Abstract
Introduction
Angiotensin receptor blockers are widely used antihypertensive drugs in South Korea. In 2021, the Korea Ministry of Food and Drug Safety acknowledged the need for national compensation for a drug-induced liver injury (DILI) after azilsartan use. However, little is known regarding the association between angiotensin receptor blockers and DILI.
Objective
We conducted a retrospective cohort study in incident users of angiotensin receptor blockers from a common data model database (1 January, 2017–31 December, 2021) to compare the risk of DILI among specific angiotensin receptor blockers against valsartan.
Methods
Patients were assigned to treatment groups at cohort entry based on prescribed angiotensin receptor blockers. Drug-induced liver injury was operationally defined using the International DILI Expert Working Group criteria. Cox regression analyses were conducted to derive hazard ratios and the inverse probability of treatment weighting method was applied. All analyses were performed using R.
Results
In total, 229,881 angiotensin receptor blocker users from 20 university hospitals were included. Crude DILI incidence ranged from 15.6 to 82.8 per 1000 person-years in treatment groups, most were cholestatic and of mild severity. Overall, the risk of DILI was significantly lower in olmesartan users than in valsartan users (hazard ratio: 0.73 [95% confidence interval 0.55–0.96]). In monotherapy patients, the risk was significantly higher in azilsartan users than in valsartan users (hazard ratio: 6.55 [95% confidence interval 5.28–8.12]).
Conclusions
We found a significantly higher risk of suspected DILI in patients receiving azilsartan monotherapy compared with valsartan monotherapy. Our findings emphasize the utility of real-world evidence in advancing our understanding of adverse drug reactions in clinical practice.
Similar content being viewed by others
Angiotensin receptor blockers such as azilsartan can cause drug-induced liver injury. |
The cholestatic type of liver injury was most the common, and the majority of the cases were of mild severity. |
The risk of drug-induced liver injury was lower in olmesartan users than in valsartan users. |
1 Introduction
Angiotensin receptor blockers (ARBs) are first-line treatments for hypertension, and they are one of the most used antihypertensive drugs in South Korea [1,2,3]. They lower blood pressure by antagonizing the effect of angiotensin II on AT1 receptors, effectively blocking the downstream renin-angiotensin-aldosterone system [4, 5]. Common adverse drug reactions associated with ARBs include hyperkalemia, hypotension, and renal dysfunction [5].
In 2020, a request for national compensation for a azilsartan-induced liver injury case was submitted to the Korea Institute of Drug Safety and Risk Management (KIDS). The case was investigated according to the national regulations on the adverse drug reaction relief system [6], and the need for compensation was acknowledged in 2021. Azilsartan is the newest among ARBs, approved by the US Food and Drug Administration on 25 February, 2011, and by the Korea Ministry of Food and Drug Safety on 26 May, 2017 [7, 8]. Moreover, this drug has been approved by the European Medicines Agency [9], and Japanese Pharmaceuticals and Medical Devices Agency [10] and is used worldwide. As of 2022, azilsartan is the only drug in its class in South Korea with no warning against possible liver dysfunction on its product label [8]. The situation is similar in other countries, including the USA and several European countries [9, 11].
Drug-induced liver injuries (DILI) are rare, with an incidence of 2.4–13.9 per 100,000 globally [12]. It is a major concern in the pharmaceutical industry as it is the most frequent cause of post-market safety-related drug withdrawals yet rarely detected in randomized controlled trials [12, 13]. Some forms of DILI can be life threatening, which are often unpredictable and independent of the dose, route, or duration of exposure [12, 13]. Drug-induced liver injury mimics a wide spectrum of liver diseases, and their biological mechanisms are poorly understood [12, 13].
Little is known about ARB-induced liver injuries, and only a handful of individual case reports are available [14,15,16]. Motivated by the authority’s decision to compensate for the azilsartan-induced liver injury, we aimed to compare the risk of DILI among specific ARBs against valsartan by analyzing an electronic healthcare record-based common data model (CDM) database. The findings of this study can provide methodological insights into comparing the risk of DILI using big data and provide real-world evidence for enhancing patient safety.
2 Methods
2.1 Data Source
This study utilized the medical record observation and assessment of drug safety (MOA) CDM, a standardized and distributed data network that allows for a multicenter data analysis using de-identified electronic healthcare record data collected from university hospitals (MOA CDM data partners) in South Korea [17]. The MOA CDM is coordinated by KIDS, a national organization affiliated with the Korea Ministry of Food and Drug Safety, and contains medical records of over 37 million patients from 30 data partners as of 2023 [17]. Patients are registered in the database on the day of their first visit to a hospital. For this study, we specifically collaborated with 20 data partners of which 10 are in the capital city (Seoul) and 7 in the nearby province (Gyeonggi-do).
2.2 Study Design and Population
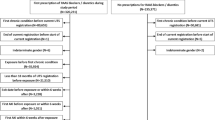
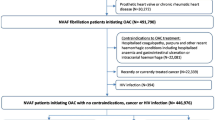
We performed a retrospective cohort study with incident ARB users who were at least 18 years of age and had initiated ARB treatment between 1 January, 2018 and 30 June, 2021. The first prescription date of the ARB was defined as the index date. Patients were censored when they experienced the study outcome, switched to another ARB, or at 6 months from the treatment initiation, as the azilsartan-induced liver injury case submitted to KIDS occurred approximately 6 months after the first use of the drug [18].
We excluded patients who were younger than 18 years of age or were prescribed multiple ARBs at the index date. Additionally, patients were excluded if they were prescribed any ARBs 3 months before the index date, had a suspected DILI (study outcome) or clinically significant conditions that may interfere with the interpretation of the study results 1 year before the index date, or had a serious hepatobiliary condition or were pregnant, which is a contraindication for ARB use 1 year before the index date or during the study period (study figure available in the Electronic Supplementary Material [ESM]). The decision to exclude pregnant patients was based on the significant contraindication of ARBs during pregnancy, as discontinuation of ARBs is a common practice when planning pregnancy. The exclusion was applied to mitigate a potential serious mis-estimation of follow-up time in these cases. To further validate this approach, we examined the number of pregnant patients during the entire data period, which was 0.028% among ARB users and would not have a significant impact on the overall study results.
2.3 Exposure Variable (ARBs)
Patients were assigned to treatment groups according to the ARB prescribed at the index date. Nine ARBs were marketed in South Korea during the study period: azilsartan, eprosartan, telmisartan, fimasartan, valsartan, olmesartan, losartan, irbesartan, and candesartan.
2.4 Outcome Variable (Suspected DILI)
We operationally defined DILI by adapting the clinical chemistry criteria provided by the International DILI Expert Working Group: alanine aminotransferase (ALT) ≥ 5× upper limit of normal (ULN), alkaline phosphatase (ALP) ≥ 2× ULN, or ALT ≥3× ULN and total bilirubin (TBL) > 2× ULN. After investigating the ULN standards of the data partners, the following values were selected: ALT, 40 U/L; ALP, 117 U/L; and TBL 1.2 mg/dL. Aspartate aminotransferase levels were not assessed as they may not specifically indicate liver injury [19].
The type of DILI was classified using the R ratio ([ALT/ALT ULN]/[ALP/ALP ULN]) as follows: hepatocellular (R ratio ≥ 5), mixed (R ratio 2–5), or cholestatic (R ratio ≤ 2) [19]. The severity of DILI was classified as follows: mild (ALT ≥ 5× ULN or ALP ≥2× ULN and TBL < 2× ULN), moderate-severe: (ALT ≥ 5× ULN or ALP ≥2× ULN and TBL ≥ 2× ULN), and fatal: any all-cause death within 1 year after the incident DILI [19]. The operational definitions for DILI were further reviewed by clinical experts and researchers with expertise in liver injury. As any patient identification information is pseudonymized in the CDM database, medical charts reviews were not feasible. Therefore, we inform that the cases detected in our study are all suspected cases for which no additional validation was conducted.
2.5 Alternative Causes of Liver Injury
As the diagnosis of DILI mostly depends on the exclusion of alternative causes of liver injury, we listed clinical conditions that could be potential alternative causes of liver injury based on the previous literature [13]. With a review by clinical experts, the conditions were categorized as follows: to be excluded at baseline and adjusted for during the follow-up (clinically significant conditions), to be completely excluded at baseline and the follow-up (serious hepatobiliary conditions), and others to be adjusted for at baseline and the follow-up. In addition, we adjusted for hepatotoxic drugs by class at baseline and the follow-up, which were defined as drugs with a LiverTox DILI-likelihood score of A (well known) or B (known or highly likely) [ESM].
2.6 Covariates
Patient demographics (sex, age, and enrollment year), encounter records (hospitalizations, days outpatient visits, and emergency room visits), Charlson Comorbidity Index, comorbidities, and prescription histories were included as baseline covariates. Additionally, predetermined potential alternative causes of liver injury and anti-hypertensive drugs class prescribed during the follow-up were also included as covariates.
2.7 Statistical Analysis
Descriptive analyses were conducted to summarize the patient characteristics, treatment patterns, and characteristics of DILI. The number of patients by status over time was collected from each data partner to calculate pooled incidence rates. Cox proportional hazards models were used to derive hazard ratios (HRs) of DILI and to compare the risk among specific ARBs against valsartan. Subgroup analyses were conducted by treatment patterns and data partners to compare study populations. Valsartan was selected as the reference drug as it is the most used ARB in Korea [21]. To minimize selection bias, we applied propensity score-based inverse probability of treatment weighting (IPTW) and derived the average treatment effect [22, 23]. Additionally, covariates collected during the follow-up were included in the regression model to reduce confounding. From each data partner, coefficients, standard errors, and confidence intervals (CIs) from Cox proportional hazards models were collected to conduct the meta-analysis. We evaluated the average treatment effect by a random-effect model in the meta-analysis to consider population variance of each data source.
All statistical analyses were performed using R [24] and the level of statistical significance was set at p < 0.05 (two-sided). R packages ‘DatabaseConnector,’ ‘SqlRender,’ ‘plyr,’ ‘dplyr,’ ‘DBI,’ ‘odbc,’ ‘lubridate,’ and ‘nnet’ for database connection and preprocessing, ‘WeightIt’ and ‘cobalt’ for IPTW, ‘survival’ and ‘survminer’ for the survival analysis, and ‘meta’ for meta-analyses were used [25,26,27,28,29,30,31,32,33,34,35,36,37].
3 Results
3.1 Characteristics of the Study Population
A total of 229,881 ARB users were included in this study. Of these, valsartan (21.9%) was most used, followed by telmisartan (17.9%), olmesartan (14.6%), and losartan (14.6%); azilsartan (1.1%) and eprosartan (0.4%) were least common (Fig. 1). The mean age of the study population was 64.8 years, with a range from 61.3 years (azilsartan) to 68.7 years (eprosartan). There were slightly more men than women (53.2%). More than half of the patients had a history of hospitalization (59.2%) and fewer than five outpatient visits (53.7%), whereas the majority had no history of emergency room visits (89.4%). The mean Charlson Comorbidity Index of the total population was 1.5, which was within the range of mild severity [38]. Overall, diabetes without chronic complications (16.9%) was the most common comorbidity, followed by diabetes with chronic complications (15.7%), malignant tumors (14.4%), and cerebrovascular diseases (11.2%). In the treatment groups, there were significant differences in the prevalence of comorbidities likely reflecting indications approved for each ARB (p < 0.001). Overall, the patients took a mean of 17 prescribed medications (Table 1).
After IPTW, standardized mean differences between treatment groups were well within the recommended ranges, indicating that the baseline characteristics were well balanced, except in the eprosartan group likely owing to the small sample size, which was less than 1% of the study population (Table 1). Across data partners, the median follow-up duration varied from 61.5 days to 96 days (details on the study population comparison by data partners available in the ESM).
3.2 Anti-Hypertensive Treatment Patterns During the Follow-Up
In total, 37.1% had less than 1 defined daily dose (DDD), 35.5% had 1–2 DDDs, and 27.4% had more than 2 DDDs of ARBs. Notably, the proportion of high-dose prescriptions (>2 DDDs/day) was significantly higher in the azilsartan and candesartan groups, and the proportion of long-term prescriptions (≥60 days) was the highest in the azilsartan group. The overall proportion of monotherapy was 27.5%, and the proportion was significantly higher in the irbesartan and azilsartan groups than other groups. In total, 41,270 patients (18.0%) were lost to the follow-up owing to switching within the ARB class; the proportion was the highest in the eprosartan group (38.5%) [all p < 0.001] (Table 2).
3.3 Safety of ARBs Versus Valsartan
Overall, the crude incidence of DILI was 48.4 (per 1000 person-years) in ARB users. Most were cholestatic and of mild severity (Table 3). Notably, DILI frequency was the highest within the first 4 weeks except in the azilsartan group (ESM).
As a result of the IPTW Cox proportional hazards model analysis with additional covariate adjustment at the follow-up, the risk was significantly lower in olmesartan users than in valsartan users (HR: 0.73 [95% CI 0.55–0.96]). No significant differences were observed among the other treatment groups (Table 4).
By anti-hypertensive treatment patterns, in patients receiving azilsartan monotherapy, the risk was significantly higher than that in those who received valsartan monotherapy (HR: 6.55 [95% CI 5.28–8.12]). A significantly higher risk was also found in patients who received azilsartan and diuretics instead of valsartan and diuretics (HR: 1.63 [95% CI 1.27–2.09]). No significant dose-dependent trend was observed across the treatment groups (see Table 5).
4 Discussion
In this large-scale observational study of 229,881 ARB users from 20 university hospitals, the most common type of liver injury was cholestatic. Importantly, we found the risk of DILI was significantly higher in patients receiving azilsartan monotherapy compared with valsartan monotherapy. Our findings add new value to current anti-hypertensive therapies as post-market data on ARB-induced liver injury are scarce. Although ARBs are generally considered safe with a low risk of liver injury, our findings can help understand ARB-induced liver injury.
We found the comparative risk of DILI was significantly higher in patients receiving azilsartan monotherapy compared with valsartan monotherapy, which may be associated with the higher proportion of long-term and high-dose prescriptions found in this group. Azilsartan has been known to have greater antihypertensive effects than other ARB, owing to its unique binding behavior to the AT1 receptor with its 5-oxo-1,2,4-oxadiazole moiety that induces stronger inverse agonism [39, 40]. The moiety also makes it more lipophilic than other ARBs and requires metabolism via cytochrome P450 2C9 [7, 41]. Despite its hepatic metabolism, no notable hepatic adverse events have been detected in randomized clinical trials and the safety profiles obtained were similar to those of other ARBs in this class [41, 42]. It is possible that the low incidence of DILI made their detection difficult in the trials.
We also found that the comparative risk of DILI was significantly lower in olmesartan users than in valsartan users. Previously, olmesartan prevented hepatic steatosis and fibrosis in diabetic mice via inhibition of apoptosis signaling. In another pre-clinical study, the administration of olmesartan significantly improved liver function and decreased hepatic oxidative stress and inflammatory cytokines [43]. Because of the current lack of real-world evidence in patients, further interpretation of the findings is limited.
As a result of the assessment of characteristics of suspected DILI by ARBs, most were cholestatic and of mild severity. Importantly, we found no differences in the type or severity of DILI by ARBs. Cholestatic liver injuries are more common in older adults and require longer days of recovery than other types. Our findings highlight close monitoring after ARB use may help prevent unnecessary progression to chronic liver disease [39]. As a result of the subgroup analysis based on the prescribed dose, no significant dose-dependent trend was observed across the treatment groups, suggesting ARB-induced liver injuries are most likely idiosyncratic, unlike DILI secondary to drug overdose [19]. The pathophysiology of idiosyncratic DILI is yet poorly understood, unexpected given the drug’s pharmacological action, and is largely dependent on patient-specific factors that increase their susceptibility to a liver injury [12, 19]. We also assessed the temporal pattern of DILI occurrence and found that the highest number of DILI occurred within the first 4 weeks except in the azilsartan group, suggesting the pathophysiology of liver injury from azilsartan may differ from that of other ARBs.
This study has some limitations. First, the results of our observational study may have been affected by residual confounding factors and biases. For example, information on over-the-counter drugs, traditional medicine, and alcohol use that was unavailable in the hospital database could have affected our study results. To minimize such risks, we applied IPTW, conducted balance diagnostics, and adjusted for patient characteristics during the follow-up. Second, our study objective was to compare the risk of DILI among specific ARBs against valsartan, which served as the control ARB in our analyses; therefore, the results provided are only a relative comparison. We acknowledge the inclusion of negative controls could have enhanced the interpretability of the study results, and their inclusion can be considered in future investigations. Third, because no further adjudication was conducted for the detected DILI, our statistics were based on the number of suspected DILI. The specificity of the detected DILI could have been improved by further adjudication using medical chart reviews. Furthermore, given the rarity of the outcome, a formal phenotyping to reflect local setting would have added value. To overcome such limitations, we have applied stringent criteria to detect DILI provided by the international DILI Expert Working Group for which superior performance was previously demonstrated when compared with other algorithms like the Council for International Organization of Medical Sciences and the Drug-Induced Liver Injury Network [20]. In addition, because of the inability to share a patient identifier across data partners, the same patient visiting more than two hospitals may result in double counting. Finally, the results should be interpreted with caution owing to the limited sample size, especially for eprosartan and azilsartan users, and the fact that our study population included patients who visited tertiary hospitals, which could limit generalizability. Despite the limitations of this study, we have successfully assessed the characteristics of suspected DILI in ARB users and derived the relative risks among ARBs in a real-world clinical setting in Korea.
5 Conclusions
We found a significantly higher risk of suspected DILI in patients receiving azilsartan monotherapy compared with valsartan monotherapy. Our findings underscore the valuable role of real-world evidence in regulatory decision making.
References
Korean Society Hypertension (KSH), Hypertension Epidemiology Research Working Group, Kim HC, Cho MC, et al. Korea hypertension fact sheet 2018. Clin Hypertens. 2018;24:13. https://doi.org/10.1186/s40885-018-0098-0.
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–324. https://doi.org/10.1161/HYP.0000000000000066.
Williams B, Mancia G. Ten commandments of the 2018 ESC/ESH HTN guidelines on hypertension in adults. Eur Heart J. 2018;39:3007–8. https://doi.org/10.1093/eurheartj/ehy439.
Brouwers S, Sudano I, Kokubo Y, Sulaica EM. Arterial hypertension. Lancet. 2021;398:249–61. https://doi.org/10.1016/S0140-6736(21)00221-X.
Terra SG. Angiotensin receptor blockers. Circulation. 2003;107:e215–6. https://doi.org/10.1161/01.CIR.0000072344.12827.13.
Korea Institute of Drug Safety & Risk Management. Introduction of ADR relief system. Available from: https://www.drugsafe.or.kr/iwt/ds/en/introduction/EgovPropelSummary.do. Accessed 2 Aug 2022.
Pradhan A, Tiwari A, Sethi R. Azilsartan: current evidence and perspectives in management of hypertension. Int J Hypertens. 2019;2019:1824621. https://doi.org/10.1155/2019/1824621.
Korea Ministry of Food and Drug Safety. Drug safety database. Available from: https://nedrug.mfds.go.kr/index. Accessed 8 Jul 2022.
European Medicines Agency. Assessment report: Edarbi (azilsartan medoxomil). 2011. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/edarbi. Accessed 12 Oct 2023.
Japan Pharmaceuticals and Medical Devices Agency (PMDA). Products approved in FY 2011. 2011. Available from: https://www.pmda.go.jp/files/000229075.pdf. Accessed 13 Oct 2023.
US FDA. Edarbi: azilsartan kamedoxomil tablet (label). 2023. Available from: https://www.accessdata.fda.gov/spl/data/2f63b0e8-977a-4d22-9550-29ad1926a9de/2f63b0e8-977a-4d22-9550-29ad1926a9de.xml. Accessed 12 Oct 2023.
Group CW. Drug-induced liver injury (DILI): current status and future directions for drug development and the post-marketing setting. 2020. Available from: https://cioms.ch/wp-content/uploads/2020/06/CIOMS_DILI_Web_16Jun2020.pdf. Accessed 12 Oct 2023.
US FDA. Guidance for industry drug-induced liver injury: premarketing clinical evaluation. 2009. Available from: https://www.fda.gov/media/116737/download. Accessed 12 Oct 2023.
Lorena M, Autolitano A, Natale G, Uberti F, Vitali F, Schiantarelli C, et al. Telmisartan/hydrochlorothiazide-induced hepatotoxicity. Arch Med Sci. 2015;11(4):893–4. https://doi.org/10.5114/aoms.2015.53311.
Al-Halawani MZ, Thawabi M, Asslo F, Shaaban H, Shamoon F, Baddoura WJ. Losartan-induced ischemic hepatocellular hepatotoxicity: a case report and literature review. J Family Med Prim Care. 2014;3(3):272. https://doi.org/10.4103/2249-4863.141635.
Park DH, Yun GY, Eun HS, Joo JS, Kim JS, Kang SH, et al. Fimasartan-induced liver injury in a patient with no adverse reactions on other types of angiotensin II receptor blockers: a case report. Medicine. 2017;96(47): e8905. https://doi.org/10.1097/MD.0000000000008905.
Korea Institute of Drug Safety & Risk Management. MOA project: medical record observation and assessment for drug safety. Available from: https://moa.drugsafe.or.kr/cs/biz/background. Accessed 2 Aug 2022.
Korea Institute of Drug Safety & Risk Management. Status of applications and deliberations for adverse drug reaction relief, in the first half of 2021. 2021. Available from: https://www.drugsafe.or.kr/iwt/ds/ko/bbs/EgovBbs.do?bbsId=BBSMSTR_000000000343&nttId=4460&pageIndex=2&searchCnd=0&searchWr. Accessed 2 Aug 2022.
Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–15. https://doi.org/10.1038/clpt.2011.58.
Tan EH, Ling ZJ, Ang PS, et al. Comparison of laboratory threshold criteria in drug-induced liver injury detection algorithms for use in pharmacovigilance. Pharmacoepidemiol Drug Saf. 2020;29(11):1480–8. https://doi.org/10.1002/pds.5099.
Lee SH CE, Jung MJ, Yoo HJ, MFDS. Drug utilization pattern of high frequency and long-term use medication (antihypertensive drugs). Available from: https://scienceon.kisti.re.kr/srch/selectPORSrchReport.do?cn=TRKO202000029997. Accessed 12 Oct 2023.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107. https://doi.org/10.1002/sim.3697.
Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001. https://doi.org/10.1023/A:1020363010465.
R Core Team R. R: a language and environment for statistical computing. 2013. Available from: https://www.R-project.org/. Accessed 30 Dec 2022.
Martijn Schuemie MS. DatabaseConnector: connecting to various database platforms. R package version 5.0.2. 2022. Available from: https://CRAN.R-project.org/package=DatabaseConnector. Accessed 30 Dec 2022.
Martijn Schuemie MS. SqlRender: rendering parameterized SQL and translation to dialects. R package version 1.9.0. 2022. Available from: https://CRAN.R-project.org/package=SqlRender. Accessed 30 Dec 2022.
Wickham H. The split-apply-combine strategy for data analysis. J Stat Soft. 2011. https://doi.org/10.18637/jss.v040.i01.
Hadley Wickham RF, Lionel Henry, Kirill Muller. Dplyr: a grammar of data manipulation. R package version 1.0.8. 2022. Available from: https://CRAN.R-project.org/package=dplyr. Accessed 30 Dec 2022.
Hadley Wickham KM, R-SIG-DB. DBI: R database interface. R package version 1.1.2. 2022. Available from: https://CRAN.R-project.org/package=DBI. Accessed 30 Dec 2022.
Jim Hester HW. ODBC: connect to ODBC compatible databases (using the DBI Interface). R package version 1.3.3. 2021. Available from: https://CRAN.R-project.org/package=odbc. Accessed 30 Dec 2022.
Garrett Grolemund HW. Dates and times made easy with lubridate. 2011. Available from: https://www.jstatsoft.org/v40/i03/. Accessed 30 Dec 2022.
Venables WN, Ripley BD. Modern applied statistics with S. 4th ed. New York: Springer; 2002.
Greifer N. WeightIt: weighting for covariate balance in observational studies. R package version 0.13.1. 2022. Available from: https://CRAN.R-project.org/package=WeightIt. Accessed 30 Dec 2022.
Therneau TM. A package for survival analysis in R. R package version 3.3-1. 2022. Available from: https://CRAN.R-project.org/package=survival. Accessed 30 Dec 2022.
Alboukadel Kassambara MK, Przemyslaw Biecek. Survminer: drawing survival curves using ‘ggplot2’. R package version 0.4.9. 2021. Available from: https://CRAN.R-project.org/package=survminer. Accessed 30 Dec 2022.
Greifer N. Cobalt: covariate balance tables and plots. R package version 4.3.2. Available from: https://CRAN.R-project.org/package=cobalt. Accessed 2 Aug 2022.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60. https://doi.org/10.1136/ebmental-2019-300117.
Huang YQ, Gou R, Diao YS, Yin QH, Fan WX, Liang YP, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15:58–66. https://doi.org/10.1631/jzus.B1300109.
Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21. https://doi.org/10.1016/j.gastro.2005.05.006.
Miura S-i, Okabe A, Matsuo Y, Karnik SS, Saku K. Unique binding behavior of the recently approved angiotensin II receptor blocker azilsartan compared with that of candesartan. Hypertens Res. 2013;36(2):134–9. https://doi.org/10.1038/hr.2012.147.
Jones JD, Jackson SH, Agboton C, Martin TS. Azilsartan medoxomil (Edarbi): the eighth angiotensin II receptor blocker. P T. 2011;36:634–40.
US FDA. Center For Drug Evaluation and Research. Application number: 200796Orig1s000 Summary review. 2011. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/200796orig1s000sumr.pdf. Accessed 2 Aug 2022.
Abo El-Nasr NME, Saleh DO, Mahmoud SS, Nofal SM, Abdelsalam RM, Safar MM, et al. Olmesartan attenuates type 2 diabetes-associated liver injury: cross-talk of AGE/RAGE/JNK, STAT3/SCOS3 and RAS signaling pathways. Eur J Pharmacol. 2020;5:874. https://doi.org/10.1016/j.ejphar.2020.173010.
Acknowledgments
We extend our sincere gratitude to the MOA CDM data partners for their invaluable cooperation and collaboration in facilitating this study.
Funding
Open Access funding enabled and organized by Seoul National University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflicts of Interest
The authors have no actual or potential conflicts of interest or have any financial relationships to disclose.
Availability of Data and Material
The MOA CDM database is available from KIDS and MOA CDM data partners on reasonable request.
Code Availability
The data analysis code is available from KIDS on reasonable request.
Ethics Approval
Ethical approval was waived by the Institutional Review Board (IRB) of KIDS in view of the retrospective nature of the study (IRB number 2022-5).
Consent to Participate
Informed consent to participate was waived by the IRB of KIDS in view of the retrospective nature of the study.
Consent for Publication
Informed consent for publication was waived by the IRB of KIDS in view of the retrospective nature of the study.
Authors’ Contributions
HK, NS, DJ, and BK contributed to the study conception and design. Data pre-processing and analysis were performed by NS. The manuscript was drafted by HK and NS and reviewed by EEL and BK. MY contributed to the supervision. IYC, WC, YWC, SWK, SB, SI, DWS, JS, M-GK, JKL, Y-GS, H-JA, YK, SC, DWJ, D-JC, SGK, SY, H-JY, IL, HJP, and J-HL contributed to the construction of the CDM database, implementation of the analysis, and manuscript review. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kim, H., Son, N., Jeong, D. et al. Angiotensin Receptor Blockers and the Risk of Suspected Drug-Induced Liver Injury: A Retrospective Cohort Study Using Electronic Health Record-Based Common Data Model in South Korea. Drug Saf (2024). https://doi.org/10.1007/s40264-024-01418-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s40264-024-01418-4