Abstract
Introduction
Stevens–Johnson syndrome and toxic epidermal necrolysis have been associated with the use of various drugs, but evidence is scarce. We studied the association between new use of outpatient drugs other than anti-epileptic drugs and antibiotics and Stevens–Johnson syndrome and toxic epidermal necrolysis.
Methods
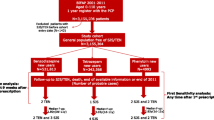
We conducted a matched (1:4) case–control analysis in 480 previously validated Stevens–Johnson syndrome/toxic epidermal necrolysis cases (1995–2013). We calculated odds ratios with 95% confidence intervals for Stevens–Johnson syndrome/toxic epidermal necrolysis in new users of drugs compared to non-users. For cases of Stevens–Johnson syndrome/toxic epidermal necrolysis diagnosed ≤ 84 days after the first use of a drug, we assessed causality between drug exposure and Stevens–Johnson syndrome/toxic epidermal necrolysis using ALDEN (algorithm of drug causality in epidermal necrolysis). We calculated absolute risks by dividing the number of Stevens–Johnson syndrome/toxic epidermal necrolysis cases ≤ 84 days after new drug exposure by the total number of new users of the drug.
Results
There was an association between Stevens–Johnson syndrome/toxic epidermal necrolysis and the use of allopurinol (odds ratio 24.51, 95% confidence interval 2.94–204.04) and cyclooxygenase-2 inhibitors (odds ratio 24.19, 95% confidence interval 2.91–200.92). Proton pump inhibitors, fluoxetine, mirtazapine, and 5-aminosalicylates (sulfasalazine, mesalamine) were also associated with an increased risk of Stevens–Johnson syndrome/toxic epidermal necrolysis, though with lower odds ratios. ALDEN score application suggests a likely causality for these associations. Absolute risks of Stevens–Johnson syndrome/toxic epidermal necrolysis were 6.0/100,000 new users for allopurinol, and 1.9–4.3/100,000 new users for cyclooxygenase-2 inhibitors and 5-aminosalicylates, and 0.2–1.6/100,000 new users for proton pump inhibitors, fluoxetine, and mirtazapine. We found no association between Stevens–Johnson syndrome/toxic epidermal necrolysis and oxicams, benzodiazepines, citalopram, sertraline, paroxetine, venlafaxine, and phosphodiesterase-5 inhibitors despite > 100,000 new users.
Conclusions
In this observational study, we observed likely causal associations between Stevens–Johnson syndrome/toxic epidermal necrolysis and use of allopurinol, cyclooxygenase-2 inhibitors, and 5-aminosalicylates, and potential associations for proton pump inhibitors, fluoxetine, and mirtazapine.

Similar content being viewed by others
References
Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015 Jul;13(7):625–45.
Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens-Johnson syndrome: a review. Crit Care Med. 2011;39(6):1521–32.
Harr T, French LE. Stevens-Johnson syndrome and toxic epidermal necrolysis. Chem Immunol Allergy. 2012 Jan;97:149–66.
Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008 Jan;128(1):35–44.
Roujeau J, Kelly J, Naldi L, Rzany B, Stern R, Anderson T, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333(24):1600–7.
Jonsson GW, Moosa MY, Jeenah FY. Toxic epidermal necrolysis and fluoxetine: a case report. J Clin Psychopharmacol. 2008;28(1):93–5.
Wolkenstein P, Revuz J, Diehl J, Langeron O, Roupie E, Machet L. Toxic epidermal necrolysis after fluvoxamine. Lancet. 1993;342(8866):304–5.
Tudela E, Villier C, Mallaret M. Toxic epidermal necrolysis associated with paroxetine. Gen Hosp Psychiatry. 2009 Jan;31(3):297–8.
Belkahia A, Hillaire-Buys D, Dereure O, Guillot B, Raison-Peyron N. Stevens-Johnson syndrome due to mirtazapine: first case. Allergy. 2009 Oct;64(10):1554.
Strawn J, Whitsel R, Nandagopal J, Delbello M. Atypical Stevens-Johnson syndrome in an adolescent treated with duloxetine. J Child Adolesc Psychopharmacol. 2011;21(1):91–2.
Weiss N, Jones L, Chamberlain J. A possible case of venlafaxine-induced Stevens-Johnson syndrome. J Clin Psychiatry. 2004;65(10):1431–3.
Casacci M, Lebas D, Decamps F, Fourrier F, Delaporte E. Toxic epidermal necrolysis due to omeprazole. Eur J Dermatol. 2006;16(6):699–700.
Heaton N, Edmonds E, Francis N, Bunker C, Bowling J, Morar N. Fatal toxic epidermal necrolysis due to lansoprazole. Clin Exp Dermatol. 2004;29(6):612–3.
Moubayed D, Gifuni A, Tourian L. Methotrimeprazine-associated Stevens-Johnson syndrome in 2 Ashkenazi Jewish patients. J Clin Psychopharmacol. 2017;37(1):112–3.
Kumar P, Chauhan A, Haraniya R, Ghosh A, Tandon V. Metolazone associated Stevens Johnson syndrome-toxic epidermal necrolysis overlap. J Clin Diagn Res. 2016;10(3):01–2.
Jao T, Tsai T-H, Jeng J-S. Aggrenox (Asasantin retard)-induced Stevens-Johnson syndrome. Br J Clin Pharmacol. 2009 Feb;67(2):264–5.
Struye A, Depuydt C, Abdel-Sater E, Dubois V. Toxic epidermal necrolysis related to paliperidone palmitate: first case report. J Clin Psychopharmacol. 2016;36(3):279–82.
Yang C-Y, Chen C-H, Wang H-Y, Hsiao H-L, Hsiao Y-H, Chung W-H. Strontium ranelate related Stevens-Johnson syndrome: a case report. Osteoporos Int. 2014 Jun;25(6):1813–6.
Surovik J, Riddel C, Chon S. A case of bupropion-induced Stevens-Johnson syndrome with acute psoriatic exacerbation. J Drugs Dermatol. 2010;9(8):1010–2.
Pretel-Irazabal M, Marquez-Martin L, Aguado-Gil L, Idoate-Gastearena M. Tranexamic acid-induced toxic epidermal necrolysis. Ann Pharmacother. 2013;47(3):e16.
Fukunaga K, Ohda Y, Inoue T, Kono T, Miwa H, Matsumoto T. Toxic epidermal necrosis associated with mesalamine in a patient with ulcerative colitis. Inflamm Bowel Dis. 2007 Aug;13(8):1055–6.
La Grenade L, Lee L, Weaver J, Bonnel R, Karowski C, Governale L, et al. Comparison of reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis in association with selective COX-2 inhibitors. Drug Saf. 2005;28(10):917–24.
Delesalle F, Carpentier O, Guatier S, Delaporte E. Toxic epidermal necrolysis caused by tetrazepam. Int J Dermatol. 2006;45(4):480.
Haddad C, Sidoroff A, Kardaun SH, Mockenhaupt M, Creamer D, Dunant A, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: are drug dictionaries correctly informing physicians regarding the risk? Drug Saf. 2013;36(8):681–6.
Frey N, Bodmer M, Bircher A, Rüegg S, Jick SS, Meier CR, et al. The risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptic drugs. Epilepsia. 2017;58(12):2178–85.
Frey N, Bircher A, Bodmer M, Jick SS, Meier CR, Spoendlin J. Antibiotic drug use and the risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: a population-based case-control study. J Invest Dermatol. 2018;138(5):1207–9.
Herrett E, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–36.
Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010 Jan;69(1):4–14.
Frey N, Bircher A, Bodmer M, Jick SS, Meier CR, Spoendlin J. Validation of Stevens-Johnson syndrome or toxic epidermal necrolysis diagnoses in the Clinical Practice Research Datalink. Pharmacoepidemiol Drug Saf. 2017;26(4):429–36.
Lambertini M, Del Mastro L, Gardin G, Levaggi A, Bighin C, Giraudi S, et al. Stevens-Johnson syndrome after treatment with bendamustine. Leuk Res. 2012;36(7):153–4.
Doesch J, Debus D, Meyer C, Papadopoulos T, Schultz ES, Ficker JH, et al. Afatinib-associated Stevens-Johnson syndrome in an EGFR-mutated lung cancer patient. Lung Cancer. 2016;95:35–8.
Ikeda M, Fujita T, Amoh Y, Mii S, Matsumoto K, Iwamura M. Stevens-Johnson syndrome induced by sorafenib for metastatic renal cell carcinoma. Urol Int. 2013;91(4):482–3.
Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case–control analysis. Clin Pharmacol Ther. 2010;88(1):60–8.
Mockenhaupt M, Messenheimer J, Tennis P, Schlingmann J. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics. Neurology. 2005 Apr 12;64(7):1134–8.
Miliszewski MA, Kirchhof MG, Sikora S, Papp A, Dutz JP. Stevens-Johnson syndrome and toxic epidermal necrolysis: an analysis of triggers and implications for improving prevention. Am J Med. 2016;129(11):1221–5.
Frey N, Jossi J, Bodmer M, Bircher A, Jick SS, Meier CR, et al. The epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in the UK. J Invest Dermatol. 2017;137(6):1240–7.
Burns RA, Topoz I, Reynolds SL. Tumor lysis syndrome: risk factors, diagnosis, and management. Pediatr Emerg Care. 2014;30(8):571–6.
de Souto Barreto P, Lapeyre-Mestre M, Mathieu C, Piau C, Bouget C, Cayla F, et al. Prevalence and associations of the use of proton-pump inhibitors in nursing homes: a cross-sectional study. J Am Med Dir Assoc. 2013;14(4):265–9.
Creamer D, Walsh SA, Dziewulski P, Exton LS, Lee HY, Dart JKG, et al. UK guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br J Dermatol. 2016;174(6):1194–227.
Dang CD, Beets-Shay L, Kahn EC. Toxic epidermal necrolysis triggered by clobazam: a case report in a 13-year-old girl. Pediatr Dermatol. 2015;32(3):e102–3.
Sánchez I, García-Abujeta JL, Fernández L, Rodríguez F, Quiñones D, Duque S, et al. Stevens-Johnson syndrome from tetrazepam. Allergol Immunopathol (Madr). 1998;26(2):55–7.
Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol. 2016;136:1387–97.
Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schroder W, Roujeau J-C. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Arch Dermatol. 2002;138(8):1019–24.
Acknowledgments
We thank Pascal Egger for programming and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the University of Basel Science Foundation (Grant No. DMS2297).
Conflict of Interest
Noel Frey, Michael Bodmer, Andreas Bircher, Susan S. Jick, Christoph R. Meier, and Julia Spoendlin have no conflicts of interest that are directly relevant to the content of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Frey, N., Bodmer, M., Bircher, A. et al. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis in Association with Commonly Prescribed Drugs in Outpatient Care Other than Anti-Epileptic Drugs and Antibiotics: A Population-Based Case–Control Study. Drug Saf 42, 55–66 (2019). https://doi.org/10.1007/s40264-018-0711-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0711-x