Abstract
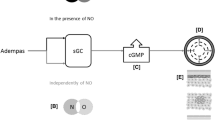
Riociguat (Adempas®), a soluble guanylate cyclase stimulator, is a new, first-in-class drug approved for the treatment of patients with chronic thromboembolic pulmonary hypertension (CTEPH) [inoperable or persistent/recurrent following surgery] or pulmonary arterial hypertension (PAH). It has been designated an orphan medicine by the European Medicines Agency and the US FDA. This article reviews the available pharmacological properties of oral riociguat and its clinical efficacy and tolerability in adults with CTEPH or PAH. Riociguat is effective and well tolerated in patients with inoperable CTEPH or persistent/recurrent CTEPH following pulmonary endarterectomy, and in patients with PAH. It has a positive result on exercise capacity and pulmonary haemodynamics, and improves WHO functional class. Most adverse events can be attributed to the vasodilatory mechanism of riociguat; however, there is a potential for serious bleeding and fetal harm, and riociguat use is contraindicated in pregnant patients. Pulmonary endarterectomy remains the first treatment of choice for CTEPH, as it is potentially curative. Head-to-head trials comparing riociguat with the approved phosphodiesterase type 5 inhibitors in patients with PAH would be of value for the placement of riociguat in the management of this disease. Riociguat is a promising addition to the treatment options for patients with CTEPH or PAH.




Similar content being viewed by others
References
McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250–94.
Galié N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493–537.
Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–50.
European Medicines Agency. Adempas (riociguat) EPAR summary for the public. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002737/WC500165037.pdf. Accessed 8 Sep 2014.
US FDA. Adempas (riociguat) FDA approval letter. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204819Orig1s000Approv.pdf. Accessed 8 Sep 2014.
Bayer Healthcare. Adempas® (riociguat tablets): US prescribing information. 2014. http://labeling.bayerhealthcare.com/html/products/pi/Adempas_PI.pdf. Accessed 9 July 2014.
European Medicines Agency. Adempas® (riociguat tablets): EU summary of product characteristics. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002737/WC500165034.pdf. Accessed 9 July 2014.
Schermuly RT, Stasch JP, Pullamsetti SS, et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J. 2008;32(4):881–91.
Becker EM, Stasch J-P, Bechem M, et al. Effects of different pulmonary vasodilators on arterial saturation in a model of pulmonary hypertension. PLoS One. 2013;8(8):e73502.
Dumitrascu R, Weissmann N, Ghofrani HA, et al. Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation. 2006;113(2):286–95.
Evgenov OV, Zou L, Zhang M, et al. Nitric oxide-independent stimulation of soluble guanylate cyclase attenuates pulmonary fibrosis [abstract no. O9]. BMC Pharmacol. 2011;11(Suppl. 1).
Geschka S, Kretschmer A, Sharkovska Y, et al. Soluble guanylate cyclase stimulation prevents fibrotic tissue remodeling and improves survival in salt-sensitive Dahl rats. PLoS One. 2011;6(7):e21853.
Kojonazarov B, Lang M, Weissmann N, et al. Effects of riociguat on pulmonary vascular remodeling in severe experimental pulmonary hypertension [abstract no. A2516]. Am J Respir Crit Care Med. 2011;183(1 MeetingAbstracts).
Lang M, Kojonazarov B, Tian X, et al. The soluble guanylate cyclase stimulator riociguat ameliorates pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS One. 2012;7(8):e43433.
Pichl A, Parajuli N, Seimetz M, et al. Stimulation of soluble guanylate cyclase by riociguat prevents tobacco smoke-induced pulmonary hypertension in mice [abstract no. A310]. Pneumologie. 2012;66(6).
Sharkovska Y, Kalk P, Lawrenz B, et al. Nitric oxide-independent stimulation of soluble guanylate cyclase reduces organ damage in experimental low-renin and high-renin models. J Hypertens. 2010;28(8):1666–75.
Weissmann N, Lobo B, Pichl A, et al. Stimulation of soluble guanylate cyclase prevents cigarette smoke-induced pulmonary hypertension and emphysema. Am J Respir Crit Care Med. 2014;189(11):1359–73.
Frey R, Muck W, Unger S, et al. Single-dose pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase stimulator BAY 63-2521: an ascending-dose study in healthy male volunteers. J Clin Pharmacol. 2008;48(8):926–34.
Grimminger F, Weimann G, Frey R, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J. 2009;33(4):785–92.
Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J. 2010;36(4):792–9.
Ghofrani H-A, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–29.
Ghofrani H-A, Galié N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–40.
Bayer HealthCare Pharmaceuticals Inc. Briefing document for the Cardiovascular and Renal Drugs Advisory Committee: riociguat (BAY 63-2521). 2013. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm363543.pdf. Accessed 5 June 2014.
Frey R, Muck W, Kirschbaum N, et al. Riociguat (BAY 63-2521) and warfarin: a pharmacodynamic and pharmacokinetic interaction study. J Clin Pharmacol. 2011;51(7):1051–60.
Frey R, Muck W, Unger S, et al. No pharmacodynamic (PD) and pharmacokinetic (PK) interaction of riociguat (BAY 63-2521) and aspirin [abstract no. P3977]. In: 21st Annual Congress of the European Respiratory Society; 24–28 Sep 2011; Amsterdam.
Galie N, Neuser D, Muller K, et al. A placebo-controlled, double-blind phase II interaction study to evaluate blood pressure following addition of riociguat to patients with symptomatic pulmonary arterial hypertension (pah) receiving sildenafil (patent Plus) [abstract no. A3530]. Am J Respir Crit Care Med. 2013;187(1_MeetingAbstracts).
Becker C, Frey R, Hesse C, et al. Absorption behavior of riociguat: bioavailability, food effects, and dose-proportionality [abstract no. P7]. BMC Pharmacol Toxicol. 2013;14(Suppl. 1):22.
Becker C, Frey R, Unger S, et al. Pharmacokinetic interaction of ketoconazole, clarithromycin, and midazolam with riociguat [abstract no. P5]. BMC Pharmacol Toxicol. 2013;14(Suppl. 1):19–22.
Rickert V, Haefeli WE, Weiss J. Pharmacokinetic interaction profile of riociguat, a new soluble guanylate cyclase stimulator, in vitro. Pulm Pharmacol Ther. 2014. doi:10.1016/j.pupt.2014.02.004.
Frey R, Becker C, Unger S, et al. Effect of omeprazole and AlOH/MgOH on riociguat absorption [abstract no. P4079]. In: 23rd Annual Congress of the European Respiratory Society; 7–11 Sep 2013; Barcelona.
Frey R, Becker C, Unger S, et al. Pharmacokinetics of the soluble guanylate cyclase stimulator riociguat in individuals with hepatic impairment [abstract no. P21]. BMC Pharmacol Toxicol. 2013;14(Suppl. 1):33–4.
Frey R, Becker C, Unger S, et al. Pharmacokinetics of the soluble guanylate cyclase stimulator riociguat in individuals with renal impairment [abstract no. P22]. BMC Pharmacol Toxicol. 2013;14(Suppl. 1):34–6.
Frey R, Lettieri J, Nadel A, et al. Effects of age and gender on the pharmacokinetics of the soluble guanylate cyclase stimulator riociguat [abstract no. P23]. BMC Pharmacol Toxicol. 2013;14(Suppl. 1):36–7.
US FDA. Center for Drug Evaluation and Research medical review document for riociguat. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204819Orig1s000MedR.pdf. Accessed 5 Sep 2014.
Ghofrani H, Hoeper M, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: long-term safety, tolerability, and efficacy [abstract no. A2370]. Am J Respir Crit Care Med. 2012;185(Online abstracts).
Ghofrani H-A, Simonneau G, Rubin LJ, et al. Riociguat for pulmonary hypertension. N Engl J Med. 2013;369(23):2266–8.
Ghofrani HA, Grimminger F, Hoeper MM, et al. Impact of riociguat on health-related quality of life (HRQoL) in patients with chronic thromboembolic pulmonary hypertension (CTEPH) [abstract no. P3418]. In: 23rd Annual Congress of the European Respiratory Society; 7–11 Sep 2013; Barcelona.
Simonneau G, D’Armini A, Ghofrani AH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension (CTEPH): 2-year results from the CHEST-2 long-term extension [abstract]. In: European Respiratory Society International Congress; 6–10 Sep 2014; Munich.
Wang C, D’Armini AM, Ghofrani H-A, et al. Long-term riociguat treatment in inoperable and persistent/recurrent CTEPH patients in WHO functional class (FC) I/II versus FC III/IV at baseline: results from the 16-week phase III CHEST-1 study and CHEST-2 open-label extension [abstract no. 535B]. Chest. 2014;145(3_MeetingAbstracts).
Galié N, Grimminger F, Grünig E, et al. Correlation of improvements in hemodynamics and exercise capacity in patients with PAH: Results from the phase III PATENT-1 study [abstract no. P1784]. In: 23rd Annual Congress of the European Respiratory Society; 7–11 Sep 2013; Barcelona.
Rubin L, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension (PAH): 2-year results from the PATENT-2 long-term extension [abstract] In: European Respiratory Society International Congress; 6–10 Sep 2014; Munich.
Meyer G, Galié N, Grimminger F, et al. Long-term riociguat treatment in PAH patients in WHO functional class (FC) I/II versus FC III/IV at baseline: results from the 12-week phase III PATENT-1 study and PATENT-2 open-label extension [abstract no. 513A]. Chest. 2014;145(3_MeetingAbstracts).
Mittendorf J, Weigand S, Alonso-Alija C, et al. Discovery of riociguat (BAY 63-2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. Chem Med Chem. 2009;4(5):853–65.
European Medicines Agency. Adempas (riociguat): Committee for Medicinal Products for Human Use assessment report. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002737/WC500165036.pdf. Accessed 8 July 2014.
European Medicines Agency. Committee for Medicinal Products for Human Use guideline on the clinical investigations of medicinal products for the treatment of pulmonary arterial hypertension. 2009. http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500016686. Accessed 3 Oct 2014.
Archer SL. Riociguat for pulmonary hypertension–a glass half full. N Engl J Med. 2013;369(4):386–8.
Bayer. Riociguat clinical effects studied in patients with insufficient treatment response to phosphodiesterase-5 inhibitor (RESPITE) [ClinicalTrials.gov identifier NCT02007629]. US National Institutes of Health, ClinicalTrials.gov. 2014. http://www.clinicaltrials.gov. Accessed 8 Sep 2014.
Bayer. Riociguat in patients with chronic thromboembolic pulmonary hypertension (CTEPH) (EAS) [ClinicalTrials.gov identifier NCT01784562]. US National Institutes of Health, ClinicalTrials.gov. 2014. http://www.clinicaltrials.gov. Accessed 8 July 2014.
Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation. 2013;128(5):502–11.
Bonderman D, Pretsch I, Steringer-Mascherbauer R, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;. doi:10.1378/chest.14-0106.
Ghofrani HA, Staehler G, Gruenig E, et al. The effect of the soluble guanylate cyclase stimulator riociguat on hemodynamics in patients with pulmonary hypertension due to chronic obstructive pulmonary disease [abstract no. A6127]. Am J Respir Crit Care Med. 2011;183(1_MeetingAbstracts).
Hoeper MM, Halank M, Wilkens H, et al. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J. 2013;41(4):853–60.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit. K. P. Garnock-Jones is a salaried employee of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garnock-Jones, K.P. Riociguat: A Review of Its Use in Patients with Chronic Thromboembolic Pulmonary Hypertension or Pulmonary Arterial Hypertension. Drugs 74, 2065–2078 (2014). https://doi.org/10.1007/s40265-014-0317-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0317-2