Abstract
Introduction
Non-steroidal anti-inflammatory drugs are associated with a dose and duration-dependent coronary risk. There is little information concerning analgesic-dose ibuprofen, among the most widely used drugs worldwide.
Objective
Our objective was to measure the risks of acute coronary syndrome (ACS) after dispensing of ibuprofen, versus paracetamol.
Methods
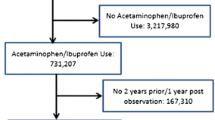
Propensity score 1:2-matched cohorts of ibuprofen or paracetamol treatment episodes (TEs) in Echantillon Généraliste de Bénéficiaires (EGB), the 1/97 sample of Système National des Données de Santé (SNDS), the French nationwide claims database, from 2009 to 2014, were compared. Outcomes were hospital admissions for ACS during the 3 months after the dispensing of ibuprofen or paracetamol. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated overall and stratified on low-dose aspirin dispensing.
Results
A total of 315,269 ibuprofen TEs in 168,400 persons were matched to 630,457 paracetamol TEs in 395,952 patients. Event rates were 50–100 times higher in low-dose aspirin users (27 vs 0.28 per 1000 patient years). Overall there was no difference in risk of ACS at 3 months (HR 0.94, 95% CI 0.74–1.20) despite a transient increase in the first 2 weeks in ibuprofen users (HR 1.70, 95% CI 1.11–2.59). In the stratified analysis, this short-term risk was only found in aspirin users (5% of population, HR 1.84, 95% CI 1.24–3.24), but not in non-aspirin users (HR 1.09, 95% CI 0.40–2.94).
Conclusions
There was no evidence for an increased risk of ACS in patients dispensed ibuprofen compared to paracetamol.



Similar content being viewed by others
References
Coxib and traditional NSAID Trialists (CNT) Collaboration, Bhala N, Emberson J, Merhi A, Abramson S, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–79.
Garcia Rodriguez LA, Tacconelli S, Patrignani P. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J Am Coll Cardiol. 2008;52(20):1628–36.
Varas-Lorenzo C, Riera-Guardia N, Calingaert B, Castellsague J, Salvo F, Nicotra F, et al. Myocardial infarction and individual nonsteroidal anti-inflammatory drugs meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. 2013;22(6):559–70.
Bally M, Dendukuri N, Rich B, Nadeau L, Helin-Salmivaara A, Garbe E, et al. Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ. 2017;9(357):j1909.
Lindhardsen J, Gislason GH, Jacobsen S, Ahlehoff O, Olsen AM, Madsen OR, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis. 2014;73(8):1515–21.
Liao KP, Solomon DH. Traditional cardiovascular risk factors, inflammation and cardiovascular risk in rheumatoid arthritis. Rheumatology (Oxf). 2013;52(1):45–52.
Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010;87(4):407–16.
Gonzalez-Gay MA, Gonzalez-Juanatey C. Inflammation: NSAIDs and cardiovascular risk in arthritis. Nat Rev Cardiol. 2017;14(2):69–70.
Pontes C, Marsal JR, Elorza JM, Aragon M, Prieto-Alhambra D, Morros R. Analgesic use and risk for acute coronary events in patients with osteoarthritis: a population-based, nested case-control study. Clin Ther. 2018;40(2):270–83.
Garcia-Poza P, de Abajo FJ, Gil MJ, Chacon A, Bryant V, Garcia-Rodriguez LA. Risk of ischemic stroke associated with non-steroidal anti-inflammatory drugs and paracetamol: a population-based case-control study. J Thromb Haemost. 2015;13(5):708–18.
Spiler NM, Rork TH, Merrill GF. An old drug with a new purpose: cardiovascular actions of acetaminophen (paracetamol). Curr Drug Targets Cardiovasc Haematol Disord. 2005;5(5):419–29.
Moore RA, Wiffen PJ, Derry S, Maguire T, Roy YM, Tyrrell L. Non-prescription (OTC) oral analgesics for acute pain—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;11(11):CD010794.
Depont F, Fourrier A, Merliere Y, Droz C, Amouretti M, Begaud B, et al. Channelling of COX-2 inhibitors to patients at higher gastrointestinal risk but not at lower cardiovascular risk: the Cox2 inhibitors and tNSAIDs description of users (CADEUS) study. Pharmacoepidemiol Drug Saf. 2007;16(8):891–900.
Duong M, Salvo F, Pariente A, Abouelfath A, Lassalle R, Droz C, et al. Usage patterns of ‘over-the-counter’ vs. prescription-strength nonsteroidal anti-inflammatory drugs in France. Br J Clin Pharmacol. 2014;77(5):887–95.
Hasford J, Moore N, Hoye K. Safety and usage pattern of low-dose diclofenac when used as an over-the-counter medication: results of an observational cohort study in a community-based pharmacy setting. Int J Clin Pharmacol Ther. 2004;42(8):415–22.
Moore N. Place of OTC analgesics and NSAIDs in osteoarthritis. Inflammopharmacology. 2003;11(4):355–62.
Moore N, Salvo F, Duong M, Blin P, Pariente A. Cardiovascular risks associated with low-dose ibuprofen and diclofenac as used OTC. Expert Opin Drug Saf. 2014;13(2):167–79.
Duong M, Gulmez SE, Salvo F, Abouelfath A, Lassalle R, Droz C, et al. Usage patterns of paracetamol in France. Br J Clin Pharmacol. 2016;82(2):498–503.
Moore N, Van Ganse E, Le Parc J, Wall R, Schneid H, Farhan M, et al. The PAIN study: Paracetamol, Aspirin and Ibuprofen New Tolerability study. A large scale, randomized clinical trial comparing the tolerability of aspirin, ibuprofen and paracetamol for short-term analgesia. Clin Drug Invest. 1999;18:89–98.
Moore N, Verschuren X, Montout C, Callens J, Kong SX, Begaud B. Excess costs related to non-steroidal anti-inflammatory drug utilization in general practice. Therapie. 2000;55(1):133–6.
Gulmez SE, Larrey D, Pageaux GP, Bernuau J, Bissoli F, Horsmans Y, et al. Liver transplant associated with paracetamol overdose: results from the seven-country SALT study. Br J Clin Pharmacol. 2015;80(3):599–606.
Gulmez SE, Larrey D, Pageaux GP, Lignot S, Lassalle R, Jove J, et al. Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case-population SALT study. Drug Saf Int J Med Toxicol Drug Exp. 2013;36(2):135–44.
Bezin J, Duong M, Lassalle R, Droz C, Pariente A, Blin P, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26(8):954–62.
Bezin J, Pariente A, Lassalle R, Dureau-Pournin C, Abouelfath A, Robinson P, et al. Use of the recommended drug combination for secondary prevention after a first occurrence of acute coronary syndrome in France. Eur J Clin Pharmacol. 2014;70(4):429–36.
Blin P, Dureau-Pournin C, Lassalle R, Jove J, Thomas-Delecourt F, Droz-Perroteau C, et al. Outcomes in patients after myocardial infarction similar to those of the PEGASUS-TIMI 54 trial: a cohort study in the French national claims database. Br J Clin Pharmacol. 2017;83(9):2056–65.
Rao GH, Johnson GG, Reddy KR, White JG. Ibuprofen protects platelet cyclooxygenase from irreversible inhibition by aspirin. Arteriosclerosis. 1983;3(4):383–8.
Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345(25):1809–17.
MacDonald TM, Wei L. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet. 2003;361(9357):573–4.
Wertheimer AI. The defined daily dose system (DDD) for drug utilization review. Hosp Pharm. 1986;21(3):233–4, 9–41, 58.
Sturmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ. Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol. 2005;161(9):891–8.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–61.
Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26(4):734–53.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Nissen SE, Yeomans ND, Solomon DH, Luscher TF, Libby P, Husni ME, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375(26):2519–29.
MacDonald TM, Hawkey CJ, Ford I, McMurray JJV, Scheiman JM, Hallas J, et al. Randomized trial of switching from prescribed non-selective non-steroidal anti-inflammatory drugs to prescribed celecoxib: the Standard care vs. Celecoxib Outcome Trial (SCOT). Eur Heart J. 2017;38(23):1843–50.
de Abajo FJ, Gil MJ, Garcia Poza P, Bryant V, Oliva B, Timoner J, et al. Risk of nonfatal acute myocardial infarction associated with non-steroidal antiinflammatory drugs, non-narcotic analgesics and other drugs used in osteoarthritis: a nested case–control study. Pharmacoepidemiol Drug Saf. 2014;23(11):1128–38.
Urquhart G, Sinclair HK, Hannaford PC. The use of non-prescription medicines by general practitioner attendees. Pharmacoepidemiol Drug Saf. 2004;13(11):773–9.
Moore N, Gulmez SE, Larrey D, Pageaux GP, Lignot S, Lassalle R, et al. Choice of the denominator in case population studies: event rates for registration for liver transplantation after exposure to NSAIDs in the SALT study in France. Pharmacoepidemiol Drug Saf. 2013;22(2):160–7.
Bezin J, Groenwold RH, Ali MS, Lassalle R, Robinson P, de Boer A, et al. Comparative effectiveness of recommended versus less intensive drug combinations in secondary prevention of acute coronary syndrome. Pharmacoepidemiol Drug Saf. 2017;26(3):285–93.
Laharie D, Droz-Perroteau C, Benichou J, Amouretti M, Blin P, Begaud B, et al. Hospitalizations for gastrointestinal and cardiovascular events in the CADEUS cohort of traditional or Coxib NSAID users. Br J Clin Pharmacol. 2010;69(3):295–302.
Moore N, Tubert-Bitter P, Fourrier A, Begaud B. A simple method to estimate sample sizes for safety equivalence studies using inverse sampling. J Clin Epidemiol. 2003;56(5):433–5.
Acknowledgements
The authors wish to thank ADERA, the non-profit organization that provides legal, administrative and human resources support to Bordeaux PharmacoEpi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Mai Duong received a doctoral grant from the French Embassy in Hanoi.
Conflict of interest
There are no conflicts of interest reported related to the present paper, which was funded internally by Bordeaux PharmacoEpi. Bordeaux PharmacoEpi is a public research platform of the University of Bordeaux and INSERM that has done many post-authorization studies over the years at the request of regulatory authorities, often funded by various pharmaceutical companies, but none to date concerning NSAIDs or their cardiovascular risks. Some of these studies concerned pharmaceutical companies that also manufacture NSAIDs or paracetamol. Nicholas Moore has in the past provided personal and consulting advice to pharmaceutical companies on post-authorization studies and the risks associated with NSAIDs or paracetamol, and as part of his work on data safety management boards. This includes manufacturers of ibuprofen, ketoprofen, diclofenac, naproxen, celecoxib, rofecoxib, and paracetamol, though none were involved in the present study. Mai Duong, Abdelilah Abouelfath, Regis Lassalle, Cécile Droz, Patrick Blin have no conflicts of interest that are directly relevant to the content of this study. The present study was entirely initiated, designed, implemented and reported independently of any pharmaceutical company.
Ethics considerations
This study used secondary anonymized data from the 1/97 sample of the French National Healthcare database, respecting all regulatory and legal requirements. By law, INSERM research teams can access that data without having to request further authorization from the national data protection committee, CNIL. The study protocol was deposited at INSERM before starting data access and extractions for a nihil obstat.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duong, M., Abouelfath, A., Lassalle, R. et al. Coronary Events After Dispensing of Ibuprofen: A Propensity Score-Matched Cohort Study Versus Paracetamol in the French Nationwide Claims Database Sample. Drug Saf 41, 1049–1058 (2018). https://doi.org/10.1007/s40264-018-0686-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0686-7