:37–46.
Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110:984–92.
Herper M. Why amarin has to finish its big fish oil study. http://www.forbes.com/sites/matthewherper/2013/10/21/why-it-would-be-morally-wrong-for-amarin-to-stop-its-big-trial-of-heart-drug-vascepa/. Accessed 20 Sep 2014.
Husten L. Amarin says it will complete cardiovascular outcomes trial for its fish oil pill. http://www.forbes.com/sites/larryhusten/2014/09/16/amarin-says-it-will-complete-cardiovascular-outcomes-trial-for-its-fish-oil-pill/. Accessed 10 Mar 2015.
NICE. MI—secondary prevention. Secondary prevention in primary and secondary care for patients following a myocardial infarction National Institute for Health and Care Excellence (NICE) Clinical Guidelines CG172, November, 2013. http://www.nice.org.uk/guidance/cg172/resources/guidance-mi-secondary-prevention-pdf. Accessed 15 Feb 2015.
NICE. Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. NICE Clinical Guideline CG181, July 2014. http://www.nice.org.uk/guidance/cg181/resources/guidance-lipid-modification-cardiovascular-risk-assessment-and-the-modification-of-blood-lipids-for-the-primary-and-secondary-prevention-of-cardiovascular-disease-pdf. Accessed 15 Feb 2015.
Omega-3 fatty acid supplements, Nice Advisory KTT4, January 15, 2015. https://www.nice.org.uk/advice/ktt4. Accessed 24 June 2015.
Kastelein JJ, Maki KC, Susekov A, Ezhov M, Nordestgaard BG, Machielse BN, et al. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: the EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8:94–106.
Offman E, Marenco T, Ferber S, Johnson J, Kling D, Curcio D, et al. Steady-state bioavailability of prescription omega-3 on a low-fat diet is significantly improved with a free fatty acid formulation compared with an ethyl ester formulation: the ECLIPSE II study. Vasc Health Risk Manag. 2013;9:563–73.
Maki KC, Orloff DG, Nicholls SJ, Dunbar RL, Roth EM, Curcio D, Johnson J, et al. A highly bioavailable omega-3 free fatty acid formulation improves the cardiovascular risk profile in high-risk, statin-treated patients with residual hypertriglyceridemia (the ESPRIT trial). Clin Ther. 2013;35:1400–11.
de Lorgeril M, Salen P, Defaye P, Rabaeus M. Recent findings on the health effects of omega-3 fatty acids and statins, and their interactions: do statins inhibit omega-3? BMC Med. 2013;11:5.
Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, et al. Effects of statins on serum n-3 to n-6 polyunsaturated fatty acid ratios in patients with coronary artery disease. J Cardiovasc Pharmacol Ther. 2013;18:320–6.
Jia L, Betters JL, Yu L. Niemann-Pick C1-Like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Ann Rev Physiol. 2011;73:239–59.
Phan BA, Dayspring TD, Toth PP. Ezetimibe therapy: mechanism of action and clinical update. Vasc Health Risk Manag. 2012;8:415–427.
Sudhop T, Reber M, Tribble D, Sapre A, Taggart W, Gibbons P, et al. Changes in cholesterol absorption and cholesterol synthesis caused by ezetimibe and/or simvastatin in men. J Lipid Res. 2009;50:2117–23.
Pandor A, Ara RM, Tumur I, Wilkinson AJ, Paisley S, Duenas A, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265:568–80.
Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, ENHANCE Investigators, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–43.
Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, SEAS Investigators, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–56.
Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, SHARP Investigators, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92.
Brown G, Taylor AJ. Does ENHANCE diminish confidence in lowering LDL or in ezetimibe? N Engl J Med. 2008;358:1504–7.
Drazen JM, Jarcho JA, Morrissey S, Curfman GD. Cholesterol lowering and ezetimibe. N Engl J Med. 2008;358:1507–8.
Jackevicius CA, Tu JV, Ross JS, Ko DT, Krumholz HM. Use of ezetimibe in the United States and Canada. N Engl J Med. 2008;358:1819–28.
Krumholz HM. Emphasizing the burden of proof: the American College of Cardiology 2008 Expert Panel comments on the ENHANCE trial. Circ Cardiovasc Qual Outcomes. 2010;3:565–7.
Institute of Medicine of the National Academies (Committee on Qualification of Biomarkers and Surrogate Endpoints in Chronic Disease). Evaluation of biomarkers and surrogate endpoints in chronic disease. Washington, DC: Institute of Medicine; 2010.
Lu L, Krumholz HM, Tu JV, Ross JS, Ko DT, Jackevicius CA. Impact of drug policy on regional trends in ezetimibe use. Circ Cardiovasc Qual Outcomes. 2014;7:589–96.
Ross JS, Frazee SG, Garavaglia SB, Levin R, Novshadian H, Jackevicius CA, et al. Trends in use of ezetimibe after the ENHANCE trial, 2007 through 2010. JAMA Intern Med. 2014;174:1486–93.
Cannon CP, Giugliano RP, Blazing MA, Harrington RA, Peterson JL, Sisk CM, et al. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J. 2008;156:826–32.
Farnier M, Guyton JR, Jensen E, Polis AB, Johnson-Levonas AO, Brudi P. Effects of ezetimibe, simvastatin and ezetimibe/simvastatin on correlations between apolipoprotein B, LDL cholesterol and non-HDL cholesterol in patients with primary hypercholesterolemia. Atherosclerosis. 2013;229:415–22.
Laufs U, Descamps OS, Catapano AL, Packard CJ. Understanding IMPROVE-IT and the cardinal role of LDL-C lowering in CVD prevention. Eur Heart J. 2014;35:1996–2000.
Cannon CP. IMPROVE-IT trial: a comparison of ezetimibe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes after acute coronary syndromes. American Heart Association 2014 Scientific Sessions; November 17, 2014; Chicago, IL. [late breaking abstract session LBCT.02]. http://www.abstractsonline.com/pp8/#!/3547/presentation/49570. Accessed 20 Feb 2015.
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Therouxet P, et al. For the IMPROVE-IT investigators. Ezetimibe added to statin therapy after acute coronary syndromes. New Engl J Med. 2015;372:2387–97.
Myocardial Infarction Genetics Consortium Investigators. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med. 2014;371:2072–82.
Ference BA, Flack JM. Effect of naturally random allocation to lower LDL-C mediated by polymorphisms in NPC1L1, HMGCR or both on the risk of coronary heart disease: a 2×2 factorial Mendelian randomization study [poster no. 2258]. Chicago: American Heart Association Scientific Sessions 2014; 15–19 Nov 2014.
Gudzune KA, Monroe AK, Sharma R, Ranasinghe PD, Chelladurai Y, Robinson KA. Effectiveness of combination therapy with statin and another lipid-modifying agent compared with intensified statin monotherapy. A systematic review: effectiveness of combination therapy with statin. Ann Intern Med. 2014;160:468–76.
Zhao YT, Chen Q, Sun YX, Li XB, Zhang P, Xu Y, et al. Prevention of sudden cardiac death with omega-3 fatty acids in patients with coronary artery disease: a meta-analysis of randomized controlled trials. Ann Med. 2009;41:301–10.
von Schacky C. Omega-3 fatty acids in cardiovascular disease—an uphill battle. Prostaglandins Leukot Essent Fatty Acids. 2015;92:41–7.
Sahebkar A, Watts GF. New LDL-cholesterol lowering therapies: pharmacology, clinical trials, and relevance to acute coronary syndromes. Clin Ther. 2013;35:1082–98.
Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72.
Bjørklund MM, Hollensen AK, Hagensen MK, Dagnæs-Hansen F, Christoffersen C, Mikkelsen JG, et al. Induction of atherosclerosis in mice and hamsters without germline genetic engineering. Circ Res. 2014;114:1684–9.
Alborn WE, Cao G, Careskey HE, Qian YW, Subramaniam DR, Davies J, et al. Serum proprotein convertase subtilisin kexin type 9 is correlated directly with serum LDL cholesterol. Clin Chem. 2007;53:1814–9.
Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol. 2014;11:563–75.
Ridker PM. LDL cholesterol: controversies and future therapeutic directions. Lancet. 2014;384:607–17.
Stein EA, Swergold GD. Potential of proprotein convertase subtilisin/kexin type 9 based therapeutics. Curr Atheroscler Rep. 2013;15:310.
Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, for the The DESCARTES Investigators, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–19.
Raal FJ, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, et al. The addition of evolocumab (AMG 145) allows the majority of familial hypercholesterolemic patients to achieve low-density lipoprotein cholesterol goals: results from the phase 3 randomized, double-blind placebo-controlled study [abstract no. 5037]. Washington, DC: American College of Scientific Sessions 2014; 29 Mar 2014.
Cho L, Rocco M, Colquhoun D, Rosenson RS, Dent R, Zue A, et al. Design and rationale of the GAUSS-2 study trial: a double-blind, ezetimibe-controlled phase 3 study of the efficacy and tolerability of evolocumab (AMG 145) in subjects with hypercholesterolemia who are intolerant of statin therapy. Clin Cardiol. 2014;37:131–9.
Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, et al. GAUSS-2 Investigators. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–8.
Amgen. Further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk (FOURIER) [ClinicalTrials.gov. identifier NCT01764633]. Bethesda: US National Institutes of Health. http://clinicaltrials.gov/show/NCT01764633. Accessed 20 Sep 2014.
Roth EM, Taskinen M, Ginsberg H, et al. A 24-week study of alirocumab as monotherapy versus ezetimibe: the first Phase 3 data of a proprotein convertase subtilisin/kexin type 9 inhibitor. J Am Coll Cardiol. 2014;63(12_S).
Farnier M, Kastelein JJP, Roth E, Taskinen MR, Ginsberg HN, Colhoun HM, et al. Relationship between alirocumab, PCSK9 and LDL-C levels: results from the ODYSSEY MONO Phase 3 trial of alirocumab 75 mg every 2 weeks [abstract no. EAS-0758]. Madrid: EAS; 31 May–3 Jun 2014.
Sanofi. Phase III study to evaluate alirocumab in patients with hypercholesterolemia not treated with a statin (ODYSSEY CHOICE II) [ClinicalTrials.gov. identifier NCT02023879]. Bethesda: US National Institutes of Health. https://clinicaltrials.gov/ct2/show/NCT02023879. Accessed 10 Mar 2015.
Sanofi. Long-term safety and tolerability of alirocumab SAR236553 (REGN727) versus placebo on top of lipid-modifying therapy in high cardiovascular risk patients with hypercholesterolemia (ODYSSEY Long Term) [ClinicalTrials.gov. identifier NCT01507831]. Bethesda: US National Institutes of Health. http://clinicaltrials.gov/ct2/show/NCT01507831. Accessed 10 Mar 2015.
Cholesterol drug halves heart attack and stroke in early test. The N. Y. Times via Reuters. August 31, 2014. http://www.nytimes.com/2014/09/01/business/international/cholesterol-drug-halves-heart-attack-and-stroke-in-early-test.html. Accessed 19 Mar 2015.
Sanofi. The efficacy and safety of alirocumab SAR236553 (REGN727) versus ezetimibe on top of statin in high cardiovascular risk patients with hypercholesterolemia (ODYSSEY Combo II) [ClinicalTrials.gov. identifier NCT01644188]. Bethesda: US National Institutes of Health. http://clinicaltrials.gov/ct2/show/NCT01644188. Accessed 10 Mar 2015.
Sanofi. ODYSSEY Outcomes: evaluation of cardiovascular outcomes after an acute coronary syndrome during treatment with alirocumab SAR236553 (REGN727). [ClinicalTrials.gov. identifier NCT01644188]. Bethesda: US National Institutes of Health. http://clinicaltrials.gov/show/NCT01663402. Accessed 10 Mar 2015.
Moriarty PM, Jacobson TA, Bruckert E, Thompson PD, Guyton JR, Baccara-Dinet MT, et al. Efficacy and safety of alirocumab, a monoclonal antibody to PCSK9, in statin-intolerant patients: design and rationale of ODYSSEY ALTERNATIVE, a randomized phase 3 trial. J Clin Lipidol. 2014;8:554–61.
Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, et al. ODYSSEY ALTERNATIVE: efficacy and safety of the proprotein convertase subtilisin/kexin type 9 monoclonal antibody, alirocumab, versus ezetimibe, in patients with statin intolerance as defined by a placebo run-in and satin rechallenge arm. Late-Breaking Clinical Trial Abstracts. Circulation. 2014;2(130):2108–9.
Ballantyne CM, Neutel J, Cropp A, Duggan W, Wang E, Plowchalk D, et al. Efficacy and safety of bococizumab (RN316/PF-04950615), a monoclonal antibody against proprotein convertase subtilisin/kexin type 9 in statin-treated hypercholesterolemic subjects: results from a randomized, placebo-controlled, dose-ranging study (NCT: 01592240) [abstract no. 1183-129]. Washington, DC: American College of Cardiology Scientific Session, 29–31 Mar 2014.
Pfizer. The evaluation of PF-04950615 (RN316), in reducing the occurrence of major cardiovascular events in high risk subjects (SPIRE-1) [ClinicalTrials.gov. identifier NCT01975376]. Bethesda: US National Institutes of Health. http://clinicaltrials.gov/ct2/show/NCT01975376. Accessed 10 Mar 2015.
Pfizer. The evaluation of PF-04950615 (RN316) in reducing the occurrence of major cardiovascular events in high risk subjects (SPIRE-2) [ClinicalTrials.gov. identifier NCT01975389]. Bethesda: US National Institutes of Health. https://clinicaltrials.gov/ct2/show/results/NCT01975389. Accessed 10 Mar 2015.
Sheridan C. Phase 3 data for PCSK9 inhibitor wows. Nat Biotech. 2013;31:1057–8.
Beasley D. FDA probes cognitive impact of new cholesterol drugs. March 7, 2014. http://www.reuters.com/article/2014/03/07/us-regeneron-cholesterol-idUSBREA261KU20140307. Accessed 19 Mar 2015.
Norata GD, Tibolla G, Catapano AL. PCSK9 inhibition for the treatment of hypercholesterolemia: promises and emerging challenges. Vascul Pharmacol. 2014;62:103–11.
Seidah NG, Awan Z, Chrétien M, Mbikay M. PCSK9. A key modulator of cardiovascular health. Circ Res. 2014;114:1022–36.
Shapiro MD, Fazio S, Tavori H. Targeting PCSK9 for therapeutic gains. Curr Atheroscler Rep. 2015;17:499.
Rader DJ, Kastelein JJP. Lomitapide and mipomersen. Two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014;129:1022–32.
Wong E, Goldberg T. Mipomersen (Kynamro): a novel antisense oligonucleotide inhibitor for the management of homozygous familial hypercholesterolemia. PT. 2014;39:119–22.
Bell DA, Hooper AJ, Watts GF, Burnett JR. Mipomersen and other therapies for the treatment of severe familial hypercholesterolemia. Vasc Health Risk Manag. 2012;8:651–9.
Crooke ST, Geary RS. Clinical pharmacological properties of mipomersen (Kynamro), a second generation antisense inhibitor of apolipoprotein B. Br J Clin Pharmacol. 2013;76:269–76.
Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006.
Stein EA, Dufour R, Gagne C, Gaudet D, East C, Donovan JM, Chin W, et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation. 2012;126:2283–92.
McGowan MP, Tardif JC, Ceska R, Burgess LJ, Soran H, Gouni-Berthold I, et al. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS One. 2012;7:e49006.
Santos RD, Raal FJ, Catapano AL, Witztum JL, Steinhagen-Thiessen E, Tsimikas S. Mipomersen, an antisense oligonucleotide to apolipoprotein B-100, reduces lipoprotein(a) in various populations with hypercholesterolemia: results of 4 phase III trials. Arterioscler Thromb Vasc Biol. 2015;35:689–99.
Kynamro (mipomersen sodium) injection solution for subcutaneous injection [prescribing information]. Cambridge: Genzyme Corp.; 2013. http://www.kynamro.com/~/media/Kynamro/Files/kynamro-pi.pdf. Accessed 26 Feb 2015.
Wong E, Goldberg T. Mipomersen (kynamro): a novel antisense oligonucleotide inhibitor for the management of homozygous familial hypercholesterolemia. P T. 2014;39:119–22.
deGoma EM. Lomitapide for the management of homozygous familial hypercholesterolemia. Rev Cardiovasc Med. 2014;15:109–118.
Raal FJ. Lomitapide for homozygous familial hypercholesterolaemia. Lancet. 2013;381:7–8.
Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–56.
Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Phase 3 HoFH Lomitapide Study Investigators, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–6.
Cuchel M, Blom D, Averna MR, Meagher EA, Theron HD, Sirtori CR, et al. Sustained LDL-C lowering and stable hepatic fat levels in patients with homozygous familial hypercholesterolemia treated with the microsomal triglyceride transfer protein inhibitor, lomitapide: results of an ongoing long-term extension study. Circulation. 2013;128:A16516.
Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006.
Kones R. Primary prevention of coronary heart disease: integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey. Drug Des Devel Ther. 2011;5:325–380.
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25.
Rohla M, Weiss TW. Adipose tissue, inflammation and atherosclerosis. Clin Lipidol. 2014;9:71–81.
Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B Cells and humoral immunity in atherosclerosis. Circ Res. 2014;114:1743–56.
Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:19–46.
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–6.
Ghattas A, Griffiths HR, Devitt A, Lip GYH, Shantsila E. Monocytes in coronary artery disease and atherosclerosis. Where are we now? J Am Coll Cardiol. 2013;62:1541–51.
Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol Mech Dis. 2014;9:73–102.
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
Giunzioni I, Bonomo A, Bishop E, Castiglioni S, Corsini A, Bellosta S. Cigarette smoke condensate affects monocyte interaction with endothelium. Atherosclerosis. 2014;234:383–90.
Nordestgaard BG, Palmer TM, Benn M, Zacho J, Tybjærg-Hansen A, et al. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a mendelian randomisation approach. PLoS Med. 2012;9:e1001212.
Thomsen M, Nordestgaard BG. Myocardial infarction and ischemic heart disease in overweight and obesity with and without metabolic syndrome. JAMA Intern Med. 2014;174:15–22.
Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308.
Jørgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjarg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34:1826–33.
Varbo A, Benn M, Tybjarg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–309.
Benn M, Tybjaerg-Hansen A, McCarthy MI, Jensen GB, Grande P, Nordestgaard BG. Nonfasting glucose, ischemic heart disease, and myocardial infarction: a Mendelian randomization study. J Am Coll Cardiol. 2012;19(59):2356–65.
Kones R. Molecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: relationship with subclinical disease, undertreatment, and poor adherence: implications of new evidence upon optimizing cardiovascular patient outcomes. Vasc Health Risk Manag. 2013;9:617–70.
Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012;125:1147–56.
Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–92.
Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–13.
Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114:1867–79.
Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;11(339):166–72.
Fredman G, Ozcan L, Tabas I. Common therapeutic targets in cardiometabolic disease. Science Transl Med. 2014;6:1–5.
Dinarello CA. A clinical perspective of interleukin-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–17.
Kones R. Rosuvastatin, inflammation, C-reactive protein, JUPITER, and primary prevention of cardiovascular disease—a perspective. Drug Des Devel Ther. 2010;4:383–413.
Satoh K, Shimokawa H. High-sensitivity C-reactive protein: still need for next-generation biomarkers for remote future cardiovascular events. Eur Heart J. 2014;35:1776–8.
Braunwald E. Creating controversy where none exists: the important role of C-reactive protein in the CARE, AFCAPS/TexCAPS, PROVE IT, REVERSAL, A to Z, JUPITER, HEART PROTECTION, and ASCOT trials. Eur Heart J. 2012;33:430–2.
Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40.
Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–9.
Wilson PW, Pencina M, Jacques P, Selhub J, D’Agostino R Sr, O’Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes. 2008;1:92–7.
Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35:578–89.
Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet. 2012;379:1214–24.
IL6R Genetics Consortium Emerging Risk Factors Collaboration, Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–13.
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJP, for the JUPITER Study Group JUPITER Study Group, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207.
Henao-Mejia J, Elinav E, Thaiss CA, Flavell RA. Inflammasomes and metabolic disease. Annu Rev Physiol. 2014;76:57–78.
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61.
Imazio M, Brucato A, Ferrazzi P, Pullara A, Adler Y, Barosi A, for the COPPS-2 Investigators, et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. JAMA. 2014;312:1016–23.
Nidorf SM, Eikelboom JW, Thompson PL. Colchicine for secondary prevention of cardiovascular disease. Curr Atheroscler Rep. 2014;16:391.
Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1b inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162:597–605.
Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, on behalf of the CANTOS Pilot Investigative Group, et al. Effects of interleukin-1 inhibition with canakinumab on hemoglobin A1c, lipids, C-eactive protein, interleukin-6, and fibrinogen. A phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–48.
Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782–91.
Ridker PM. Targeting inflammatory pathways for the treatment of cardiovascular disease. Eur Heart J. 2014;35:540–3.
Everett BM, Pradhan A, Solomon DH, Paynter N, MacFadyen J, Zaharris E, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166(199–207):e15.
Ridker P. Cardiovascular Inflammation Reduction Trial (CIRT) [Clinicaltrials.gov identifier NCT01594333]. Bethesda: US National Institutes of Health. http://clinicaltrials.gov/show/NCT01594333. Accessed 24 June 2015.
Pagnoux C, Goulet M. Role and place of methotrexate in vasculitis management. Int J Clin Rheumtol. 2009;4:697–715.
Olbetam capsules 250. SPC. http://www.medicines.org.uk/emc/medicine/5355. Accessed 24 Sep 2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
No external funding was used for this work.
Rights and permissions
About this article
Cite this article
Kones, R., Rumana, U. Current Treatment of Dyslipidemia: Evolving Roles of Non-Statin and Newer Drugs. Drugs 75, 1201–1228 (2015). https://doi.org/10.1007/s40265-015-0429-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0429-3