Abstract
Introduction
Drug–drug interactions (DDIs) have potential to cause patient harm, including lowering therapeutic efficacy. This study aimed to (i) determine the prevalence of potential DDIs (pDDIs); clinically relevant DDIs (cDDIs), that is, DDIs that could lead to patient harm, taking into account a patient’s individual clinical profile, drug effects and severity of potential harmful outcome; and subsequent actual harm among hospitalized patients and (ii) examine the impact of transitioning from paper-based medication charts to electronic medication management (eMM) on DDIs and patient harms.
Methods
This was a secondary analysis of the control arm of a controlled pre-post study. Patients were randomly selected from three Australian hospitals. Retrospective chart review was conducted before and after the implementation of an eMM system, without accompanying clinical decision support alerts for DDIs. Harm was assessed by an expert panel.
Results
Of 1186 patient admissions, 70.1% (n = 831) experienced a pDDI, 42.6% (n = 505) a cDDI and 0.9% (n = 11) an actual harm in hospital. Of 15,860 pDDIs identified, 27.0% (n = 4285) were classified as cDDIs. The median number of pDDIs and cDDIs per 10 drugs were 6 [interquartile range (IQR) 2–13] and 0 (IQR 0–2), respectively. In cases where a cDDI was identified, both drugs were 44% less likely to be co-administered following eMM (adjusted odds ratio 0.56, 95% confidence interval 0.46–0.73).
Conclusion
Although most patients experienced a pDDI during their hospital stay, less than one-third of pDDIs were clinically relevant. The low prevalence of harm identified raises questions about the value of incorporating DDI decision support into systems given the potential negative impacts of DDI alerts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Prevalence of DDIs and harm in hospital: 70% of patients experienced a potential DDI, 40% a clinically relevant DDI and < 1% an actual harm. |
In total, < 30% of potential DDIs were clinically relevant. |
1 Introduction
Drug–drug interactions (DDIs) have the potential to cause patient harm, including lowering therapeutic efficacy [1,2,3], and are associated with greater length of hospital stay and healthcare costs [4]. A US national study of more than 2 billion patient visits revealed that nearly two-thirds of patients were on multiple medications, and 23% were prescribed high-risk medications [5]. Risk of potential DDIs increases with the number of medications prescribed, polypharmacy, older age, renal impairment, declining hepatic function and the presence of comorbid disease [4,5,6,7,8]. As a result of the ageing population and rising rates of drug prescriptions, the prevalence of DDIs is increasing [6, 9].
Polypharmacy creates many opportunities for DDIs. A 2018 systematic review and meta-analysis estimated that 33% of general inpatients and 67% of intensive care unit (ICU) patients experience at least one DDI during their hospital stay [10]. However, the likelihood of harm associated with DDIs is dependent on a range of contextual factors related to the drug, patient and clinical setting. For example, in some cases, a DDI is unlikely to be clinically significant when a drug is given as a one-off dose, for example, the concurrent use of prednisone and once-only ibuprofen. Hence, it is valuable to identify clinically relevant DDIs (cDDIs), defined as DDIs that could lead to patient harm, taking into account a patient’s individual clinical profile, drug effects and severity of any potential harmful outcome. Nonetheless, only a limited number of studies have examined DDIs that are clinically relevant among hospitalised patients [10]. One previous study reported the prevalence of cDDIs [11], but clinical and pharmacological risk factors related to DDIs identified in this study were not specified or comprehensive. Only one study was identified to have examined actual harm to patients as a result of DDIs [12].
Electronic medication management (eMM) systems (or computerised provider order entry systems, CPOE), have been increasingly implemented in hospitals worldwide. These systems replace paper-based medication charts and allow computerised prescribing, review and administration of medications. eMM systems typically include clinical decision support (CDS) to assist clinicians in managing the risk of DDIs. Alerts identifying potential DDIs are triggered in high numbers at the point of prescribing, raising concerns around compliance to alert recommendations, alert fatigue and effectiveness [13]. DDI alerts have been the focus of much research [14, 15], however, there have been limited controlled studies examining the effectiveness of DDI alerts to reduce cDDIs and patient harm [13]. We undertook a large, controlled pre-post study across five hospitals to determine the effectiveness of DDI alerts [16], and in this current paper we report detailed results from the control arm of this study. In particular, our control hospitals transitioned from paper-based prescribing to eMM systems, but did not implement DDI alerts. This presented us with an opportunity to generate evidence on how the transition from paper to eMM without accompanying CDS impacts DDIs and patient harms.
This paper primarily aimed to report on the prevalence and severity of cDDIs and subsequent actual harm during admission among hospitalised patients and to identify contextual factors associated with cDDIs. As data collection occurred at pre- and post-eMM periods, we performed a secondary analysis with an aim to examine the impact of the transition from paper-based medication charts to eMM without accompanying CDS on DDIs and patient harm.
2 Methods
2.1 Study Design, Setting and Population
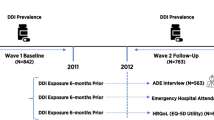
A multi-site before and after retrospective audit was undertaken at three public hospitals in New South Wales, Australia. Hospitals A and B were regional acute hospitals with 250 and 300 beds, respectively. Hospital C was a metropolitan principal referral hospital with 820 beds. Data were collected from study hospitals before and after the implementation of each hospital’s eMM system. The eMM allowed for electronic prescribing, review and administration of medications, but included no specific CDS for DDIs. Thus, the prescribing of two potentially interacting medications did not result in the triggering of a DDI alert for prescribers. The same eMM system was introduced across hospitals A and B (Cerner Millennium, https://www.cerner.com/solutions/health-systems), whilst hospital C implemented a different system (MedChart, https://www.medchart.com/).
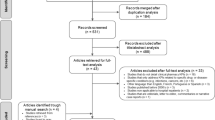
Study patients were randomly selected from all admitted patients during two time periods: pre- and post-eMM implementation (Fig. 1). Patients were excluded if (i) their hospital stay was longer than 3 months, (ii) they were in a ward/unit without eMM being implemented or with a different eMM system (e.g. chemotherapy units, intensive care units and some psychiatric wards) or (iii) they were in ambulatory care to receive regular intravenous infusions, for example, blood or blood product transfusions or dialysis. In addition, anaesthetic charts were excluded from chart review due to variations in documentation of anaesthetic medications across the study sites. Ethics approval was obtained from one of the hospitals’ Human Research Ethics Committee (reference no.: 8/02/21/4.07), and site-specific governance approval was subsequently obtained from all sites.
2.2 Drug–Drug Interaction (DDI) Classification—Potential and Clinically Relevant DDIs
All medication orders that were active on a patient’s medication chart on the same calendar date were screened for DDIs using Stockley’s Drug Interactions Checker [17], a standard international reference used in Australian hospital practice. All moderate or severe DDIs specified by Stockley’s were classified as a potential DDI (pDDI). This term was used to distinguish DDIs from cDDIs.
The clinical relevance of each pDDI, that is, the probability and potential for harm to each patient, was assessed by trained clinical pharmacists. The pharmacists classified the likelihood of clinical relevance as either very unlikely, unlikely, possible or likely. In this review process, they identified 26 contextual factors, including 11 drug factors, 11 patient factors, two settings factors and two other factors that determined whether a pDDI was clinically relevant. See Online Resource Table S1 for detailed definitions and examples of these contextual factors that were considered when determining clinical relevance of each pDDI. All pDDIs with possible or likely clinical relevance were categorised as cDDIs. A step-by-step workflow of the DDI assessment process is presented in Online Resource Fig. S1.
2.3 Data Collection
Clinical pharmacists who were trained in the procedures described in the study protocol reviewed medication orders and medical records [16]. Five clinical pharmacist reviewers undertook chart review across the three study hospitals. All five reviewers were experienced hospital pharmacists, but were independent from study hospitals. Reviewers documented patient demographics including date of birth, sex, admission and discharge date and diagnoses. For each patient reviewed during the pre-eMM period, details of every medication prescribed during the patient’s admission were recorded, including drug name, route, dose, frequency and start and end dates. For post-eMM patients, medication data were extracted from eMM systems.
Reviewers collected information on whether each drug in a DDI pair was administered. A drug was recorded as ‘not administered’ if the drug was not administered at all during the admission.
2.4 Inter-Rater Reliability Assessment
Inter-rater reliability testing between the five pharmacist reviewers was conducted before commencing independent data collection. This was to ensure that all reviewers were consistent in identifying contextual factors related to clinical significance and classifying the likelihood of clinical relevance of pDDIs. The reviewers were required to achieve a minimum Cohen’s Kappa score of 0.7 on contextual factor categorisation and 0.8 on clinical significance level, indicating moderate-to-strong level of agreement [18], before starting independent data collection.
2.5 Harm Assessment
Medication charts were reviewed by the study pharmacists to determine whether medications involved in a cDDI were administered to patients concomitantly. The clinically relevant time frame of two interacting drugs was assessed by pharmacist reviewers with respect to the pharmacokinetic and pharmacodynamic characteristics of each agent on the basis of published online databases (MIMS Australia [19] and Micromedex [20]). If both medications were administered within an estimated clinically relevant time frame, pharmacist reviewers proceeded to identify whether there was evidence that the possible harms associated with the cDDI, as described in Stockley’s, had manifested in the patient. An in-depth review of patient records was undertaken to identify signs of possible harm to the patient from the cDDI during that hospital admission. This included, for example, abnormal findings from clinical examinations or vital signs, pathology results, patient symptoms or interruption to a treatment plan (e.g. prescribing of an antidote). For those patients who were identified to have experienced potential harm, study pharmacist reviewers prepared case studies detailing all relevant information. These case studies were reviewed by two clinical pharmacologists (physicians) to determine whether actual harm had occurred from cDDIs. On the basis of published classifications of DDI severity and resulting potential harms [21, 22], the clinical pharmacologists independently reviewed the material and made two assessments: (i) plausibility that the cDDI caused the identified harm (unlikely, possible, probable or certain) and (ii) severity of harm experienced (no harm, minor, moderate, serious or severe). Definitions of each category are in Online Resource Tables S2 and S3. The independent assessments were compared. Panels, involving the clinical pharmacologists and study pharmacist reviewers, were convened to discuss all disagreements and reach consensus on plausibility and severity of patient harms in all cases.
2.6 Statistical Analysis
Descriptive summaries of patient demographic and clinical characteristics were produced for both pre- and post-eMM periods. Summary statistics on pDDIs and cDDIs were presented, including contextual factors identified when classifying pDDIs as either cDDIs or non-cDDIs. The distributions of pDDIs and cDDIs per admission by age, sex and eMM were displayed in clustered box plots. The same plots were created for the distributions of pDDIs and cDDIs per ten unique drugs prescribed during hospital stay, that is, per ten active ingredients per admission. Among the pDDIs identified from patient records, the primary outcome examined was occurrence of a cDDI. A cDDI was classified as leading to actual harm when its plausibility was rated as probable or certain, and severity as minor or above. Sensitivity analyses were conducted and presented using different levels of harm plausibility and severity.
To examine the effect of the introduction of eMMs without DDI decision support, three multilevel regression models were applied to three outcomes of interest (i.e. dependent variable of each model): (1) the occurrence of a cDDI, (2) the administration of both medicines identified as contributing to cDDIs and (3) the occurrence of actual harm. Mixed-effect multivariable logistic regression models were developed with consideration of the correlation of multiple DDIs for the same patients. Study period (pre- versus post-eMM) was an explanatory variable of interest. Other explanatory variables included study hospital, patient’s age group, number of unique drugs prescribed during the admission and number of medication orders and relevant contextual factors identified by the pharmacist reviewers. These variables in the multivariable models were selected on the basis of prior knowledge to control for potential confounding. Backwards elimination and forwards addition steps were used to arrive at final models. Total number of medication orders was excluded from final models due to collinearity with number of unique drugs prescribed. Number of unique drugs prescribed was found to have a linear association with the outcome and thus kept as a continuous variable. Correlation and variance inflation factors were examined, and no other collinearity was detected in final models. All statistical analyses were performed using SAS 9.4.
3 Results
A total of 1186 patient admissions from 1170 patients were included in the study (Table 1). Overall, 55.9% of patients were female. Most (79.7%, n = 933) were adults (aged 18 years and older). The median number of medication orders (prescriptions) was 9 orders per admission [interquartile range (IQR) 3–18], which is slightly higher than the number of unique drugs prescribed (median 7, IQR 3–13). A total of 1030 admissions (86.8%) had at least two drugs prescribed during their hospital stay. The distributions of site, age, sex, type of admission and medication orders were similar across pre- and post-eMM periods.
3.1 Prevalence of pDDIs and cDDIs
A total of 15,860 pDDIs were identified. At least one pDDI was identified in 70.1% of admissions (n = 831), and the median number of pDDIs per admission was 4 (IQR 0–13; Table 1). Among all pDDIs, 27.0% (n = 4285) were classified as cDDIs; 42.6% (n = 505) of admissions had a cDDI, and the median number of cDDIs per admission was 0 (IQR 0–2). The median number of pDDIs per admission increased with age (Fig. 2A-1) and the median number of cDDIs remained low for those aged < 45 years (Fig. 2B-1). Patients aged 80+ years had the highest number of pDDIs and cDDIs per admission (median 10, IQR 3–24 and 2 IQR 0–5, respectively). The number of pDDIs per 10 drugs prescribed increased with age and peaked for those aged 65–79 years (median 9 IQR 4–17), then decreased slightly for those aged 80+ (median 8, IQR 5–15; Fig. 2C-1). However, patients aged 80+ still had the highest number of cDDIs per 10 drugs (median 1.7, IQR 0–3.3, Fig. 2D-1). The distributions of DDIs were similar for male and female patients.
3.2 Contextual Factors Related to pDDIs Being Classified as cDDIs
Overall, drug dose was identified as the most frequently occurring contextual factor affecting clinical relevance of pDDIs (22.1%, n = 3500; Table 2). Dose was the most common factor used to determine that a pDDI was unlikely to be a cDDI (29.7% of all non cDDIs).
In contrast, ‘patient taking concomitant drugs beyond the drug pair identified that contribute to the same pharmacodynamic effect’ (further explanation, see Table 2) was the factor most associated with a pDDI being classified as a cDDI (55.3% of all cDDIs). The next four most common factors associated with a cDDI were: (i) ‘DDI is relevant based on the pharmacology of both drugs and the severity of the potential outcome (21.0%); (ii) ‘patient has renal/hepatic impairment’ (19.6%); (iii) age (14.0%); and (iv) ‘patient has a medical condition that may increase significance of DDI’ (13.6%).
3.3 Actual Harm Experienced by Patients Due to DDIs
Of all 4285 cDDIs, in 2904 cases (67.8%) both medications involved in the cDDI were administered to patients within a clinically relevant time frame depending on the pharmacokinetic and pharmacodynamic characteristics of each medication (as explained in the Sect. 2). These patient records were further reviewed to identify any evidence of harm as described in the Sect. 2.
According to our study definition of actual harm, which is cDDIs with probable or certain plausibility of causing the harm experienced by the patient, 76 cDDIs (1.8% of all 4285 cDDIs; Table 3) in 11 patients (0.9% of 1170 patients) led to actual harm with a severity of minor or above. Broadening the definition of actual harm to include cDDIs with possible, probable or certain plausibility found that 3% of cDDIs in 27 patients led to actual harm with a severity of minor or above. Narrowing the definition of actual harm to cases with certain plausibility identified actual harm from 0.6% of cDDIs in three patients. Furthermore, no patients experienced serious or severe actual harm with certain plausibility.
Table 4 presents examples of cDDIs that resulted in patient harm. A range of examples was chosen to demonstrate harms of different severity and plausibility. Information on drugs involved in cDDIs, plausibility of harm and level of harm are also included in this table.
3.4 Distribution of DDIs and associated patient harms before and after introduction of the electronic medication management (eMM) system
The distributions of pDDIs and cDDIs across study periods (pre- and post-eMM) were similar, as presented in Table 1 and shown in Fig. 2. In the pre-eMM period, 27.1% of pDDIs were classified as cDDIs and a similar proportion (26.9%) was found in the post-eMM period (Table 5). The implementation of eMM was not associated with significant changes in the occurrence of cDDIs on the basis of the multilevel model after accounting for patient-level cluster and adjusting for hospital, patient age, number of drugs and relevant contextual factors [adjusted odds ratio (AOR) 1.14, 95% CI 0.73–1.77].
Among all cDDIs, the proportion of cases in which both drugs were administered decreased from 72.9% before the implementation of eMM to 61.9% after the implementation (Table 5). The likelihood of both drugs being administered was significantly reduced by 44% in the post-eMM period (AOR 0.56, 95% CI 0.43–0.73, p < 0.0001) on the basis of the multilevel model after adjusting for other relevant factors.
A small reduction in cDDIs that led to actual harm was observed using the study definition of probable or certain plausibility and severity minor or above, from 2.5% pre-eMM (95% CI 2.0–3.3% n = 57) to 0.9% post-eMM (95% CI 0.6–1.5% n = 19; Table 3). The occurrence of cDDIs that led to actual harm when broadening plausibility to possible, probable or certain harm of severity minor or above was reduced from 3.9% (95% CI 3.2–4.8%, n = 88) in the pre-eMM period to 2.0% post-eMM (95% CI 1.5–2.7%, n = 40; Table 3), but this change was not significant after adjusting for other relevant factors (AOR 0.61, 95% CI 0.32–1.21; Table 5). There was no significant difference when further limiting plausibility to certain (from 1.1%, 95% CI 0.7–1.6%, n = 24 in pre-eMM to 0%, 95% CI 0–0.2%, n = 0 in post-eMM; Table 3).
4 Discussion
In this large-scale multisite study of 1170 patients, we found that 70% of inpatients experienced a pDDI during their hospital stay, 43% experienced a cDDI and < 1% experienced probable or certain harm from a cDDI with severity minor or above. The prevalence of pDDI in our study is significantly higher than the 33% reported in a 2018 meta-analysis (reported rate from 0.3–71.1% among 11 included studies) [10], 15–45% reported in a narrative review [23] and 58% from a systematic review of ICU patients [24], while close to the 71% reported in a 2013 Romanian study of 305 hospital internal medicine patients in a single hospital [12]. The number of pDDIs per patient in our study (mean 13 per patient) was also higher than the 0.3–4.5 reported for general hospital inpatients and 1.7–5.0 reported for ICU patients in a systematic review [10]. This likely reflects the variability in definitions and methods used in identifying pDDIs across different studies and differences in study populations. Our study included all inpatients with limited exclusion criteria, and thus is more representative of patients admitted to these public hospitals than previous studies that have typically restricted inclusion to patients prescribed at least one or two drugs, or to particular patient cohorts [2, 10]. The inclusion of multiple hospitals of different sizes and locations further strengthens the generalisability of the results. Careful assessment by experienced pharmacists and clinical pharmacologists is another strength of this study, as previous studies rarely used a pharmacist or physician to evaluate identified pDDIs and actual harm [10].
Our study identified cDDIs and reported their prevalence, which has rarely been done in previous research [10, 23]. Our cDDI prevalence rate of 43% is much higher than 8.8% reported in a Norwegian study of 827 patients [11], which investigated a broad range of 13 drug-related problems, including cDDIs, but did not provide a specific method for identifying cDDIs. The difference could be partially explained by the lower mean number of drugs for the 827 patients in this Norwegian study (4.6 vs. 9.0 in our study). Polypharmacy is well established as a strong risk factor for DDIs [25]. We reported the average number of cDDIs per 10 drugs (as 2.5, median 0), but this was not reported in the Norwegian study, preventing a comparison. Older patients experienced a relatively high number of cDDIs per ten drugs (median 1.6–1.7) for those aged 65 years and older and age was a contextual factor accounting for classification of 14% of pDDIs as cDDIs. To the best of our knowledge, this information has not been reported in previous studies for hospitalized patients.
We further examined the proportion of pDDIs that constituted cDDIs for patients. In both pre- and post-eMM periods, only 27% of all identified pDDIs were classified as cDDIs, indicating that pDDIs are not an accurate measure of risk to a patient from drug interactions. Although pDDIs are quick to identify, designing clinical decision support (CDS) systems to target pDDIs rather than cDDIs would likely lead to alert fatigue, as 73% of pDDIs in our sample were not clinically relevant when patient, drug, setting and other contextual factors were considered.
Another major strength of this study was the extensive chart review undertaken to identify drug, patient, setting and other contextual factors related to the clinical relevance of DDIs. These could be used when designing future CDS systems to improve alert effectiveness. Relevant demographic and clinical information in electronic medical records, such as pathology results and vital signs, could be automatically updated in real time to be available at the point of prescribing. In particular, the key contextual factors identified in our study, for example, renal/hepatic impairment, age and comorbidity, should be considered when developing alert algorithms. Dose and route information would also be critical information to incorporate into CDS, as nearly half of pDDIs were classified as non cDDIs because of these two drug factors (29.7% dose and 10.1% route), and so would reduce false positive DDI alerts being generated.
In cases where a cDDI was identified, both drugs were less likely to be administered by nurses in the post-eMM period compared with the pre-eMM period, although no DDI alerts or specific CDS were incorporated into the eMM systems in this study. It is possible that eMM systems improve visibility of medicine information [26], which may make it easier for clinicians to detect DDIs when prescribing, reviewing or administering medications. Easy access to reference material (e.g. DDI databases) at the point of care is also a frequently reported benefit of electronic systems [27]. All staff at study sites also had online access to several interaction checkers that are not integrated with the eMM system, although we did not investigate utilisation of this function during the study. Both improved visibility of medication information and easy access to online interaction checkers may have improved DDI identification and reduced concurrent administration of interacting drug pairs, resulting in fewer cDDIs and related harm after the eMM was introduced. Further investigation would be needed to shed light on the reasons behind this change.
Our research went further than most previous studies and investigated actual harm during hospital admission related to cDDIs. We found that 76 cDDIs resulted in actual harm with plausibility limited to probable or certain for 11 (0.9%) of 1170 patients, which is comparable to the 2.0% (6 out of 305 patients) reported in a Romanian study [12]. Only about 35% of our randomly selected study patients were aged 65 years and older, which might contribute to this low rate of harm, as old age is a known risk factor for DDIs and related harm [6]. However, only four of these patients were found to experience serious or severe actual harm (with 30 cDDIs; Table 3). With plausibility limited to certain, no patients experienced serious or severe actual harm. Given the relatively low rates of serious or severe harm, these findings raise questions regarding the relative value of implementing DDI alerts without considering clinical relevance and contextual factors including age, renal/hepatic impairment and other comorbidities, given the monetary investments in such decision support systems and the negative impacts alerts can produce (e.g. frustration, alert fatigue, time lost) [28].
This study, and particularly harm identification, was limited by information contained in medical records during patient hospital stays. Any harms not documented or evident in patients’ medical records would have been missed, which could be due to limited documentation or to harm occurring after hospital discharge. Lack of reported harm may not have always reflected the importance of the DDI identified and instead could have been due to the two interacting drugs not being administered together in a clinically relevant time frame. Identification of pDDIs relied on Stockley’s Drug Interaction Checker and DDIs were assessed only on the basis of the information presented by Stockley’s and classified by Stockley’s as moderate or severe, which could have resulted in some DDIs being missed and in some interactions being classified as DDIs that were actually drug–disease interactions (Table 4). Despite inconsistencies across compendia in how DDIs are identified and classified [29], Stockley’s is one of the most reputable and frequently used sources for identifying DDIs in Australian healthcare settings. Furthermore, nearly two-thirds of all randomly selected patients in our study were under 65 years of age, which reflected the age distribution of patients in our study hospitals. As older patients are more likely to experience DDIs and DDI-related harm [2, 30], caution should be taken when interpreting or comparing our results with other studies of particular patient cohorts, for example, age groups and medical conditions.
In conclusion, a large number of inpatients experienced potential DDIs but nearly three-quarters of pDDIs were not clinically relevant to patients. Contextual factors associated with cDDIs identified in this study could be used to design more targeted interventions, such as DDI alerts, which trigger only when DDIs are relevant to the patient (e.g. age, hepatic/renal impairment, comorbidities) and context. In a representative sample of all patients admitted to three Australian hospitals, very few cDDIs were associated with serious or severe actual harm during hospital admission. This warrants further thought and investigation on targeting the adoption of clinical decision support systems for DDIs to those situations where they are most likely to be clinically relevant, given the resources required for implementation and known negative impact of alerts.
References
Grannell L. Stockley’s drug interactions. 11th edition. Aust Prescriber. 2016;39(5):179.
Juurlink DN, Mamdani M, Kopp A, et al. Drug–drug interactions among elderly patients hospitalized for drug toxicity. J Am Med Assoc. 2003;289(13):1652–8.
Strandell J, Bate A, Lindquist M, et al. Drug–drug interactions—a preventable patient safety issue? Br J Clin Pharmacol. 2008;65(1):144–6.
Moura CS, Acurcio FA, Belo NO. Drug–drug interactions associated with length of stay and cost of hospitalization. J Pharm Pharm Sci. 2009;12(3):266–72.
Young EH, Pan S, Yap AG, et al. Polypharmacy prevalence in older adults seen in United States physician offices from 2009 to 2016. PLoS ONE. 2021;16(8): e0255642.
Doan J, Zakrzewski-Jakubiak H, Roy J, et al. Prevalence and risk of potential cytochrome P450-mediated drug–drug interactions in older hospitalized patients with polypharmacy. Ann Pharmacother. 2013;47(3):324–32.
Lubinga SJ, Uwiduhaye E. Potential drug–drug interactions on in-patient medication prescriptions at Mbarara Regional Referral Hospital (MRRH) in western Uganda: prevalence, clinical importance and associated factors. Afr Health Sci. 2011;11(3):499–507.
Mousavi S, Ghanbari G. Potential drug–drug interactions among hospitalized patients in a developing country. Caspian J Intern Med. 2017;8(4):282–8.
Guthrie B, Makubate B, Hernandez-Santiago V, et al. The rising tide of polypharmacy and drug–drug interactions: population database analysis 1995–2010. BMC Med. 2015;13:74.
Zheng WY, Richardson LC, Li L, et al. Drug–drug interactions and their harmful effects in hospitalised patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2018;74(1):15–27.
Blix HS, Viktil KK, Reikvam A, et al. The majority of hospitalised patients have drug-related problems: results from a prospective study in general hospitals. Eur J Clin Pharmacol. 2004;60(9):651–8.
Bucsa C, Farcas A, Cazacu I, et al. How many potential drug–drug interactions cause adverse drug reactions in hospitalized patients? Eur J Intern Med. 2013;24(1):27–33.
Nabovati E, Vakili-Arki H, Taherzadeh Z, et al. Information technology-based interventions to improve drug-drug interaction outcomes: a systematic review on features and effects. J Med Syst. 2017;41(1):12.
Reese T, Wright A, Liu S, et al. Improving the specificity of drug–drug interaction alerts: can it be done? Am J Health Syst Pharm. 2022;08:08.
Van De Sijpe G, Quintens C, Walgraeve K, et al. Overall performance of a drug–drug interaction clinical decision support system: quantitative evaluation and end-user survey. BMC Med Inf Decis Mak. 2022;22(1):48.
Baysari MT, Zheng WY, Li L, et al. Optimising computerised decision support to transform medication safety and reduce prescriber burden: study protocol for a mixed-methods evaluation of drug–drug interaction alerts. BMJ Open. 2019;9(8): e026034.
Preston CL (ed.). Stockley’s Interactions Checker (online): London: Pharmaceutical Press. Available from: http://www.medicinescomplete.com.
McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–82.
MIMS Online [Internet]. Crows Nest (NSW): MIMS Australia; 2022. Available from: www.mimsonline.com.au. Accessed 8 Dec 2022.
Merative Micromedex Web Application Access [Internet]. Merative US L.P. 1973, 2024. Available from https://www.micromedexsolutions.com/home/dispatch. Accessed 8 Dec 2022.
Hire RC, Kinage PJ, Gaikwad NN. Causality assessment in pharmacovigilance: a step towards quality care. Scholars J Appl Med Sci. 2013;1(5):386–92.
World Health Organization Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment 2013. Available from https://who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf. Accessed 2 May 2022.
Espinosa-Bosch M, Santos-Ramos B, Gil-Navarro MV, et al. Prevalence of drug interactions in hospital healthcare. Int J Clin Pharm. 2012;34(6):807–17.
Fitzmaurice MG, Wong A, Akerberg H, et al. Evaluation of potential drug-drug interactions in adults in the intensive care unit: a systematic review and meta-analysis. Drug Saf. 2019;42(9):1035–44.
Johnell K, Klarin I. The relationship between number of drugs and potential drug–drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30(10):911–8.
Baysari MT, Hardie RA, Lake R, et al. Longitudinal study of user experiences of a CPOE system in a pediatric hospital. Int J Med Inform. 2018;109:5–14.
Baysari MT, Reckmann MH, Li L, et al. Failure to utilize functions of an electronic prescribing system and the subsequent generation of “technically preventable” computerized alerts. J Am Med Inform Assoc. 2012;19(6):1003–10.
Baysari MT, Dort BAV, Zheng WY, et al. Prescribers’ reported acceptance and use of drug–drug interaction alerts: an Australian survey. Health Inf J. 2022;28(2):14604582221100678.
Meslin SMM, Zheng WY, Day RO, et al. Evaluation of clinical relevance of drug–drug interaction alerts prior to implementation. Appl Clin Inf. 2018;9(4):849–55.
Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ. 2004;329(7456):15–9.
Acknowledgements
The research team acknowledge support and assistance from Kevin Gunn, Donna Dorrington, Peter Gates and Kasun Rathnayake for their expertise and assistance with different aspects of our study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work is supported by the National Health and Medical Research Council (partnership grant APP1134824) in partnership with eHealth NSW and eHealth QLD, Australia. JIW is supported by an NHMRC Elizabeth Blackburn Leadership Fellowship (1174021).
Conflict of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Availability of Data
The datasets generated during the current study are not publicly available to be accessed from researchers as this is not covered by the project’s Human Research Ethics Application. The data that support the findings of this study are available from the authors, but restrictions apply for future use, including appropriate ethics approval.
Ethics Approval
Ethics approval was obtained from one of the hospitals’ Human Research Ethics Committee (reference no.: 8/02/21/4.07), and site-specific governance approval was subsequently obtained from all sites.
Consent to Participate
Individual patient consent to access retrospective medication and clinical records was waived by the human research ethics committee.
Consent to Publish
Not applicable.
Code Availability
Not applicable.
Author Contributions
L.L., M.B., J.W., S.H. and R.D. conceived of and designed the study. R.Q., D.D., M.M. and A.S. participated in data collection with input from B.V., W.Z., A.H. and P.D.; S.H. and R.D. led the harm classification process. L.L., J.B. and N.A. designed and conducted data analysis. All authors provided input on the interpretation of findings. L.L. prepared the first draft of the manuscript and all authors provided input and approved the final version of the manuscript and agree to be accountable for the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, L., Baker, J., Quirk, R. et al. Drug–Drug Interactions and Actual Harm to Hospitalized Patients: A Multicentre Study Examining the Prevalence Pre- and Post-Electronic Medication System Implementation. Drug Saf 47, 557–569 (2024). https://doi.org/10.1007/s40264-024-01412-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-024-01412-w