Abstract
Introduction
The Chinese National Medical Products Administration (NMPA) has emphasized proactive pharmacovigilance throughout the product life cycle in recent years. However, the safety-related withdrawal of drugs from the Chinese market has received less attention.
Objectives
The primary aim of the study was to investigate the context of withdrawing a drug for safety reasons in China (between 1999 and 2021).
Methods
Withdrawn drugs were first identified from the Chinese NMPA and United States (US) Food and Drug Administration websites and the World Health Organization’s (WHO’s) consolidated list of products, WHO Drug Information, and WHO Pharmaceuticals Newsletter. We then searched the China National Knowledge Internet database, Chongqing VIP information database, Wanfang database, PubMed, and Google Scholar for drug withdrawal details. We used the Oxford Centre for Evidence-Based Medicine criteria to assess the levels of evidence that support withdrawing a drug.
Results
A total of 30 drugs were withdrawn from the Chinese market between 1999 and 2021. The number of withdrawals increased during the stable Chinese drug surveillance period (2012–2021). Evidence from case-series or case–control studies was primarily used to determine the withdrawals of 16 drugs (53.3%). Fifteen drugs were withdrawn from the markets of China and the US, including five drugs (5/15, 33.3%) that were withdrawn in the same year in China and the US.
Conclusions
The promulgation of regulations and development of advanced passive and active systems have enhanced pharmacovigilance in China. High-quality evidence, coordination with other regulatory authorities, and communication and information sharing should be strengthened to optimize drug safety surveillance and risk management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Thirty drugs were withdrawn from the Chinese market between 1999 and 2021, with 14 drugs withdrawn during the stable Chinese drug surveillance period (2012–2021). |
Robust evidence contributed to the drug withdrawal decisions following the development of pharmacovigilance programs in China. |
The development of regulations and advanced passive and active systems has enhanced the pharmacovigilance in China. |
1 Introduction
The Chinese National Medical Products Administration (NMPA) attaches great importance to public health through adverse drug reaction (ADR) monitoring, safety surveillance, and risk management following drug marketing approval.
ADR monitoring was initiated in 1988 in China, and the National Center for ADR Monitoring, which was established in 1989, is responsible for drug safety [1]. In 1999, the Measures for Monitoring and Management of ADRs (for Trial Implementation) was enacted as the first regulation for post-marketing ADR surveillance in China, and it is devoted to monitoring the safety of post-marketed drugs [2]. Prior to 2003, ADRs were reported to provincial ADR centers via paper reports, fax, or telephone. In 2003, a nationwide China Adverse Drug Reaction Monitoring System (CADRMS), which is an online spontaneous reporting system, was established [3]. In 2016, the NMPA created the China ADR Sentinel Surveillance Alliance (CASSA) program, which provides a solid basis for active drug safety monitoring using electronic medical records [4]. After more than 30 years of development, a relatively mature pharmacovigilance regulation and management system has been established [5].
Post-marketing withdrawal of a drug in China occurs when the drug is suspected to have caused serious adverse reactions, especially when the risks outweigh the benefits, and such withdrawals may represent a measure of successful pharmacovigilance [6]. In the United States (US), 32 drugs were withdrawn between 1975 and 2009 [7]. A recent study stated that 133 drugs were withdrawn from the market worldwide due to safety reasons from 1990 to 2010 [8]. The objective of the present study was to investigate the context of drug withdrawal for safety reasons in China between 1999 and 2021, identify the scientific evidence that leads to drug withdrawals, and perform a comparative analysis of drug safety withdrawals between China and the US.
2 Methods
2.1 Data Source
We chose the study period between January 1999 and December 2021 because it corresponds to China’s modern era of drug surveillance. We categorized the pharmacovigilance development of China into three periods: the initial Chinese drug safety surveillance development period (1999–2004), the rapid Chinese drug safety surveillance development period (2005–2011), and the stable Chinese drug safety surveillance period (2012–2021) [9].
We obtained a list of drugs from the NMPA and US Food and Drug Administration (FDA) websites and the World Health Organization’s (WHO’s) consolidated list of products for which the consumption and/or sale has been banned, withdrawn, severely restricted, or not approved by governments (issues 6, 8, 12, and 14, the updated version of issue 14, 2010–2018), WHO Drug Information (2005–2021), and WHO Pharmaceuticals Newsletter (2002–2021). For each drug withdrawal, we searched the China National Knowledge Internet (CNKI), Chongqing VIP information, and Wanfang databases, which are the three scientific databases in China. We also searched PubMed and Google Scholar for drug withdrawal details.
2.2 Inclusion/Exclusion Criteria
In the present study, the term ‘drug’ means active ingredient. Thus, we did not calculate the total number of marketed drugs and generics, but only the number of active ingredients. We included drugs that were withdrawn by regulatory authorities and those that were previously withdrawn (by regulatory authorities) because of ADRs but then re-introduced with a restriction of use. When only one formulation of a drug was withdrawn, the drug was included in the list, and the formulation was noted; however, if all formulations of the drug were subsequently withdrawn, we used the earliest date, irrespective of formulation, as the year of first withdrawal.
Drugs were excluded for the following reasons: documented evidence is available of voluntary withdrawal by marketing authorization holders (MAHs) for commercial reasons; withdrawal was only based on insufficient efficacy (not due to adverse reactions) or contamination of the active ingredient by other agents; and withdrawal was based on certain indications or specific populations. We also excluded vaccines, traditional Chinese medicine drugs, non-human medicines, and dietary supplements.
2.3 Data Extraction and Analysis
For each withdrawn drug, we extracted the following data: Anatomic Therapeutic Chemical (ATC) classification (first level); year of marketing (year of marketing authorization, launch date, or date of first recorded use); year of withdrawal (year of first withdrawal); and ADRs that were most related to the drug’s withdrawal. The highest quality level of available evidence supporting the withdrawal of drugs was assessed based on the Oxford Centre for Evidence-Based Medicine (OCEBM) criteria. OCEBM criteria are as follows: level 1, systematic reviews (highest); level 2, randomized clinical trials (RCT); level 3, non-randomized, cohort, or follow-up studies; level 4, case-series or case-control studies; and level 5, mechanism-based reasoning (lowest) [10]. One reviewer (YRL) extracted the data, and a second reviewer (YJ) independently verified the data, with discrepancies between the reviewers resolved through discussion. We compared the withdrawal information both available in China and the US for drugs that were withdrawn in China in the study periods. Data were analyzed using descriptive analysis.
3 Results
We identified 30 drugs withdrawn from the Chinese market between 1999 and 2021 (Table 1). The most frequent ATC classification (first level) of these drugs was “Antiinfectives for systemic use” (9/30, 30.0%) (Table 1).
3.1 Number of Drug Withdrawals According to the Study Periods
Fourteen drugs were withdrawn (46.7%) during the stable Chinese drug safety surveillance period (2012–2021), whereas nine and seven drugs were withdrawn (30.0% and 23.3%) during the initial Chinese drug safety surveillance development period (1999–2004) and the rapid Chinese drug safety surveillance development period (2005–2011), respectively (Fig. 1).
3.2 Types of Adverse Drug Reactions
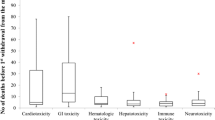
Cardiovascular and hematological toxicities (5/30; 16.7%) were the most reported ADRs that led to withdrawals, followed by drug abuse, neurotoxicity, and carcinogenicity (3/30; 10.0%) (Fig. 2).
3.3 Evidence for Drug Withdrawal
The quality of available evidence that triggered drug withdrawal decisions according to the OCEBM criteria is shown in Fig. 3. Of the 30 included drugs, case-series or case–control studies were used as evidence for withdrawals in 16 drugs (53.3%), while systematic reviews were used in five drug withdrawals (16.7%) (Fig. 3). The OCEBM criteria supporting drug withdrawals during the study periods are shown in Fig. 4. More robust evidence contributed to regulatory decisions following the three periods.
Evidence for drug withdrawal. OCEBM criteria [10]: level 1, systematic reviews; level 2, randomized clinical trials; level 3, non-randomized, cohort, or follow-up studies; level 4, case-series or case–control studies; and level 5, mechanism-based reasoning. OCEBM Oxford Centre for Evidence-Based Medicine
Evidence for drug withdrawal according to the study period. OCEBM criteria [10]: level 1, systematic reviews; level 2, randomized clinical trials; level 3, non-randomized, cohort, or follow-up studies; level 4, case-series or case–control studies; and level 5, mechanism-based reasoning. OCEBM Oxford Centre for Evidence-Based Medicine
3.4 Comparison of Drug Withdrawals in China and the United States
Fifteen drugs were withdrawn in both China and the US. Phenylpropanolamine, aprotinin, pergolide mesylate, tegaserod, and sibutramine (5/15, 33.3%) were withdrawn from the Chinese and US markets in the same year, while dextropropoxyphene was withdrawn from the Chinese market in 2011, which was a year after its withdrawal from the US market (2010) (Table 2).
Figure 5 also provides data on the duration of marketing before drug withdrawal in China and the US. Compared with the US, China had longer exposure durations to seven drugs (aprotinin, fenfluramine, pemoline, phenformin, terfenadine, furazolidone, and oxyphenbutazone) (Fig. 5. We excluded metamizole in Fig. 5 as the marketing year of US was unavailable).
4 Discussion
Drug withdrawal decisions are based on re-evaluations of the benefit-to-risk balance when rare and new ADRs occur following large-scale use after drug approval. Our study showed that a total of 30 drugs were withdrawn in China between 1999 and 2021. The number of drug withdrawals increased during the stable Chinese drug safety surveillance period (2012–2021). Case-series or case–control studies were mostly used as the evidence for withdrawals in 16 drugs (53.3%). Fifteen products were withdrawn from both the Chinese and US markets, including five products (5/15, 33.3%) that were withdrawn in the same year in China and the US.
China has a relatively mature passive spontaneous reporting system that includes a four-level network consisting of one national center, 34 provincial centers, and > 400 municipal centers [9]. Healthcare professionals (physicians, nurses, pharmacists), MAHs, and drug distributors are allowed to report ADRs. ADRs and/or adverse drug events were initially mainly reported by medical institutions, with fewer reports by MAHs and drug distributors. Currently, ADR reporting is mandatory for both MAHs and medical institutions, which is different from that in the US [2, 44]. There is currently no dedicated patient reporting system in China and patients report ADRs mainly through medical institutions or MAHs. The NMPA has recognized the importance of patient reporting and is considering to establish an ADR patient reporting system. CADRMS received a total of 1.962 million ADR reports in 2021, and the average number of reports per million people was 1392 [45]. In 2016, the NMPA created the CASSA program, which provides a solid basis for national active drug safety monitoring with electronic medical records [4]. Provincial active surveillance programs have also been developed in China. For example, the Guangdong Provincial Center for ADR Monitoring has developed an ADR Quick Reporting and Intelligent Scanning System to promote active pharmacovigilance [46]. The combination of active and passive monitoring strengthens the post-marketing surveillance of drugs. The NMPA implements risk management approaches when serious safety issues are identified by post-marketing surveillance, including MAH communication meetings, and these methods include modification of package inserts, restriction of use, and suspension or withdrawal of marketing authorization. A drug is withdrawn from the market when the NMPA determines that the benefit–risk assessment is not acceptable.
It is important to note that the sources of evidence used in our study include evidence originating from the literature, pharmaceutical company notices, and regulatory authority websites. For example, the decision to revoke tegaserod was based on a systematic review of data from 29 premarketing trials by Novartis [23]. Our study found that sixteen withdrawals (53.3%) were supported solely by case-series or case–control studies. In line with our study, Olivier et al. showed that twelve withdrawals in France (57%) were based on spontaneous reports and case series between 1998 and 2004 [47]. Charles et al. reported that most of the medicine withdrawals (63%) from the worldwide market were based on case reports between 1953 and 2014 [48]. However, a recent study suggested that the use of RCTs increased as supporting evidence of withdrawn, revoked, or suspended regulatory actions due to safety reasons increased to 72.2% within the EU in the period from 1 July 2012 to 31 December 2016 [49]. Similarly, more robust evidence has contributed to regulatory decisions following the development of pharmacovigilance programs in China. An example is ketoconazole, which was marketed as an antifungal treatment. After confirming the risk of ketoconazole to the liver, the NMPA issued an ‘ADR Information Bulletin’ to alert the public of this risk in 2002. In 2012, the NMPA warned the public again that ketoconazole can cause severe liver injuries that may potentially result in liver transplantation or death, and it advised patients to monitor liver functions. In 2013, the NMPA added ketoconazole to the list of drugs for intensive ADR monitoring, which strengthened the frequency of monitoring of this drug. Meanwhile, the NMPA commissioned Peking University to re-evaluate the hepatotoxicity of ketoconazole [50]. A systematic review showed that the hepatotoxicity incidence of ketoconazole was 3.6–4.2%, and this value was higher in patients who were prescribed this drug for off-label use [51]. With the above evidence, ketoconazole was finally withdrawn from the Chinese market in 2015. This example implies that a gradual shift has occurred towards using more robust evidence to support drug withdrawal regulatory actions.
Our study suggested that five drugs were withdrawn from the Chinese and US markets in the same year, which may be explained by information sharing between international countries. For example, based on the result of the Sibutramine Cardiovascular Outcome Trial (SCOUT) study, the NMPA concluded that the benefits of sibutramine do not outweigh the cardiovascular risks and recommended the withdrawal of sibutramine in China in the same year as it was withdrawn in the US [26]. In 1998, the National Center for ADR Monitoring became a member of the WHO International Drug Monitoring Program [52]. Then the National Center for ADR Monitoring began reporting ADRs to the WHO center, and coordinated with other WHO members in Uppsala. The drug regulatory authorities work together to ensure that regulatory authorities are continuously updated on any emerging safety issue. Delays until drug withdrawal have been observed in China because drug withdrawal decisions depend on the duration before drug withdrawal, the number of patients exposed to unsafe drugs, the indications for therapy, the frequency of the ADRs, the severity of the ADRs, the benefits of the drug involved, and the availability of safer alternative drugs [50, 53,54,55]. Other possible explanations for the different drug withdrawal times between China and the US include the different regulatory systems, different economic considerations, and different willingness of the regulatory authorities to act [6, 7].
This study is the first study to report on drug withdrawals for safety reasons in China. We used robust methods to search for drugs withdrawn for safety reasons and assessed data from a variety of sources. However, our study also had certain limitations. First, despite our intensive effort to conduct an exhaustive review of all available data, errors may have occurred because we only analyzed pharmacovigilance public data and data obtained from indirect sources (e.g., year of marketing) such as the published literature. In addition, we did not provide the number of drugs withdrawn during the three time periods as a percentage of the number of drugs approved during the three periods because we could not obtain the number of drugs approved before 2012, due to opaque information. Furthermore, although post-marketing drug withdrawals can be a measure of successful pharmacovigilance, they may also indicate a failure of the drug approval system to identify safety issues in the pre-market phase. The relationship between post-marketing drug withdrawals and China’s drug approval system is an important issue, and further investigations should be performed.
5 Conclusions
This study describes drugs that were withdrawn from the market in China for safety reasons. The promulgation of regulations and development of advanced passive and active systems have enhanced pharmacovigilance in China. High-quality evidence, coordination with other regulatory authorities, and communication and information sharing should be strengthened to optimize drug safety surveillance and risk management. Moreover, active pharmacovigilance has now been introduced into monitoring and evaluation systems based on the integration of multiple databases, which can facilitate the early detection of drug safety risks and establish high-quality evidence for regulatory decision-making.
References
Li LS, Yin J. Drug safety evaluation in China. Curr Allergy Asthma Rep. 2019;19(9):39. https://doi.org/10.1007/s11882-019-0872-4.
Ministry of Health (MOH), China Food and Drug Administration (CFDA), Measures for Monitoring and Management of ADRs (for Trial Implementation). 2011. https://www.nmpa.gov.cn/xxgk/fgwj/bmgzh/20110504162501325.html. Accessed 12 Apr 2022.
Zhang L, Yan JB, Liu XM, et al. Pharmacovigilance practice and risk control of traditional Chinese medicine drugs in China: current status and future perspective. J Ethnopharmacol. 2012;140(3):519–25. https://doi.org/10.1016/j.jep.2012.01.058.
Zhao Y, Wang TS, Li GY, et al. Pharmacovigilance in China: development and challenges. Int J Clin Pharm. 2018;40(4):823–31. https://doi.org/10.1007/s11096-018-0693-x.
Biswas P. Pharmacovigilance in Asia. J Pharmacol Pharmacother. 2013;4(Suppl 1):S7–19. https://doi.org/10.4103/0976-500X.120941.
Aronson JK. Post-marketing drug withdrawals: pharmacovigilance success, regulatory problems. Therapie. 2017;72(5):555–61. https://doi.org/10.1016/j.therap.2017.02.005.
Frank C, Himmelstein DU, Woolhandler S, et al. Era of faster FDA drug approval has also seen increased black-box warnings and market withdrawals. Health Aff (Millwood). 2014;33(8):1453–9. https://doi.org/10.1377/hlthaff.2014.0122.
Craveiro NS, Lopes BS, Tomás L, et al. Drug withdrawal due to safety: a review of the data supporting withdrawal decision. Curr Drug Saf. 2020;15(1):4–12. https://doi.org/10.2174/1574886314666191004092520.
Zhang L, Wong LYL, He Y, et al. Pharmacovigilance in China: current situation, successes and challenges. Drug Saf. 2014;37(10):765–70. https://doi.org/10.1007/s40264-014-0222-3.
Onakpoya IJ, Heneghan CJ, Aronson JK, et al. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC Med. 2016;14:10. https://doi.org/10.1186/s12916-016-0553-2.
Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343(25):1826–32. https://doi.org/10.1056/NEJM200012213432501.
Hu RJ, Zhan SZ, Wu GL, et al. Epidemiological investigation on acute leukemia induced by ethylimine and bimolane for psoriasis. Tianjin Med J. 1989;02:118–20.
Xue YQ, Lu D, Guo Y, et al. Specific chromosomal translocations and therapy-related leukemia induced by bimolane therapy for psoriasis. Leuk Res. 1992;16(11):1113–23. https://doi.org/10.1016/0145-2126(92)90050-h.
Mi FN, Yin DH, Liu CL, et al. Acute agranulocytosis induced by ethoxide: a case report. J Mudanjiang Med Univ. 1988;03:001. https://doi.org/10.13799/j.cnki.mdjyxyxb.1988.03.001.
Golanov VS, Ia VV. Agranulocytic reaction following the use of ethoxide in a patient with pulmonary tuberculosis. Vrach Delo. 1975;3:88–9.
Li ZS. Ototoxicity of systemic ototoxic antibiotics. Chin J Med. 1991;02:4–7.
Duan YY, Zhang Y, Bao YY, et al. Clinical countermeasure against adverse drug reaction of antimicrobial agents. Chin J Antibio. 2002;27(5):316–20.
Ma JN, Li SS, Wang X, et al. Deafness induced by ototoxic antibiotics: 253 case reports. Chin J Appl Clin Pediatr. 1991;6(6):321.
De Bont B, Lauwerys R, Govaerts H, et al. Yellow mercuric oxide ointment and mercury intoxication. Eur J Pediatr. 1986;145(3):217–8. https://doi.org/10.1007/BF00446069.
Lu ZT. Summary of adverse drug reactions of WHO Drug Information. Chin Pharm Aff. 1990;S2:104–28.
National Medical Products Administration (NMPA). Notice on temporarily suspending the sales and use of aprotinin. 2007. https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjyp/20071217174401700.html. Accessed 12 Apr 2022.
Waller EA, Kaplan J, Heckman MG. Valvular heart disease in patients taking pergolide. Mayo Clin Proc. 2005;80(8):1016–20. https://doi.org/10.4065/80.8.1016.
World Health Organization (WHO). WHO Pharmaceutical Newsletter. No. 2, 2007. 2007. https://apps.who.int/iris/handle/10665/255709?search-result=true&query=WHO+pharmaceuticals+newsletter&scope=&rpp=10&sort_by=score&order=desc&page=9. Accessed 12 Apr 2022.
Loke YK, Derry S, Pritchard-Copley A, et al. Appetite suppressants and valvular heart disease—a systematic review. BMC Clin Pharmacol. 2002;2:6. https://doi.org/10.1186/1472-6904-2-6.
McCann UD, Seiden LS, Rubin LJ, et al. Brain serotonin neurotoxicity and primary pulmonary hypertension from fenfluramine and dexfenfluramine. A systematic review of the evidence. JAMA. 1997;278(8):666–72.
National Medical Products Administration (NMPA). Notice on withdrawing the production and sales of sibutramine. 2010. https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/fgwj/gzwj/gzwjyp/20101030110901266.html. Accessed 12 Apr 2022.
US Food and Drug Administration (FDA). FDA Drug Safety Communication: FDA recommends against the continued use of propoxyphene. 2010. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-recommends-against-continued-use-propoxyphene. Accessed 12 Apr 2022.
National Medical Products Administration (NMPA). Notice on withdrawing the production and sales of clenbuterol hydrochloride. 2011. https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/fgwj/gzwj/gzwjyp/20110923105801254.html. Accessed 12 Apr 2022.
Bucolo C, Longo L, Camillieri G, et al. Safety profile assessment of buflomedil: an overview of adverse reactions between 1975 and 2011. Pharmacoepidemiol Drug Saf. 2012;21(11):1190–6. https://doi.org/10.1002/pds.3328.
Mounier B, Pons B, Delavenne X, et al. Severe meprobamate poisoning: description of 146 cases in a French department. Therapie. 2012;67(2):183–9. https://doi.org/10.2515/therapie/2012019.
Yan JY, Nie XL, Tao QM, et al. Ketoconazole associated hepatotoxicity: a systematic review and meta-analysis. Biomed Environ Sci. 2013;26(7):605–10. https://doi.org/10.3967/0895-3988.2013.07.013.
Wang D, Yang Y, Song QJ, et al. Liver damage induced by pemoline and post-marketing actions on it. Chin J Pharmacov. 2006;3(1):36–48.
Escousse A, Jean-Pastor MJ, Kreft-Jais C, et al. Retrospective of national pharmacovigilance surveys on drug-induced bullous, vesicular eruptions: methods and results. Therapie. 2002;57(3):269–72.
Cavallo-Perin P, Aluffi E, Estivi P, et al. The hyperlactatemic effect of biguanides: a comparison between phenformin and metformin during a 6-month treatment. Riv Eur Sci Med Farmacol. 1989;11(1):45–9.
Smirnova LI. Drug agranulocytosis. Feldsher Akush. 1981;46(9):58–62.
Hanrahan JP, Choo PW, Carlson W, et al. Terfenadine-associated ventricular arrhythmias and QTc interval prolongation. A retrospective cohort comparison with other antihistamines among members of a health maintenance organization. Ann Epidemiol. 1995;5(3):201–9. https://doi.org/10.1016/1047-2797(94)00039-v.
Tholen S. Drug-induced pemphigus. Z Hautkr. 1986;61(10):719–23.
Guan L, Chen J, Wu XA, et al. Systematic evaluation of nervous toxicity and mental disorders induced by furazolidone. Chin J New Drugs Clin Rem. 2015;34(6):436–40. https://doi.org/10.14109/j.cnki.xyylc.2015.06.008.
Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146(9):657–65. https://doi.org/10.7326/0003-4819-146-9-200705010-00009.
Chaplin S. Bone marrow depression due to mianserin, phenylbutazone, oxyphenbutazone and chloramphenicol–part 1. Adv Drug React Ac Pois Rev. 1986;5(2):97–136.
Borodaĭ EM, Davydovskaia EP, Golotenko NI. Case of combined acute methemoglobinemia and agranulocytosis caused by the intake of sulfadimezine. Vrach Delo. 1976;1:122–3.
Smirnov AN. Acute drug-induced hemolytic anemia. Feldsher Akush. 1982;47(8):54–8.
Dunnick JK, Hailey JR. Phenolphthalein exposure causes multiple carcinogenic effects in experimental model systems. Cancer Res. 1996;56(21):4922–6.
National Medical Products Administration (NMPA). Announcement on the issuance of the Good Pharmacovigilance Practice. 2021. https://www.nmpa.gov.cn/yaopin/ypggtg/20210513151827179.html. Accessed 12 Apr 2022.
National Medical Products Administration (NMPA). Annual Report for National Adverse Drug Reaction Monitoring. 2021. https://www.cdr-adr.org.cn/drug_1/aqjs_1/drug_aqjs_sjbg/202203/t20220330_49586.html. Accessed 12 Apr 2022.
Li XL, Li HN, Deng JX, et al. Active pharmacovigilance in China: recent development and future perspectives. Eur J Clin Pharmacol. 2018;74(7):863–71. https://doi.org/10.1007/s00228-018-2455-z.
Olivier P, Montastruc JL. The nature of the scientific evidence leading to drug withdrawals for pharmacovigilance reasons in France. Pharmacoepidemiol Drug Saf. 2006;15(11):808–12. https://doi.org/10.1002/pds.1248.
Charles O, Onakpoya I, Benipal S, et al. Withdrawn medicines included in the essential medicines lists of 136 countries. PLoS ONE. 2019;14(12): e0225429. https://doi.org/10.1371/journal.pone.0225429.
Lane S, Lynn E, Shakir S. Investigation assessing the publicly available evidence supporting postmarketing withdrawals, revocations and suspensions of marketing authorisations in the EU since 2012. BMJ Open. 2018;8(1): e019759. https://doi.org/10.1136/bmjopen-2017-019759.
Wang D, Wu GZ, Nie XL. Reasons for Nizoral withdrawal from the market and reflections on risk management. Chin J Pharmacov. 2014;11(9):550–3. https://doi.org/10.19803/j.1672-8629.2014.09.010.
Tao QM, Nie XL, Cheng G, et al. Systematic review of oral ketoconazole adverse reactions and hepatotoxicity incidence. Chin J Pharmacov. 2013;10(12):719–22. https://doi.org/10.19803/j.1672-8629.2013.12.005.
World Health Organization (WHO). The WHO Programme for International Drug Monitoring. 2022. https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/health-professionals-info/pidm. Accessed 12 Apr 2022.
Onakpoya IJ, Heneghan CJ, Aronson JK, et al. Delays in the post-marketing withdrawal of drugs to which deaths have been attributed: a systematic investigation and analysis. BMC Med. 2015;13:26. https://doi.org/10.1186/s12916-014-0262-7.
Bakke OM, Wardell WM, Lasagna L. Drug discontinuations in the United Kingdom and the United States, 1964 to 1983: issues of safety. Clin Pharmacol Ther. 1984;35(5):559–67. https://doi.org/10.1038/clpt.1984.78.
Schick A, Miller KL, Lanthier M, et al. Evaluation of pre-marketing factors to predict post-marketing boxed warnings and safety withdrawals. Drug Saf. 2017;40(6):497–503. https://doi.org/10.1007/s40264-017-0526-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Yanrong Li, Yang Jiang, Haixue Wang, Li Zhang, and Yue Yang have no conflicts of interest that are directly relevant to the content of this study.
Author contributions
All of the authors contributed as follows: YY developed the study protocol; YRL and YJ collected data and did analysis; YRL wrote the article; YJ, HXW, LZ, and YY reviewed the article before submission. All authors read and approved the final version.
Ethics approval
Not applicable because the data in this article were provided by public websites and scientific literature.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Informed consent
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, Y., Jiang, Y., Wang, H. et al. Safety-Related Drug Withdrawals in China Between 1999 and 2021: A Systematic Investigation and Analysis. Drug Saf 45, 737–745 (2022). https://doi.org/10.1007/s40264-022-01185-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01185-0