Abstract
Introduction
Statin discontinuation can have major negative health consequences. Studying the reasons for discontinuation can be challenging as traditional data collection methods have limitations. We propose an alternative approach using social media.
Methods
We used natural language processing and machine learning to extract mentions of discontinuation of statin therapy from an online health forum, WebMD (http://www.webmd.com). We then extracted data according to themes and identified key attributes of the people posting for themselves.
Results
We identified 2121 statin reviews that contained information on discontinuing at least one named statin. Sixty percent of people posting declared themselves as female and the most common age category was 55–64 years. Over half the people taking statins did so for < 6 months. By far the most common reason given (90%) was patient experience of adverse events, the most common of which were musculoskeletal and connective tissue disorders. The rank order of adverse events reported in WebMD was largely consistent with those reported to regulatory agencies in the US and UK. Data were available on age, sex, duration of statin use, and, in some instances, adverse event resolution and rechallenge. In some instances, details were presented on resolution of the adverse event and rechallenge.
Conclusion
Social media may provide data on the reasons for switching or discontinuation of a medication, as well as unique patient perspectives that may influence continuation of a medication. This information source may provide unique data for novel interventions to reduce medication discontinuation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adverse events are the most common reason cited on WebMD for the discontinuation or switching of statin medication. |
Details of the types of adverse events leading to discontinuation or switching of statin medication are available on social media along with key demographics. |
The types of statin adverse events mentioned on WedMD generally follow a similar pattern to reports from regulatory data. |
1 Introduction
Cardiovascular disease (CVD) is the number one cause of death globally [1], with an estimated 75% of premature cases being preventable [2]. Hypercholesterolaemia, especially elevated low-density lipoprotein cholesterol (LDL-C) levels, has long been recognized as a risk factor for the development of CVD [3]. Statin therapy has been shown to reduce serum cholesterol, leading to a reduction in the incidence of heart disease and stroke [4].
Although the benefits of lifelong adherence to statins are well-established, worldwide rates of persistence (the duration of time from initiation to discontinuation of therapy) for statins are suboptimal [5,6,7,8,9,10,11]. A recent meta-analysis revealed that after 1 year, 23% of patients aged 65 years and over had discontinued their therapy at 1 year, and 32 and 39% had discontinued at 2 and 4 years, respectively [12]. Other studies have reported rates of switching from one statin to another of 20% [13] and 28% [14] within people over 65 years of age and in the general population in Australia, respectively. Discontinuation of statins can be responsible for the failure to achieve and maintain optimal LDL-C levels [15]. Discontinuation of statins is mainly patient-initiated [16, and may not be disclosed to the prescriber [17]. Switching statins may be one alternative to discontinuation for patients, particularly in relation to certain adverse events [18] or cost [19].
Despite being well-tolerated in trials, many patients have reported that adverse effects are a major contributor to statin discontinuation [16] or switching [20]. A better understanding of why some patients discontinue their statins would provide healthcare professionals with an opportunity to understand patient perspectives and potentially help to design interventions to address these reasons.
Measuring the reasons for statin switching or discontinuation is challenging [21]. For instance, reasons for drug discontinuations are rarely available in pharmacy data or primary care prescribing data, and if present, are often in narrative form [21, 22]. Reasons for non-adherence and persistence to statins have been explored using cross-sectional surveys [10, 17, 19, 23,24,25,26,27,28,29,30,31,32], with occasional qualitative approaches undertaken [33, 34], or examination of electronic health records [21].
While cross-sectional surveys are relatively quick, simple and cheap to administer, their accuracy remains unclear, as they are at risk of recall and social desirability bias and limited by questionnaire design and delivery [35,36,37]. Interviews, on the other hand, are also prone to interviewer bias, relating to the way the interviewer asks questions and responds to answers, as well as their identity or behaviour [38]. For example, an interviewer may have preconceived ideas about who or why statins are discontinued and the patient may be prone to deviate from the truth due to social desirability [38]. Substantial disagreement has been observed between patient questionnaires and interviews and pharmacy data [22]. Social media posts tend to be contemporaneous to the event studied and are without the need for interrogation by a researcher, potentially reducing these biases.
We assess the reasons for discontinuation or switching of statin therapy as reported by patients on social media.
2 Methods
We selected WebMD (http://www.webmd.com) as our data source. WebMD is a popular American healthcare website, which along with providing health information also provides open access to anonymous online drug reviews posted by the public in a free-text form. Named medications, such as simvastatin, can be searched for or browsed for using an alphabetical list. A review is assigned to exactly one drug and is composed of three scores evaluating the satisfaction, effectiveness, and ease of use of the drug. The scores range from 1 to 5 stars, with 1 star being the lowest value. The overall workflow is presented below and in Fig. 1.
2.1 Dataset
We collected a complete set of 343,459 WebMD reviews for all medications posted from September 2007 to August 2021 using scraping tools [39]. For each review, we collected the free-text comment section as well as the date, author age, author sex, time on drug, author role (options being ‘Patient’ and ‘Caregiver’), condition, overall rating, effectiveness, ease of use, and satisfaction.
All data used in this study were collected according to the WebMD terms of use and were publicly available at the time of collection and analysis. All quotes are paraphrased. We have an Institutional Review Board certificate of exemption from the University of Pennsylvania.
In an earlier study, we trained a classifier to identify posts mentioning changes to medication treatment [39]. In order to train the classifier, we manually annotated for medication change using a corpus (‘WebMD-BIN’), which consists of a randomly selected subset of 12,972 WebMD reviews, with a satisfaction rating of 1 or 2 stars (the two options indicating the lowest satisfaction). We chose reviews where users reported low satisfaction to create the annotated corpus used to train our classifier because they were more likely to also report stopping or a change of medications, thus providing enough positive examples. However, we did not restrict our final set by star rating; the low satisfaction scores were only used to more efficiently create our training corpus. The performance of the classifier was also validated for the reviews with 3–5 stars, although it was trained with only 1–2 stars. For the current study, we created a second corpus (‘WebMD-NER’) consisting of 2837 reviews, a random subset of reviews in WebMD-BIN, which were further annotated to extract the span of text that mentions the reason(s) for discontinuing or changing the medication. We used these annotated datasets to train a pipeline to identify when the reasons for treatment change were expressed.
2.2 Extracting Reasons for Medication Change in WebMD Reviews
To automate the annotation of the reasons for the change or discontinuation of medications, we implemented a pipeline composed of a classifier and a sequence labeler, sequentially applied. Given an unlabelled review, we first applied our classifier to categorize whether or not the review mentions a change or discontinuation of a medication. If the review is predicted to mention a change in medications, we applied the sequence labeler to extract the spans of text explaining the reasons for these changes. For our classifier, we chose a deep neural network with a standard architecture for classification tasks in natural language processing (NLP) [40]. We used bidirectional encoder representations from transformer (BERT)-base contextual embeddings as inputs to a fully connected layer followed by a softmax layer to predict the probability of a review to mention a medication change. We trained the classifier using the WebMD-BIN corpus on a subset of 90% (11,675 reviews with a satisfaction rating of 1 or 2 stars) and evaluated its performance on the remaining 10% (1297 reviews). Our classifier achieved good performance, with a 0.871 precision, 0.876 recall, and 0.874 F1 score (the harmonic mean of precision and recall). We also designed our sequence labeler as a deep neural network following a standard architecture for spans extraction tasks; we used BERT-base contextual embeddings as inputs to a bidirectional-long short-term memory layer. A technical presentation of this neural network is out of the scope of this study, but interested readers can find more details in the article by Li et al. [41]. Our neural network, following the IO annotation schema, predicts the probabilities for all tokens of a review to be either inside (I) in a phrase mentioning a reason for a medication change, or outside (O). We trained our sequence labeler on 80% (2269 reviews) of the WebMD-NER corpus. The sequence labeler achieved moderate performance with a 0.6964 F1 score when we evaluated it on the 20% (568 reviews) of remaining reviews.
2.3 Automatic and Manual Extraction of Reasons for Medication Changes in Statin Medication Reviews
To study the reason stated for the switching from one statin to another or discontinuing statins altogether, we applied our pipeline to WebMD reviews of any star rating (1–5) after selecting the subset of reviews of eight statin medications, denoted by their generic or common brand names. We used the automated extracted reasons for change to help manually annotate the reviews.
For each review identified by the pipeline, two authors (SG and KO) first checked whether the review was a true mention of a medication change, and if that change was switching from one statin to another or a discontinuation. We then extracted data on the specific statins mentioned and the stated reasons for switching or discontinuation. We amalgamated the results for both discontinuation of a specific named statin in preference for another statin (switching) and discontinuation of a specific named statin. In instances where adverse events were cited as the reason for discontinuation, the adverse event terms were manually normalized to Medical Dictionary for Regulatory Activities (MedDRA®) Lowest-Level Terms (LLTs) and Preferred Term (PT) codes. To facilitate comparison with other sources, the PT codes were then assigned to one of 27 MedDRA® broader categories of primary System Organ Class (SOC) codes. For each drug review stating adverse events, we collected data on the time from starting the medication until the adverse event occurred in days, whether the adverse event stopped after discontinuation, and whether the patient attempted to restart the drug, and if so, did the adverse event re-occur. In addition, we noted any instances of any other commonly mentioned themes using a qualitative content analysis (QCA) approach [42]. This approach focuses on creating conceptual frameworks or theories through inductive analysis from the data, thus we did not seek to confirm or refute an existing hypothesis but rather to explore emerging themes [43]. We first immersed ourselves in the data and then categorized the themes that emerged and reported on the most common themes. Due to the small numbers of reviewers describing any one common theme (< 6%), we did not investigate any themes relating to different characteristics or behaviours of the people posting.
In many instances, the posted text described other statins that had been prescribed in addition to the named statin of the review. We therefore extracted information on previous statins or subsequent statins taken, as well as reasons for their discontinuation, from each post.
We collected data from spontaneous reporting systems (the US FDA Adverse Event Reporting System [FAERS] and the UK Medicines and Healthcare products Regulatory Agency [MHRA]) for comparison of the relative frequency of adverse events reported on WebMD and those reported in FAERS and MHRA data [44].
3 Results
We collected 5156 statin reviews from WebMD posted from September 2007 to August 2021. Our pipeline identified that 2458 reviews most likely mentioned a medication change.
After manually checking all 2458 reviews, 235 (9.6%) were excluded because they did not indicate any change, seven (0.3%) were excluded because they never initiated the statin due to concerns about adverse events, and three (0.1%) were excluded because they were duplicate posts. A further 92 (3.7%) posts mentioned a change in dosage but no indication whether the statin was ceased.
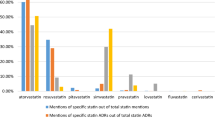
Of the remaining 2121 posts included, the most common statin review was for simvastatin (41%, 860/2121), followed by atorvastatin (22%, 469/2121); 38–49% of WebMD statin drug reviews mentioned drug switching or drug discontinuation with statins (electronic supplementary material [ESM] Table 1). This was relatively consistent among the different statins.
3.1 Patient Characteristics
Most people posting (96%, 2027/2121) were patients, with some caregivers (1.5%, 32/2121) and a few unknown roles (3%, 62/2121); 60% (1283/2121) were female, 33% (708/2121) were male and 6% (130/2121) did not declare their gender. Almost all people posting (99%) declared their age category, with the most common category being 55–64 years of age (40%) [Fig. 2]. Over half of the people taking statins had done so for <6 months (Fig. 2). The average star ratings for the statins was 2.59 for effectiveness, 3.42 for ease of use, 1.54 for satisfaction, and 2.47 for the overall rating. Each rating had entries ranging from 1 to 5.
The main reason for taking statins was selected from a drop-down selection, with ‘high cholesterol’ (59%, 1261/2121), ‘combined high blood cholesterol and triglyceride level’ (18%, 392/2121), ‘homozygous inherited high blood cholesterol’ (5%, 112/2121), ‘increased triglycerides and cholesterol’ (5%, 108/2121), and ‘treatment to prevent a heart attack’ (3%, 69/2121) being the most common.
Although the drug reviews posted on WebMD are mostly likely to be from people living in the US, as an online resource, people from other countries are also able to post reviews; however, the posts are biased towards the English language.
3.2 Reasons for Discontinuation/Switching
Some people had tried multiple statins. Within the 2121 statin drug reviews, there were 2552 mentions of a named statin. In 150 instances, the statin switched to was not discontinued at the time of posting, thus there were a total of 2402 mentions of discontinuation (1976) or switching (426) of a named statin.
The most common reason given for discontinuation was adverse events experienced (90%, 2156/2402), followed by insurance or cost (4%, 92/2402) and lack of effectiveness (3%, 62/2402). Other reasons stated were concern for potential adverse events (0.5%, 12/2402), forgetting (0.2%, 5/2402), drug interactions (0.2%, 4/2402), lack of availability (0.1%, 2/2402), had been effective in lowering cholesterol (0.1%, 2/2402) and being put off by too much advertising (0.04%, 1/2402). In some instances, no reason was given.
When the reasons for stopping a statin medication are grouped according to whether a switch to another statin occurred, the most common reason for switching was still adverse events experienced (61%, 260/426); however, a larger proportion cited insurance/cost (17%, 73/426) and ineffectiveness (9%, 37/426) as reasons for switching.
3.3 Adverse Events
Ninety percent (2156/2402) of discontinuations or switching of statin medication were reported to be due to adverse events or intolerance. The posts often described the severity of the adverse events in terms of their quality of life. Phrases such as “I would rather live with high cholesterol”, “Living like this is just not worth it” or “the cure is worse than the disease” were used. Many expressed concerns that the drug had aged them, i.e. “I feel more like 102 than 42”, and that they were worried the adverse events were irreversible.
In almost half (47%, 1017/2156) of these instances, this was due to more than one adverse event from different MedDRA primary SOC codes. In 510 cases (24%), the adverse events were from two SOC codes, in 299 cases (14%) they were from three SOC codes, and in 129 cases (6%), the adverse events were from four SOC codes. The highest number of adverse event SOC codes categorized from just one patient for one statin was eight (two cases). In 25 cases (1.2%), the number of adverse events experienced could not be determined due to phrases such as “‘I experienced terrible side effects so stopped taking this statin”.
In total, 3971 adverse events from the different SOC codes were mentioned. The most common category of adverse events leading to discontinuation were ‘Musculoskeletal and connective tissue disorders’, followed by ‘General disorders and administration site conditions’. These posts most commonly referred to muscle pain or muscle aches leading to problems in movement and fatigue or malaise.
The relative frequency of the different adverse events from WebMD were consistent with spontaneous reporting data available from the regulatory agencies FDA and MHRA. The rank order of adverse events from posts on WebMD, MHRA and FDA were in direct agreement for the top four adverse events (Table 1). There was slight disagreement with only two adverse event categories: ‘Investigations’ (such as test results—for example, blood sugar levels—or weight increased) and ‘Injury, poisoning and procedural complications’ (such as accidental exposure or intentional overdose), with both these categories being higher in the regulatory agency data than on social media.
We were able to categorize the posts according to the named statin medication, such as simvastatin or atorvastatin. ESM Fig. 1 shows similar categories of adverse events were reported in WebMD independent of the particular named statin prescribed.
3.4 Time to Event
The time to adverse event was recorded in 689 (32%) posts and ranged from 1 day to 12 years, with a median of 14 days (with a 25th percentile of 3 days and 75th percentile of 2 months). The time to event varied by adverse event, with ‘Respiratory, thoracic and mediastinal disorders’ having a quicker onset time than ‘Musculoskeletal and connective tissue disorders’ and ‘Cardiac disorders’ (ESM Table 2).
3.5 Recovery from Adverse Events
Sixty percent (1278/2121) of people stated whether they had recovered from the adverse events they experienced, with two-thirds (66%, 842/1278) describing a full recovery, 23% (295/1278) describing a partial recovery and 11% (146/1278) describing no recovery at the time of posting (ESM Table 3).
Eight hundred and forty-two people stated how many days had passed since they discontinued the statin. Of those who completely recovered or partially recovered the time passed since stopping medication was shorter than those who did not recover (ESM Table 4).
3.6 Rechallenge
One hundred and twenty-one people who posted stated that they purposefully rechallenged their particular statin medication to determine a definite link to the adverse event. Of these, 107 (88.4%) people stated that their symptoms returned, 8 (6.6%) stated that their symptoms did not return, and 6 (5.0%) people did not report whether the symptoms had returned (in some cases they reported that they had only just restarted their medication and in others they only reported their change in cholesterol levels).
3.7 Other Issues Noted (Table 2)
Six percent (128/2121) of people posting stated a distrust of their doctor or healthcare professional prescribing their statin medication. They mostly identified problems of not being believed by their doctor or their adverse events being dismissed, with some stating that this led them to changing doctors.
Five percent (96/2121) of people posting described statins as poisonous or dangerous, with some stating that they should be taken off the market; 5.3% (113/2121) of people posting directly stated that they had found the WebMD website useful in helping them to confirm their adverse events and reaching a decision to discontinue with statins.
4 Discussion
Statins are a lifelong medication, with discontinuation leading to a return of high cholesterol levels. The majority of people posting on WebMD discontinued their statin medication because of adverse events they attributed to the statins. In nearly half of cases this was more than one type of adverse event. There was some consideration as to why these people attributed the adverse events to statins. Some even rechallenged their medication and some sought other explanations such as aging or a pre-existing medical condition. People posting often stated that they did not feel that the benefits of statins outweighed the adverse events.
Previous research evaluating reasons for discontinuing or switching statins has used medical records (both structured data and unstructured with the help of NLP [21, 45], patient surveys [19], interviews [33], or a combination of medical records and telephone surveys [29]. Similar to our findings, most studies identified adverse events to be the most common reason for discontinuing statins [19, 29, 33, 45]. Although Zhang et al. found the default ‘no longer necessary’ most prominent, they state that as many as 87% of statin discontinuations are attributable to adverse events, which is similar to the 90% in our study [21].
In previous studies, cost or insurance was the second most common reason identified for both discontinuation and switching [19, 33, 45], followed by lack of efficacy [19]; however, Wei et al. found cost and lack of efficacy to be more widely cited than in our sample (16 vs. 4% for cost, and 13 vs. 3% for efficacy) [19]. Nonetheless, our sample, while biased towards the US, is not limited to the US alone, where cost may be more of an issue than in countries with free or heavily subsidized at-the-point-of-access healthcare, such as with the National Health Service (NHS) in the UK.
The rank order of the adverse events mentioned on WebMD was consistent with the rank order for spontaneous reporting data from the MHRA and FDA [44] and indeed previous observational studies [21, 46, 47] and social media studies [44]. Musculoskeletal events have been found to be the most predominant, accounting for 40–60% of all adverse events [19, 21, 33]. Zhang et al. also identified musculoskeletal and connective tissue disorders to be by far the most prominent adverse event resulting in discontinuation, followed by general disorders [21]. As social media reviews provide us with a similar rank order as regulatory capture and observational studies, this suggests that our methods are able to yield data that is a fair reflection of spontaneous reports collected by regulators, thus enhancing confidence in the validity of our methods.
Prior studies have suggested the potential value of using social media (such as the Stroke Association patient forum) to identify the barriers and facilitators of medication uptake and continuation (including statins) [48]. Our study provides more detail in further disentangling the barriers for statins, and also goes beyond those of other studies that have used social media to identify adverse events associated with statins, as we focused on which adverse events led to behaviour change and the time to event, which could provide useful data for devising pharmacoepidemiologic studies or real-world evaluations. Previous comparisons of adverse events reported on social media and those reported in regulatory data have not always demonstrated such a high level of agreement, with mild symptom-related adverse events reported more frequently on social media [49]. For instance, posts on the ‘Ask a Patient’ website (http://www.askapatient.com) focused on fewer and less serious adverse events of statins than reported to the FAERS [50], which was partly attributed to a younger population using ‘Ask a Patient’. Interestingly, our statin users were older (at least 64% were 55 years of age or older) than those on ‘Ask a Patient’ in 2014 (mean 53.9 years).
There was no clear pattern of differing adverse events associated with discontinuing different types of statins. Very little research has previously examined the relationship of the particular statin prescribed and discontinuation among patients or the clinical benefit of switching [14, 51]. The STOMP trial, for instance, found that more patients taking atorvastatin reported myalgia than those taking placebo, but did not compare with other statins [52]. Our research suggests that different statins are broadly similar in terms of adverse event categories among patients who report on WebMD that they switched or discontinued a statin. The data on switching confirms this, as, of the 150 patients who did not discontinue any statin medication, nearly half switched due to insurance/cost. In addition, the similar patterns of adverse event reporting among the different statins gives some validity to social media data, since if it was inundated by one disgruntled patient or if one review led to a surge in similar reviews, we would have expected to see a different pattern among the different statin medications.
Previous studies have suggested that rates of successful statin reattempt after an adverse event are high [47]. Our data on reattempt with the same statin do not suggest this to be the case; however, many people posting also reported switching statin medication or changing their dose, which may have led to better tolerability.
Some dissatisfaction was reported with doctors prescribing statins, including mistrust. This theme has been identified previously using social media but not when interviews were used in the same study [53]. Patients who are dissatisfied with or distrust their healthcare professional are less likely to be adherent with statins [19, 33].
Exploring the content of online posts, such as in this study, is not only useful to identify reasons for switching or discontinuing medication therapy but also to explore the information available to patients. Research suggests that patients are influenced by online information and are often influenced by it more than they are by the information provided by healthcare professionals [54]. In our study, some of the people posting sought confirmation of their suspicions through the WebMD forum and used the reviews to inform their decision making. Reading about adverse events online has also been linked to a decreased uptake or continuation of statins [54], and since many of the reviews on WebMD focus on adverse events, this may impact on patient behaviour. Some people posting explicitly stated this to be the case.
This research provides the starting point for further research using social media to quickly and effectively identify reasons for discontinuation of medications, without participant burden. This information may be helpful for drug manufacturers, researchers and public health messaging, and to improve the conversation between healthcare professionals and patients. With a larger sample, more in-depth analysis could be undertaken into who is discontinuing their medication and why.
5 Strengths and Limitations
We also had some limitations. Our data were obtaOur study had many strengths. We were able to develop computational methods for identifying reviews with information on statin discontinuation or switching with a high level of precision. We were also able to extract data on the named statin medication and the reasons for medication change in the vast majority of posts, and, in the case of adverse events, detail on the type and, often, duration. Demographic data and duration of statin intake were also available to us as well as more nuanced information provided by users.
ined from a single source (WebMD), however future research could consider using multiple social media platforms. The statin users captured from social media may not be representative of all statin users. Extraction of the demographic data available suggests that our sample overrepresents females and a younger population. Previous research has identified female sex to be associated with higher non-adherence to statins than males [55,56,57]. In addition, those motivated to write a review on a statin may be more likely to have had a particularly negative experience. It is already known that discontinuers are more likely to use the internet to research statins than continuers [19].
We also trained our model using only reviews that received a 1–2 rating for satisfaction, which could bias our predictions or lead to a reduced performance on other reviews. To assess the effect of this, we performed a post hoc analysis of classified reviews with 3–5 satisfaction ratings, which indicated only a slight drop (− 0.05 F1 score) in performance from the test set, with an accuracy of 90%, indicating good generalizability of the model over all reviews.
We did not extract information on the seriousness of each adverse event reported in WebMD or whether the medication change was discussed with a healthcare professional. While there may not have been enough detail in many reviews, others gave clear indications of seriousness or lack of disclosure to a healthcare professional by stating that ‘they were unable to walk’, ‘could hardly move’ or ‘there is no way I am telling my physician’. We also did not obtain a control group of patients who did not discontinue their statin therapy.
We did not identify much information on uptake or refusal of statins. Future research should determine the extent to which data on other social media platforms can be used to distinguish between primary non-adherence and discontinuation.
6 Conclusion
This study gives an indication as to reasons for statin discontinuation that patients felt important enough to report in social media drug reviews, with 90% of switch discontinuations attributed to adverse events. The rank order of the adverse events stated in social media is also consistent with that from spontaneous reporting systems. Further research should be undertaken to assess the generalizability of our results and to compare the results more extensively with other traditional data sources.
This study indicates the breadth and depth of information available on an open health forum (WebMD) and the type of data available to patients who may be considering statin medication uptake or considering discontinuing or switching their statin medication. The influence of the information in WebMD was explicitly apparent in some posts.
References
World Health Organization. Cardiovascular diseases (CVDs). Fact sheet. 2017. Available at: http://www.who.int/mediacentre/fact-sheets/fs317/en.
Stewart J, Addy K, Campbell S, Wilkinson P. Primary prevention of cardiovascular disease: updated review of contemporary guidance and literature. JRSM Cardiovasc Dis. 2020;9:2048004020949326.
Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–81.
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
Avorn J, Monette J, Lacour A, Bohn RL, Monane M, Mogun H, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279(18):1458–62.
Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–61.
Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med. 2004;19(6):638–45.
Hirsh BJ, Smilowitz NR, Rosenson RS, Fuster V, Sperling LS. Utilization of and adherence to guideline-recommended lipid-lowering therapy after acute coronary syndrome: opportunities for improvement. J Am Coll Cardiol. 2015;66(2):184–92.
Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288(4):462–7.
Maddox TM, Chan PS, Spertus JA, Tang F, Jones P, Ho PM, et al. Variations in coronary artery disease secondary prevention prescriptions among outpatient cardiology practices: insights from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2014;63(6):539–46.
Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4(3):206–13.
Ofori-Asenso R, Jakhu A, Zomer E, Curtis AJ, Korhonen MJ, Nelson M, et al. Adherence and persistence among statin users aged 65 years and over: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2018;73(6):813–9.
Ofori-Asenso R, Ilomaki J, Tacey M, Zomer E, Curtis Andrea J, Si S, et al. Switching, discontinuation, and reinitiation of statins among older adults. J Am Coll Cardiol. 2018;72(21):2675–7.
Talic S, Marquina C, Ofori-Asenso R, Petrova M, Liew D, Owen AJ, et al. Switching, persistence and adherence to statin therapy: a retrospective cohort study using the Australian national pharmacy data. Cardiovasc Drugs Ther. 2021. https://doi.org/10.1007/s10557-021-07199-7 (Epub 7 Jun 2021).
Bermingham M, Hayden J, Dawkins I, Miwa S, Gibson D, McDonald K, et al. Prospective analysis of LDL-C goal achievement and self-reported medication adherence among statin users in primary care. Clin Ther. 2011;33(9):1180–9.
Krüger K, Leppkes N, Gehrke-Beck S, Herrmann W, Algharably EA, Kreutz R, et al. Improving long-term adherence to statin therapy: a qualitative study of GPs’ experiences in primary care. Br J Gen Pract. 2018;68(671):e401–7.
Brinton EA. Understanding patient Adherence and Concerns with STatins and MedicatION discussions with physicians (ACTION): a survey on the patient perspective of dialogue with healthcare providers regarding statin therapy. Clin Cardiol. 2018;41(6):710–20.
Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med. 2009;150(12):858–68.
Wei MY, Ito MK, Cohen JD, Brinton EA, Jacobson TA. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol. 2013;7(5):472–83.
Arca M, Pigna G. Treating statin-intolerant patients. Diabetes Metab Syndr Obes. 2011;4:155–66.
Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–34.
Liu F, Pradhan R, Druhl E, Freund E, Liu W, Sauer BC, et al. Learning to detect and understand drug discontinuation events from clinical narratives. J Am Med Inform Assoc. 2019;26(10):943–51.
Blalock DV, Bosworth HB, Reeve BB, Voils CI. Co-occurring reasons for medication nonadherence within subgroups of patients with hyperlipidemia. J Behav Med. 2019;42(2):291–9.
Bradley CK, Wang TY, Li S, Robinson JG, Roger VL, Goldberg AC, et al. Patient-reported reasons for declining or discontinuing statin therapy: insights from the PALM Registry. J Am Heart Assoc. 2019;8(7): e011765.
Fung V, Graetz I, Reed M, Jaffe MG. Patient-reported adherence to statin therapy, barriers to adherence, and perceptions of cardiovascular risk. PLoS ONE. 2018;13(2): e0191817.
Harrison TN, Derose SF, Cheetham TC, Chiu V, Vansomphone SS, Green K, et al. Primary nonadherence to statin therapy: patients’ perceptions. Am J Manag Care. 2013;19(4):e133–9.
Karalis DG, Wild RA, Maki KC, Gaskins R, Jacobson TA, Sponseller CA, et al. Gender differences in side effects and attitudes regarding statin use in the Understanding Statin Use in America and Gaps in Patient Education (USAGE) study. J Clin Lipidol. 2016;10(4):833–41.
Khanderia U, Townsend KA, Erickson SR, Vlasnik J, Prager RL, Eagle KA. Medication adherence following coronary artery bypass graft surgery: assessment of beliefs and attitudes. Ann Pharmacother. 2008;42(2):192–9.
McGinnis B, Olson KL, Magid D, Bayliss E, Korner EJ, Brand DW, et al. Factors related to adherence to statin therapy. Ann Pharmacother. 2007;41(11):1805–11.
van der Ploeg MA, Poortvliet RK, van Blijswijk SC, den Elzen WP, van Peet PG, de Ruijter W, et al. Statin use and self-reported hindering muscle complaints in older persons: a population based study. PLoS ONE. 2016;11(12): e0166857.
Wouters H, Van Dijk L, Geers HC, Winters NA, Van Geffen EC, Stiggelbout AM, et al. Understanding statin non-adherence: knowing which perceptions and experiences matter to different patients. PLoS ONE. 2016;11(1): e0146272.
Zullig LL, Sanders LL, Thomas S, Brown JN, Danus S, McCant F, et al. Health beliefs and desire to improve cholesterol levels among patients with hyperlipidemia. Patient Educ Couns. 2016;99(5):830–5.
Garavalia L, Garavalia B, Spertus JA, Decker C. Exploring patients’ reasons for discontinuance of heart medications. J Cardiovasc Nurs. 2009;24(5):371–9.
Gencer B, Rodondi N, Auer R, Räber L, Klingenberg R, Nanchen D, et al. Reasons for discontinuation of recommended therapies according to the patients after acute coronary syndromes. Eur J Intern Med. 2015;26(1):56–62.
Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscipl Healthc. 2016;9:211–7.
Caputo A. Social desirability bias in self-reported well-being measures: evidence from an online survey. Universitas Psychologica. 2017;16(2).
Latkin CA, Edwards C, Davey-Rothwell MA, Tobin KE. The relationship between social desirability bias and self-reports of health, substance use, and social network factors among urban substance users in Baltimore, Maryland. Addict Behav. 2017;73:133–6.
Frey BB, editor. The SAGE encyclopedia of educational research, measurement, and evaluation. Thousand Oaks: Sage Publications, Inc.; 2018.
Weissenbacher D, Ge S, Klein A, O’Connor K, Gross R, Hennessy S, et al. Active neural networks to detect mentions of changes to medication treatment in social media. J Am Med Inform Assoc. 2021;28(12):2551–61.
Minaee S, Kalchbrenner N, Cambria E, Nikzad N, Chenaghlu M, Gao J. Deep learning-based text classification: a comprehensive review. ACM Comput Surv. 2021;54(3):62.
Li J, Sun A, Han J, Li C. A survey on deep learning for named entity recognition. IEEE Trans Knowl Data Eng. 2022;34(1):50–70.
Drisko J, Maschi T. Qualitative content analysis. In: Drisko J, Maschi T, editors. Content analysis. New York: Oxford University Press; 2015. p. 82–120.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Golder S, Smith K, O’Connor K, Gross R, Hennessy S, Gonzalez-Hernandez G. A comparative view of reported adverse effects of statins in social media, regulatory data, drug information databases and systematic reviews. Drug Saf. 2021;44(2):167–79.
Morrison FJ, Zhang H, Skentzos S, Shubina M, Bentley-Lewis R, Turchin A. Reasons for discontinuation of lipid-lowering medications in patients with chronic kidney disease. Cardiorenal Med. 2014;4(3–4):225–33.
Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: a cohort study. Ann Intern Med. 2017;167(4):221–7.
Zhang H, Plutzky J, Ge W, Shubina M, Turchin A. Predictors of a successful statin reattempt after an adverse reaction. J Clin Lipidol. 2018;12(3):643–51.
Jamison J, Sutton S, Mant J, De Simoni A. Barriers and facilitators to adherence to secondary stroke prevention medications after stroke: analysis of survivors and caregivers views from an online stroke forum. BMJ Open. 2017;7(7): e016814.
Golder S, Norman G, Loke YK. Systematic review on the prevalence, frequency and comparative value of adverse events data in social media. Br J Clin Pharmacol. 2015;80(4):878–88.
Duh MS, Cremieux P, Audenrode MV, Vekeman F, Karner P, Zhang H, et al. Can social media data lead to earlier detection of drug-related adverse events? Pharmacoepidemiol Drug Saf. 2016;25(12):1425–33.
Kirby MG, Allchorne P, Appanna T, Davey P, Gledhill R, Green JSA, et al. Prescription switching: rationales and risks. Int J Clin Pract. 2020;74(1): e13429.
Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127(1):96–103.
Jamison J, Sutton S, Mant J, De Simoni A. Online stroke forum as source of data for qualitative research: insights from a comparison with patients’ interviews. BMJ Open. 2018;8(3): e020133.
Jones NM, Mukamel DB, Malik S, Greenfield RS, Reikes A, Wong ND, et al. The costs outweigh the benefits: seeing side-effects online may decrease adherence to statins. BMC Med Inform Decis Mak. 2020;20(1):197.
Hope HF, Binkley GM, Fenton S, Kitas GD, Verstappen SMM, Symmons DPM. Systematic review of the predictors of statin adherence for the primary prevention of cardiovascular disease. PLoS ONE. 2019;14(1): e0201196.
Ingersgaard MV, Helms Andersen T, Norgaard O, Grabowski D, Olesen K. Reasons for nonadherence to statins: a systematic review of reviews. Patient Prefer Adher. 2020;14:675–91.
Olmastroni E, Boccalari MT, Tragni E, Rea F, Merlino L, Corrao G, et al. Sex-differences in factors and outcomes associated with adherence to statin therapy in primary care: need for customisation strategies. Pharmacol Res. 2020;155: 104514.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the National Institutes of Health (NIH) National Library of Medicine under Grant number NIH NLM 1R01. The NIH National Library of Medicine funded this research but were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. SG and KO had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
Su Golder, Davy Weissenbacher, Karen O’Connor, Sean Hennessy, Robert Gross, and Graciela Gonzalez Hernandez declare no competing financial or non-financial interests.
Ethics approval
All data used in this study were collected according to the WebMD terms of use and were publicly available at the time of collection and analysis. All quotes were paraphrased. We have an Institutional Review Board certificate of exemption from the University of Pennsylvania.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material/code availability
The drug reviews included in this project are publicly available on WebMD. The coding underpinning this article will be shared upon reasonable request to the corresponding author.
Author contributions
Study conception and design: SG, DW, GG. Data collection: DW. Analysis and interpretation of results: SG, KO. Draft manuscript preparation: SG. Acquisition of funding: GG. All authors read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Golder, S., Weissenbacher, D., O’Connor, K. et al. Patient-Reported Reasons for Switching or Discontinuing Statin Therapy: A Mixed Methods Study Using Social Media. Drug Saf 45, 971–981 (2022). https://doi.org/10.1007/s40264-022-01212-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01212-0