Abstract
Background
Seasonal influenza is prevented through annual vaccination, especially in children and older adults. These vaccines are annually updated based on World Health Organization recommendations and require continuous safety monitoring.
Objective
We assessed the frequency and severity of adverse events within 7 days of administering GSK’s inactivated quadrivalent seasonal influenza vaccine (IIV4) in Belgium, Germany, and Spain during the 2022/2023 influenza season.
Methods
In this enhanced safety surveillance study, adults who received GSK’s IIV4 and parents/guardians/legally acceptable representatives of vaccinated children (aged 6 months–17 years) were invited to complete adverse drug reaction cards reporting adverse events within 7 days post-vaccination.
Results
In total, 1332 participants (53.6% female) received at least one dose of GSK’s IIV4, including 43 children who received two doses. Overall, 97.8% of adverse drug reaction cards were completed and returned in the study. All participants in Belgium were adults, while 54.7% and 7.4% in Spain and Germany, respectively, were pediatric participants aged 6 months–17 years. After Dose 1, across all age groups, 49.8% of participants reported at least one adverse event. The most common adverse events (cumulative frequency >5%) following Dose 1 were injection-site pain (37.6%), fatigue (15.0%), headache (13.2%), injection-site swelling (9.3%), myalgia (7.6%), and injection-site erythema (7.4%). Across all countries, adverse events were most common in adults aged 18–65 years (59.7%), followed by those aged 3–17 years (47.0%), >65 years (35.7%), and 6–35 months (23.5%). After Dose 2, 18.6% of participants reported at least one adverse event, with general disorders and administration site conditions again being the most frequent.
Conclusions
Across all age and risk groups for serious disease, no serious adverse events related to GSK’s IIV4 were reported within 7 days post-vaccination. This study supports and confirms the acceptable safety profile of GSK’s IIV4 across all recommended age groups.
Clinical Trial Registration
ClinicalTrials.gov number: not applicable.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
GSK’s inactivated quadrivalent seasonal influenza vaccine (IIV4) demonstrated a favorable safety profile, with no serious adverse events reported within 7 days post-vaccination across all age groups, including at-risk individuals. |
After a single dose, approximately 50% of participants experienced at least one adverse event, with the most common events being injection-site and general disorders. A second dose was administered to children under 9 years old who had not been vaccinated against influenza in preceding seasons, and approximately 18.6% of those experienced mild adverse events. |
The study emphasizes that, during the period in which GSK monitored the safety of IIV4 vaccination in Europe, reported adverse events were mild to moderate in both pediatric and adult populations, including those at high risk for severe influenza. |
1 Introduction
Seasonal influenza is a highly contagious acute respiratory illness triggered by influenza viruses. In most cases, individuals infected with influenza tend to recover without any complications; however, in some individuals, especially the elderly and those with underlying medical conditions, infection may lead to severe illness or even death if left untreated [1]. The World Health Organization estimates that yearly influenza outbreaks result in approximately 1 billion infections and 3–5 million cases of severe illness [2]. In Europe, influenza places a significant burden on public health, with an estimated 4–50 million symptomatic cases and 15,000–70,000 fatalities annually, with seasonal fluctuations [3, 4]. Globally, an estimated 291,243–645,832 deaths annually are attributed to respiratory complications linked to seasonal influenza, which corresponds to a mortality rate of 4.0–8.8 per 100,000 individuals [5].
In northern hemisphere countries, influenza viruses cause seasonal epidemics between the late fall and early spring [6]. The primary strategy to protect public health from influenza remains annual vaccination, aimed at reducing infection and severity of the disease and its complications [7]. Given the ongoing evolution of influenza viruses, it is essential to annually update the composition of influenza vaccines. This practice is crucial for ensuring protection against strains that experts predict will be prevalent during the upcoming influenza season [3, 8, 9]. The formulation of these vaccines is guided by World Health Organization recommendations [10], emphasizing the need for continuous monitoring of the benefits and risks associated with seasonal influenza vaccines.
In 2014, the European Medicines Agency (EMA) introduced updated guidelines to establish enhanced safety surveillance (ESS) for seasonal influenza vaccines across the European Union [11, 12]. These new directives have replaced the previous requirement for annual safety and immunogenicity assessments through small-scale clinical trials. The primary goal of this revised guidance is to empower marketing authorization holders to efficiently detect and manage emerging safety concerns. This involves analyzing the patterns of frequency and severity of adverse events (AEs) compared with previous seasons and determining the need for further investigation.
In line with EMA directives, GSK launched annual ESS study programs from the 2015/2016 influenza season [13,14,15], with their quadrivalent vaccine designed for active immunization in individuals aged ≥6 months, providing protection against two influenza A virus subtypes and two influenza B virus lineages. This study adhered to the EMA guidance [11] and aimed to assess the occurrence and intensity of predefined adverse events of interest (AEIs) and any other AEs within the 7-day period following vaccination with GSK’s inactivated quadrivalent seasonal influenza vaccine (IIV4: AlphaRix Tetra in Belgium; Influsplit Tetra in Germany; Fluarix Tetra in Spain) during the 2022/2023 influenza season. Additionally, the study examined the percentages of participants reporting AEs within 7 days of GSK’s IIV4 vaccination, categorized according to the Medical Dictionary for Regulatory Activities (MedDRA®) classification, stratified by age group and risk status for each country and overall.
2 Methods
2.1 Study Design and Population
This was a non-interventional, multicenter, prospective ESS study conducted from 3 October, 2022, to 15 January, 2023, at seven healthcare centers in Belgium (1), Germany (3), and Spain (3). The study included all age groups; however, because of feasibility constraints, healthcare centers in Belgium recruited participants aged ≥18 years, whereas those in Germany and Spain recruited children aged ≥6 months–17 years and adults aged 18–65 years and ≥65 years. Study personnel approached adults aged ≥18 years and legally acceptable representatives of children aged ≥6 months to assess their willingness to participate in the study after receiving GSK’s IIV4 at the study sites as part of routine healthcare. Enrolled participants, or their legal representatives who had provided written informed consent, were provided with an adverse drug reaction (ADR) card and a coronavirus disease 2019 (COVID-19) assessment card and instructed to complete and return these cards to their healthcare providers either on the day 7 visit post-vaccination (where vaccination day was day 0) or via mail. Enrolled participants were stratified into different age categories (6–35 months, 3–17 years, 18–65 years, and >65 years) and risk status (at risk, not at risk). The determination of risk status (i.e., “at risk” or “not at risk”) for influenza-associated morbidity and mortality was made by the healthcare providers based on their medical judgment and expertise.
Study staff entered the data from the completed ADR and COVID-19 assessment cards into a database using an electronic case report form. The ADR cards included a checklist of predefined local and general AEIs for participants to indicate yes/no, along with an open-text section for reporting any other AEs occurring within 7 days post-vaccination. This facilitated the assessment of the frequency and severity of both predefined AEIs and any other AEs experienced within the specified timeframe. Severity was assessed by participants/participants’ parent(s)/legally authorized representatives based on the Division of AIDS (DAIDS) Adverse Event Grading [16]. The intensity of AEs was determined as follows: mild (no/minimal interference), moderate (greater interference), severe (inability to function), and potentially life threatening (intervention required to prevent permanent impairment, disability, or death). For fever, severity was categorized based on temperature ranges: mild (38.0 to <38.6 °C), moderate (≥38.6 to <39.3 °C), severe (≥39.3 to <40.0 °C), and potentially life-threatening (≥40.0 °C). Participants were also asked to complete a COVID-19 assessment card, documenting their COVID-19 history and associated signs and symptoms. Cases of COVID-19 were identified in accordance with World Health Organization criteria [17, 18], and the impact of COVID-19 on the study was assessed.
For children aged ≥9 years, a single dose of seasonal influenza vaccine was considered sufficient if they had previously received an influenza vaccine. A second dose of GSK’s IIV4 was administered only to children aged <9 years who had not been vaccinated against influenza in preceding seasons. The recruitment period was 3 months, from 3 October, 2022, to 31 December, 2022, for participants aged ≥9 years. For participants aged <9 years who had not been vaccinated against influenza in previous seasons, recruitment occurred from 3 October, 2022, to 1 December, 2022, allowing time for data collection on the second dose, expected at least 4 weeks after the first dose. The study follow-up was 7 days for those previously vaccinated or aged ≥9 years and 35 days for children under 9 years without prior influenza vaccination. During the study period, two interim analyses were conducted (in November and December 2022) to identify any potential safety concerns or significant AEs that might pose a risk to recipients of GSK’s IIV4, ensuring continuous safety monitoring.
2.2 Statistical Methods
Approximately 1000 participants who had received GSK’s IIV4 were planned to be enrolled in the study. This sample size was chosen to enable the detection of AEIs and other AEs categorized as very common (≥1 per 10), common (≥1 per 100 to <1 per 10), and uncommon (≥1 per 1000 to <1 per 100).
All analyses were descriptive in nature. Adverse events were analyzed using the safety set, which included all enrolled participants who had received GSK’s IIV4 (Dose 1 and/or Dose 2) according to country-specific medical practices and had also returned the ADR card within the specified window (7–18 days). Additionally, a sensitivity analysis was performed using the solicited safety set, encompassing all participants from the safety set who had returned the ADR card within 18 days of receiving Dose 1 or Dose 2 (including the vaccination day) and documented the presence or absence of AEs.
Demographic characteristics and risk status for influenza-associated morbidity and mortality were summarized using frequency tables (n, %) for categorical variables and mean, standard deviation, median, minimum, and maximum for numerical data. For each vaccine dose, the cumulative percentage of participants reporting AEs within 7 days of vaccination was calculated using International Organization for Standardization (ISO) week 40, 2022, to week 01, 2023, as per the MedDRA® primary system organ class (SOC) and preferred term (PT). Furthermore, for each vaccine dose, cumulative percentages of participants reporting AEs were computed based on age categories (6–35 months, 3–17 years, 18–65 years, and >65 years) and risk status (at risk/not at risk) for influenza-associated morbidity and mortality. In both overall and country-specific analyses, exact (Clopper–Pearson) 95% confidence intervals (CIs) accounting for the clustering effect of centers were calculated for all estimated percentages. This included the cumulative percentage of participants reporting AEs by MedDRA® primary SOC and PT within 7 days after each vaccination, as recorded in the completed ADR card, throughout the study period from week 40, 2022, to week 01, 2023.
2.3 Ethical Considerations
The study protocol was approved by independent ethics committees or institutional review boards at each study center. Ethical clearance was obtained prior to the commencement of participant enrollment. Written informed consent was obtained from all participants before their participation in the study. For minors, written informed assent was obtained from their parent(s)/guardians/legally acceptable representative(s).
3 Results
3.1 Participant Disposition and Characteristics
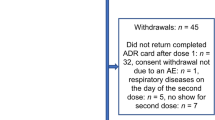
The study exceeded the planned enrollment of 1000 participants (n = 1332) owing to faster recruitment rates in some sites, while allowing for a delayed start in other sites. The additional 332 participants (+33%) strengthened the study by ensuring a sufficient sample size to capture common and uncommon AEs. A total of 1332 participants received at least one dose of GSK’s IIV4 and were enrolled across Belgium (n = 100), Germany (n = 674), and Spain (n = 558). All participants in Belgium were adults, while 54.7% and 7.4% in Spain and Germany, respectively, were pediatric participants aged 6 months–17 years. Of the enrolled participants, 1207 (90.6%) successfully completed the study, with a total of 43 (3.2%) children aged <9 years receiving two doses (Fig. 1). Overall, 97.8% of ADR cards were completed and returned in the study. The median (minimum–maximum) age of the enrolled participants was 45.0 (0.5–94.0) years (Belgium: 71.0 [33.0–91.0] years, Germany: 48.0 [1.0–94.0] years, and Spain: 12.0 [0.5–94.0] years). Most participants in Belgium (72.0% [n = 72]) were aged >65 years. In Germany, most participants (72.4% [n = 488]) were aged between 18 and 65 years, while in Spain, most participants (46.6%) were aged between 3 and 17 years. Overall, 53.6% of participants were female, 95.0% identified as White (Caucasian/European heritage), and 45.5% (606/1332) were at risk for influenza-associated morbidity and mortality, according to healthcare provider assessments. Frequently observed risk groups for influenza-associated morbidity and mortality among participants who had received their 2022/2023 GSK’s IIV4 vaccination (Dose 1) were age ≥65 years (25.2%), age ≥6 years with chronic medical conditions (24.0%), and healthcare worker status (6.4%; Table 1). Among participants who received two doses of GSK’s IIV4, the overall median age was 2.0 years, 55.8% (n = 24) were male, and 51.2% (n = 22) were considered not to be at risk for influenza-associated morbidity and mortality as assessed by healthcare providers. Overall, 18.0% of participants (234/1332) received co-administered vaccinations with GSK’s IIV4 Dose 1 during the same visit. The most frequently co-administered vaccines were COVID-19 vaccines in Germany (16.5% of participants) and Spain (17.4% of participants) and pneumococcal vaccines in Belgium (2.0% of participants). For the second dose, only one participant (9.1%) in Germany reported receiving a co-administered meningococcal vaccine on the same day as the second dose of GSK’s IIV4. Following Dose 1, all participants in the safety set from Belgium and Spain returned their completed ADR card. In Germany, 95.7% of participants returned their ADR card with documented AEs. After Dose 2, all participants in the safety set from Germany and Spain returned their completed ADR card.
3.2 AEs
Overall, 664 participants (49.8%) reported at least one AE within 7 days of Dose 1 of GSK’s IIV4 (40.0% in Belgium, 53.2% in Germany, and 47.4% in Spain). The most common AEs (cumulative frequency >5%) following Dose 1 by MedDRA® PT were injection-site pain (37.6% overall), fatigue (15.0%), headache (13.2%), injection-site swelling (9.3%), myalgia (7.6%), and injection-site erythema (7.4%; Table 2). Following Dose 2, the cumulative percentage of participants reporting any AEs within 7 days was 18.6%. The most frequently reported AE, with a cumulative frequency exceeding 5%, was pyrexia (6.9%; Table 3).
Among participants who received two doses of the vaccine, 37.5% (n = 12) in Spain and 18.1% (n = 2) in Germany reported at least one AE over the study period. In Spain, the most frequently reported AEs (occurring in more than one participant) according to MedDRA® PTs included irritability (15.6%); eye allergy, decreased appetite, pyrexia, rhinorrhea, hyperhidrosis, and rash (9.3% each); and diarrhea, vomiting, fatigue, upper respiratory tract infection, and somnolence (6.2% each). Notably, in Germany, none of the AEs was observed in more than one participant each who had received two doses of the vaccine.
Overall, following Dose 1, 28.8%, 16.7%, and 3.6% of participants experienced mild, moderate, and severe AEs, respectively. Following Dose 2, 9.0% of participants in Germany experienced moderate AEs, while 18.7% and 3.1% of participants in Spain experienced mild or moderate AEs, respectively. After receiving any vaccine dose, the cumulative percentage of participants in Spain who experienced mild, moderate, or severe AEs was 30.6%, 16.1%, and 1.4%, respectively. In Germany, the percentage of participants who experienced mild, moderate, or severe AEs was 28.4%, 17.9%, and 5.6%, respectively. Importantly, no deaths or serious AEs related to GSK’s IIV4 were reported within 7 days of vaccination in this study, and the reported AEs did not result in discontinuation of the study by any of the participants.
3.3 AEs by Age Strata and Risk Status
After Dose 1, participants aged 18–65 years experienced the highest rate of AEs (59.7%), followed by those aged 3–17 years (47.0%), >65 years (35.7%), and 6–35 months (23.5%). Among those at risk for influenza-related complications, 45.8% experienced an AE after Dose 1 and 28.5% experienced an AE after Dose 2, compared with 53.1% and 9.0%, respectively, of those not at risk. Among participants at risk, the most common AEs after Dose 1 were in the general disorders and administration-site conditions MedDRA® SOC (40.1%), followed by nervous system disorders (15.6%), musculoskeletal and connective tissue disorders (9.2%), gastrointestinal disorders (7.4%), and skin and subcutaneous tissue disorders (6.9%). After Dose 2, 19.0% of at-risk participants reported AEs in the general disorders and administration-site conditions MedDRA® SOC.
3.4 Sensitivity Analysis Using the Solicited Safety Set
In the solicited safety set, 50.0% of participants reported at least one AE after Dose 1 and 18.6% reported at least one AE after Dose 2. A sensitivity analysis demonstrated consistent AE frequencies between the safety set and solicited safety set.
3.5 COVID-19 and Its Impact on the Study
Before study enrollment (prior to 1 April, 2022), there were 135 confirmed cases of COVID-19, accounting for 10.1% of cases reported (five in Belgium, 42 in Germany, and 88 in Spain). After receiving GSK’s IIV4, there were 11 confirmed cases of COVID-19, representing 0.8% of cases reported (eight in Germany and three in Spain). Among participants with a history of COVID-19 vaccination, 48.7% (181/372) reported AEs following the first dose of GSK’s IIV4, while none reported AEs following the second dose.
4 Discussion
Findings from this ESS study of GSK’s IIV4 conducted across Belgium, Germany, and Spain during the 2022/2023 influenza season across all eligible age groups indicated for GSK’s IIV4 vaccination provide a comprehensive view of AEs encountered. Overall, 97.8% of ADR cards were completed and returned, with minimal variations among the three participating countries, demonstrating high-quality AE reporting. The type and frequency of the AEs (both AEIs and other AEs) reported in the study were consistent with those documented in the Summary of Product Characteristics for GSK’s IIV4 [19]. It is important to highlight that, during the entire study period, no serious AEs associated with GSK’s IIV4 were reported. This finding not only confirms the vaccine’s overall favorable safety but also emphasizes its appropriateness for widespread use. This provides reassurance that the vaccine’s performance aligns with established safety profiles, offering valuable insights to healthcare practitioners, regulators, and the broader medical community when considering its broader application. In a wider context, the absence of serious AEs and the alignment of reported AEs with expected rates are reassuring indicators of the vaccine’s safety across diverse population groups. These findings enhance confidence in the vaccine’s suitability for a wider range of individuals. In this study, the cumulative incidence rate of AEs was 49.8% after Dose 1. Among children who received GSK’s IIV4, 18.6% reported at least one AE after Dose 2. Our findings are consistent with those reported in a GSK-sponsored study conducted during the 2021/2022 influenza season, where 45.1% and 26.3% of participants reported AEs after Doses 1 and 2, respectively [20]. In both the earlier ESS study for the 2021/2022 season and the current ESS study, the most commonly reported AEs were from the MedDRA® SOC “general disorders and administration-site conditions. Overall, the findings of the present study are consistent with previous GSK studies [13,14,15, 20,21,22].
The safety and reactogenicity profile of GSK’s IIV4 observed in this ESS study closely mirrored the safety outcomes reported in prior ESS studies that included the same age groups. For instance, in a study involving 3036 adults aged ≥18 years who received GSK’s IIV4 (three lots), the incidence of injection-site pain was 36.4%, while the incidence of injection-site redness and swelling was 2% each [23]. In the same study, the most commonly reported solicited general AEs during the initial 7 days after GSK’s IIV4 vaccination included fatigue (15.8%), headache (15.9%), and muscle aches (16.4%) [23], which are consistent with the findings of the current ESS study. In a GSK-sponsored clinical trial involving children aged 3–8 years, the safety profile was similar to that observed in this ESS study, with 47.7% of participants reporting injection-site pain, 0.7% reporting redness, and 1.8% reporting swelling after receiving GSK’s IIV4 vaccination (N = 2584) [24]. Similarly, in another clinical trial involving children aged 6–35 months, the most frequently reported local solicited AEs within 7 days after GSK’s IIV4 vaccination (N = 6006) were pain (22.9%), redness (16.6%), and swelling (11.3%) [21, 25].
No safety concerns were observed in the interim analyses, in weekly data reviews, or at the time of study completion. This study, conducted across three European countries, enrolled over 1300 participants who had received GSK’s IIV4 as per local routine medical practices. Thus, these results are relevant for individuals receiving vaccination in Europe based on the local prescribing information across all age groups. However, it is important to acknowledge that the selection of countries and centers within countries may affect the generalizability of the findings. Additionally, the limited representation of individuals from minority geographic backgrounds could impact the applicability to non-White populations. Yet approximately 45.5% of participants were part of the “at-risk” groups for severe influenza-associated morbidity and mortality, which strengthens the reliability of the estimations for both the at-risk and not-at-risk groups. Overall, the study population closely resembled the population receiving GSK’s IIV4 per the local prescribing information in Europe, with no significant systematic differences that could substantially reduce the generalizability of the results.
A strength of this study is the inclusion of all age groups eligible for GSK’s IIV4 vaccination. This comprehensive approach provided an overall understanding of the AEs experienced, including in the pediatric population. The study rapidly exceeded its initial target enrollment, reaching a total of 1332 participants, well beyond the intended sample size of 1000. Systematic collection of medical history pertaining to COVID-19 vaccination, infections, and associated symptoms contributed to an empirical understanding of their potential impact on the safety profile of the GSK’s IIV4. Despite the limited number of reported COVID-19 cases during the follow-up, the findings were largely aligned with prior observations in the pandemic. Notably, the ADR card completion and return rates were 97.8% overall, with minimal participant attrition, which remained below 10.0%. These data are in line with response rates reported in conventional active studies [26] as well as previous ESS studies that employed paper cards for assessing seasonal influenza vaccines [22]. The utilization of standardized data collection tools such as the ADR card and an electronic data capture system facilitated efficient information extraction, cleaning, and analysis, supporting near real-time assessment. Timely encoding of electronic data by the medical team allowed ongoing evaluation, including weekly AE reviews, and prompt clarification requests from centers when necessary.
Limitations of the study include that it was not powered to detect rare AEs. However, routine pharmacovigilance activities continue to cover the detection of rare or very rare events, as the EMA guidelines did not specifically emphasize this aspect. The length of the enrollment period was constrained by the timing of vaccination campaigns across participating countries and prevented active enrollment before mid-October, particularly for the site in Belgium (where data collection commenced from ISO week 42). Although it was possible to record instances of vaccine co-administration on the same day as GSK’s IIV4, the relatively low numbers reported (n = 234, 17.6% for Dose 1 and n = 1, 2.3% for Dose 2) posed challenges for conducting a comprehensive trend analysis or assessing the impact on AE frequency. Despite these limitations, this ESS study contributes to continuous vaccine safety monitoring and complements routine pharmacovigilance efforts.
5 Conclusions
No serious AEs considered by the investigator to be associated with the vaccine were reported and no patterns of safety significance were noticed based on age group or “at-risk” status for severe influenza. Although not powered to detect rare AEs, no safety concerns were identified that alter the risk–benefit assessment of GSK’s IIV4. This study supports and confirms the acceptable safety profile of GSK’s IIV4 across all recommended age groups.
References
World Health Organization (WHO). Influenza (seasonal). Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal). [Accessed 26 Sep 2023].
Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, et al. Influenza. Nat Rev Dis Primers. 2018;4(1):3. https://doi.org/10.1038/s41572-018-0002-y.
European Centre for Disease Prevention and Control (ECDC). Factsheet about seasonal influenza. Available from: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet. [Accessed 26 Sep 2023].
Paget J, Iuliano AD, Taylor RJ, Simonsen L, Viboud C, Spreeuwenberg P, et al. Estimates of mortality associated with seasonal influenza for the European Union from the GLaMOR project. Vaccine. 2022;40(9):1361–9. https://doi.org/10.1016/j.vaccine.2021.11.080.
Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–300. https://doi.org/10.1016/S0140-6736(17)33293-2.
Larrauri A, Trilar KP. Preparing for an influenza season 2021/22 with a likely co-circulation of influenza virus and SARS-CoV-2. Euro Surveill. 2021;26(41):2100975. https://doi.org/10.2807/1560-7917.ES.2021.26.41.2100975.
World Health Organization. WHO Regional Office for Europe recommendations on influenza vaccination for the 2021/2022 season during the ongoing COVID-19 pandemic. Available from: https://iris.who.int/bitstream/handle/10665/347890/WHO-EURO-2021-3920-43679-61434-eng.pdf?sequence=1. [Accessed 26 Sep 2023].
Nypaver C, Dehlinger C, Carter C. Influenza and influenza vaccine: a review. J Midwifery Womens Health. 2021;66(1):45–53. https://doi.org/10.1111/jmwh.13203.
Becker T, Elbahesh H, Reperant LA, Rimmelzwaan GF, Osterhaus ADME. Influenza vaccines: successes and continuing challenges. J Infect Dis. 2021;224(12 Suppl. 2):S405–19. https://doi.org/10.1093/infdis/jiab269.
World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2021–2022 northern hemisphere influenza season. Available from: https://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-northern-hemisphere-recommendation-2021-2022/202102_recommendation.pdf?sfvrsn=2af603d8_12&download=true. [Accessed 26 Sep 2023].
European Medicines Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC). Interim guidance on enhanced safety surveillance for seasonal influenza vaccines in the EU. 2014. EMA/PRAC/222346/2014. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/interim-guidance-enhanced-safety-surveillance-seasonal-influenza-vaccines-eu_en.pdf. [Accessed 26 Sep 2023].
European Medicines Agency (EMA). Guideline on influenza vaccines: non-clinical and clinical module. 2016. EMA/CHMP/VWP/457259/2014. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/influenza-vaccines-non-clinical-clinical-module_en.pdf. [Accessed 26 Sep 2023].
de Lusignan S, Correa A, Dos Santos G, Meyer N, Haguinet F, Webb R, et al. Enhanced safety surveillance of influenza vaccines in general practice, winter 2015–16: feasibility study. JMIR Public Health Surveill. 2019;5(4): e12016. https://doi.org/10.2196/12016.
de Lusignan S, Ferreira F, Damaso S, Byford R, Pathirannehelage S, Yeakey A, et al. Enhanced passive surveillance of influenza vaccination in England, 2016–2017: an observational study using an adverse events reporting card. Hum Vaccin Immunother. 2019;15(5):1048–59. https://doi.org/10.1080/21645515.2019.1565258.
de Lusignan S, Damaso S, Ferreira F, Byford R, McGee C, Pathirannehelage S, et al. Brand-specific enhanced safety surveillance of GSK’s Fluarix Tetra seasonal influenza vaccine in England: 2017/2018 season. Hum Vaccin Immunother. 2020;16(8):1762–71. https://doi.org/10.1080/21645515.2019.1705112.
Division of AIDS. National Institute of Allergy and Infectious Diseases. National Institutes of Health. US Department of Health and Human Services. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events. Corrected Version 2.1. 2017. https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf
World Health Organization (WHO). Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance, 20 March 2020. Available from: https://iris.who.int/bitstream/handle/10665/331506/WHO-2019-nCoV-SurveillanceGuidance-2020.6-eng.pdf?sequence=1&isAllowed=y. [Accessed 26 Sep 2023].
World Health Organization (WHO). WHO COVID-19 case definitions. Updated in Public health surveillance for COVID-19, 22 July 2022. Available from: https://iris.who.int/bitstream/handle/10665/360579/WHO-2019-nCoV-Surveillance-Case-Definition-2022.1-eng.pdf?sequence=1. [Accessed 26 Sep 2023].
GlaxoSmithKline Biologicals. Fluarix Tetra. Quadrivalent influenza vaccine (split virion, inactivated). 2021. Available from: https://gskpro.com/content/dam/global/hcpportal/en_SG/products/PDF/Fluarix-tetra/fluarix_tetra_pi_ipi10a_si_nh_approved_24jul19.pdf. [Accessed 26 Sep 2023].
Santos GD, Eckermann T, Martínez-Gómez X, Parra J, Nwoji U, Salamanca de la Cueva I. Enhanced safety surveillance of GSK’s quadrivalent seasonal influenza vaccine in Germany and Spain (2021/2022 season) using an electronic patient-reported outcome system for vaccine safety remote monitoring. Influenza Other Respir Viruses. 2023;17(3): e13098. https://doi.org/10.1111/irv.13098.
Salamanca de la Cueva I, Cinconze E, Eckermann T, Nwoji U, Godderis L, Lu E, et al. Safety profile of GSK’s inactivated quadrivalent seasonal influenza vaccine in Belgium, Germany and Spain: passive enhanced safety surveillance study for the 2019/2020 influenza season. Drug Saf. 2021;44(12):1375–90. https://doi.org/10.1007/s40264-021-01121-8.
Dos Santos G, Wang H, Jindal P, Rybo M, Roul H, Pallem S, et al. Brand-specific enhanced safety surveillance study of GSK’s quadrivalent seasonal influenza vaccine, conducted during the COVID-19 pandemic, in Belgium, Germany and Spain, for the 2020/21 season. Infect Dis Ther. 2022;11(1):463–83. https://doi.org/10.1007/s40121-021-00571-y.
Kieninger D, Sheldon E, Lin W-Y, Yu C-J, Bayas JM, Gabor JJ, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years. BMC Infect Dis. 2013;13:343. https://doi.org/10.1186/1471-2334-13-343.
Jain VK, Rivera L, Zaman K, Espos RA Jr, Sirivichayakul C, Quiambao BP, et al. Vaccine for prevention of mild and moderate-to severe influenza in children. N Engl J Med. 2013;369(26):2481–91. https://doi.org/10.1056/NEJMoa1215817.
Claeys C, Zaman K, Dbaibo G, Li P, Izu A, Kosalaraksa P, et al. Prevention of vaccine-matched and mismatched influenza in children aged 6–35 months: a multinational randomised trial across five influenza seasons. Lancet Child Adolesc Health. 2018;2(5):338–49. https://doi.org/10.1016/S2352-4642(18)30062-2.
Spila Alegiani S, Alfonsi V, Appelgren EC, Ferrara L, Gallo T, Alicino C, et al. Active surveillance for safety monitoring of seasonal influenza vaccines in Italy, 2015/2016 season. BMC Public Health. 2018;18(1):1401. https://doi.org/10.1186/s12889-018-6260-5.
Acknowledgements
The authors thank the participants and their families for their participation in the study, the investigators and medical staff who contributed to the study implementation, and the GSK operational teams for their logistical support throughout the study preparation and implementation. The authors also thank Dr. Pia Maier, Mireia Puig, and Mª Teresa Vilella for their crucial assistance in data acquisition and Dr. Rakesh Ojha, PhD, of GSK for manuscript writing and coordination support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
GlaxoSmithKline Biologicals S.A funded this study (GlaxoSmithKline [GSK] study identifier: 213829) and was involved in all stages of the study, including analysis of the data. GlaxoSmithKline Biologicals S.A also funded all costs associated with the development and publishing of the manuscript.
Conflicts of Interest/Competing Interests
Carlos Brotons, Ignacio Salamanca, Tamara Eckermann, and Falko Panzer declare that their institution received payment from GSK for the conduct of the study. Xavier Martínez‐Gómez declares having received payment or honoraria for lectures, presentations, meetings, or educational events from AstraZeneca and Pfizer. Andrew Hastie, Jean-Yves Pirçon, Jennifer E. Gerber, Vanja Nikic, and Hannah Alsdurf were employees of the GSK group of companies. Andrew Hastie, Jean-Yves Pirçon, and Vanja Nikic possesses stock options in GSK. Paul Talsma was an employee of the GSK group of companies at the time of the study. The authors declare no other financial and non‐financial relationships and activities.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The following ethics committees, institutional review boards, and regulatory authorities were consulted in line with country requirements: Comitté d’Etique hospitalo-facultaire Erasme - Université Libre de Bruxelles in Belgium (approval received: 15 September, 2022), Ethik-Kommission der Bayerischen Landesärztekammer in Germany (approval received: 12 September, 2022), Landesärztekammer Baden-Württemberg Körperschaft des Öffentlichen Rechts Ethik-Kommission in Germany (approval received: 2 November, 2022), Comité de Ética de la Investigación Biomédica de Andalucía in Spain (approval received: 4 July, 2022), and Generalitat de Catalunya in Spain (approval received: 25 September, 2022).
Consent for Publication
Not applicable.
Consent to Participate
Written informed consent or assent was obtained from all individual participants included in this study or their parent(s), guardian(s), or legally acceptable representative(s) prior to participation in the study.
Availability of Data and Material
Anonymized individual participant data and study documents can be requested for further research from https://www.gsk-studyregister.com/en/.
Code Availability
Not applicable.
Authors’ Contributions
All authors participated in the design, implementation, analysis, and interpretation of the study and the development of this manuscript. All authors had full access to the data and approved the final manuscript prior to submission.
Trademark statement
AlphaRix Tetra, Influsplit Tetra, and Fluarix Tetra are trademarks owned by or licensed to the GSK group of companies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
de la Cueva, I.S., Gerber, J.E., Hastie, A. et al. Enhanced Safety Surveillance of GSK’s Inactivated Quadrivalent Seasonal Influenza Vaccine in Belgium, Germany, and Spain During the 2022/2023 Influenza Season. Drug Saf (2024). https://doi.org/10.1007/s40264-024-01456-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s40264-024-01456-y