Abstract
Introduction
Algorithmic Standardised MedDRA® Queries (aSMQs) are increasingly used to enhance the efficiency of safety signal detection. The manner that aSMQs affect capture of potential safety cases is unclear.
Objectives
Our objective was to characterise the performance of aSMQs with respect to their potential for double counting, the likelihood of events in aSMQ positive cases being clinically related, how frequently terms are used for algorithmically positive cases, and the face validity of positive cases based on the drug inducing events. We were also interested in what effect requiring symptoms to overlap temporally would have on performance.
Methods
We reviewed adverse event (AE) datasets of New Drug Applications and Biological License Applications and compiled a database including preferred terms and corresponding SMQs, SMQ term categories, AE start day, AE duration, drug name, and Anatomical Therapeutic Chemical class. Two reviewers independently determined if the algorithm was met and, if so, whether the broad terms overlapped temporally.
Results
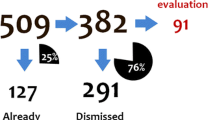
A total of 107 marketing applications were reviewed, including 103,928 patients and 277,430 AEs. Use of algorithms condensed the number of AEs to between 5 and 8% and the incidence to about 1.5% relative to when the SMQs are used without the algorithm. Certain aSMQs exhibited a potential for overcounting. Requiring symptoms to temporally overlap helped to eliminate irrelevant cases.
Conclusions
Our findings demonstrate that algorithmic and temporal assessment increased specificity of case retrieval, though the reduction in the number of terms or incidence seemed excessive for certain aSMQs. Evaluating the day of AE onset and duration improve specificity through identification of outlying events. Identification of drug classes known to cause the aSMQ’s clinical condition provides face validity for this tool, yet detection of cases associated with novel classes may provide new understanding of these disorders. Improvements in some of the SMQ term lists may improve the performance of SMQs in general.

Similar content being viewed by others
Notes
“MedDRA® the Medical Dictionary for Regulatory Activities terminology is the international medical terminology developed under the auspices of the ICH. MedDRA® trademark is owned by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) on behalf of ICH”.
References
MedDRA Maintenance and Support Services Organization. Coding with MedDRA found at the URL https://www.meddra.org/sites/default/files/training/file/000133_coding_with_meddra_f2f_course_7.pdf. Accessed 15 Jun 2018.
MedDRA Maintenance and Support Services Organization. Introductory guide for Standardised MedDRA Queries (SMQs) Version 20.1. Accessed 15 Jun 2018. In: The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, editor. Geneva, Switzerland 2017.
Chang LC, Mahmood R, Qureshi S, Breder CD. Patterns of use and impact of standardised MedDRA query analyses on the safety evaluation and review of new drug and biologics license applications. PLoS One. 2017;12(6):e0178104.
Botsis T, Woo EJ, Ball R. Application of information retrieval approaches to case classification in the vaccine adverse event reporting system. Drug Saf. 2013;36(7):573–82.
Li XJ, Cooper C, Luo Z. MAED Service: FDA-developed tool for clinical AE data signal detection. Computational Science Symposium; March 18th–19th, 2013; Silver Spring, MD 2013.
Douros A, Bronder E, Andersohn F, Klimpel A, Thomae M, Ockenga J, et al. Drug-induced acute pancreatitis: results from the hospital-based Berlin case–control surveillance study of 102 cases. Aliment Pharmacol Ther. 2013;38(7):825–34.
Nitsche CJ, Jamieson N, Lerch MM, Mayerle JV. Drug induced pancreatitis. Best Pract Res Clin Gastroenterol. 2010;24(2):143–55.
Webb LM, Lieberman P. Anaphylaxis: a review of 601 cases. Ann Allergy Asthma Immunol. 2006;97(1):39–43.
Bousquet PJ, Kvedariene V, Co-Minh HB, Martins P, Rongier M, Arnoux B, et al. Clinical presentation and time course in hypersensitivity reactions to beta-lactams. Allergy. 2007;62(8):872–6.
Bircher AJ, Scherer K. Delayed cutaneous manifestations of drug hypersensitivity. Med Clin N Am. 2010;94(4):711–25.
Michavila Gomez AV, Belver Gonzalez MT, Alvarez NC, Giner Munoz MT, Hernando Sastre V, Porto Arceo JA, et al. Perioperative anaphylactic reactions: review and procedure protocol in paediatrics. Allergol Immunopathol (Madr). 2015;43(2):203–14.
Patel RJ, Saylor T, Williams SR, Clark RF. Prevalence of autonomic signs and symptoms in antimuscarinic drug poisonings. J Emerg Med. 2004;26(1):89–94.
Hall RCW, Fox J, Stickney SK, Gardner ER, Perl M. Anticholinergic delirium—etiology, presentation, diagnosis and management. J Psychedelic Drugs. 1978;10(3):237–41.
Tazelaar HD, Linz LJ, Colby TV, Myers JL, Limper AH. Acute eosinophilic pneumonia: histopathologic findings in nine patients. Am J Respir Crit Care Med. 1997;155(1):296–302.
Jhun BW, Kim SJ, Son RC, Yoo H, Jeong BH, Chung MP, et al. Clinical outcomes in patients with acute eosinophilic pneumonia not treated with corticosteroids. Lung. 2015;193(3):361–7.
Giovannini-Chami L, Blanc S, Hadchouel A, Baruchel A, Boukari R, Dubus JC, et al. Eosinophilic pneumonias in children: a review of the epidemiology, diagnosis, and treatment. Pediatr Pulmonol. 2016;51(2):203–16.
Musselman ME, Saely S. Diagnosis and treatment of drug-induced hyperthermia. Am J Health Syst Pharm. 2013;70(1):34–42.
Burkhard PR. Acute and subacute drug-induced movement disorders. Parkinsonism Relat Disord. 2014;20(Suppl 1):S108–12.
Criscuolo M, Fianchi L, Dragonetti G, Pagano L. Tumor lysis syndrome: review of pathogenesis, risk factors and management of a medical emergency. Expert Rev Hematol. 2016;9(2):197–208.
Acknowledgements
We thank Ms. Joean James and Ms. Gretchen Kindle for their administrative support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Carolyn Tieu has no conflicts of interest that are directly relevant to the content of this study. Christopher Breder is a medical officer at the US Food and Drug Administration (FDA); he is also the FDA Topic Expert in the M1 Points to Consider Group.
Funding
Dr. Tieu’s fellowship was funded by an Oak Ridge Institute Science and Education Grant # RSR-702. The funding source had no role in the design and conduct of the study, analysis or interpretation of the data, and preparation or final approval of the manuscript prior to publication.
Disclaimer
The views expressed in this paper are the authors’ views and do not necessarily represent those of the FDA or the ICH.
Rights and permissions
About this article
Cite this article
Tieu, C., Breder, C.D. A Critical Evaluation of Safety Signal Analysis Using Algorithmic Standardised MedDRA Queries. Drug Saf 41, 1375–1385 (2018). https://doi.org/10.1007/s40264-018-0706-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0706-7