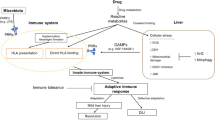
Abstract
Numerous publications contributed to the expanding knowledge base about drug-induced liver injury (DILI) in 2015. New findings from the US Drug Induced Liver Injury Network (DILIN) in their most recently updated registry include a 1- to 3-week delay in the appearance of acute DILI from short-course antibiotics such as cefazolin. They corroborated the finding that acute DILI in patients with underlying liver disease was far more severe and potentially fatal than in patients without liver disease. The only drug that seemed to have an increased risk of hepatotoxicity in these patients was azithromycin. While nearly one in six patients with acute DILI had persistently elevated liver tests at 6 months, and results for 75 % of these patients continued to be abnormal at 12 months, most of these “chronic” injury cases were relatively minor and the result of cholestatic hepatotoxins. Newly described DILI agents include tolvaptan, as well as some new direct-acting antiviral protease inhibitors for chronic hepatitis C. The latter have been associated with serious acute hepatitis, hyperbilirubinemia, and decompensation. Herbal hepatotoxicity continues to be increasingly reported, although applying causality assessment to these cases can, in fact, be more challenging than with prescription drugs. As important as cases with DILI, the class of PCSK9 inhibitors used to lower low-density lipoprotein (LDL) cholesterol have not been associated with significant liver injury, in contrast with other lipid-lowering agents. With respect to pharmacologic DILI risk factors, new data show that drugs metabolized by cytochrome P450 enzymes had a nearly four times higher likelihood of causing DILI. Interestingly, high lipophilicity, which was previously felt to be a risk factor for DILI, was not found to be associated, although more study is needed to confirm this observation. While human leukocyte antigen (HLA) genotypes have been linked to several specific agents, the role of such testing in the general population remains undefined due to the currently low positive and negative predictive values of the available tests. New DILI biomarkers, specifically microRNA-122 and keratin-18, among others, appear to have the necessary predictive value to determine the prognosis and outcome of patients with paracetamol (acetaminophen [AAP])-induced acute liver failure (ALF), and may be of great benefit in deciding who requires N-acetylcysteine (NAC), and for what duration. Treatment options for other forms of DILI remain limited; no firm conclusions can currently be drawn for the use of NAC in non-AAP ALF.
Similar content being viewed by others
References
Lewis JH. The art and science of diagnosing and managing drug-induced liver injury in 2015 and beyond. Clin Gastroenterol Hepatol. 2015;13(12):2173–89.e8. doi:10.1016/j.cgh.2015.06.017.
Senior JR. Evolution of the Food and Drug Administration approach to liver safety assessment for new drugs: current status and challenges. Drug Saf. 2014;37(Suppl 1):S9–17. doi:10.1007/s40264-014-0182-7.
Weiler S, Merz M, Kullak-Ublick GA. Drug-induced liver injury: the dawn of biomarkers? F1000Prime Rep. 2015;7:34. doi:10.12703/P7-34.
Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ, Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950–66. doi:10.1038/ajg.2014.131 (quiz 967).
Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: Critical assessment based on published case reports. Hepatology. 2016;63(2):590–603. doi:10.1002/hep.28323 (Epub 2015 Dec 21).
Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. Lippincott Williams & Wilkins; 1999.
Lewis JH. Causality assessment: which is best—expert opinion or RUCAM? Clin Liver Dis. 2014;4:4–8. doi:10.1002/cld.365.
Hayashi PH. Drug-induced liver injury network causality assessment: criteria and experience in the United States. Int J Mol Sci. 2016;17(2). doi:10.3390/ijms17020201.
Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, Drug-Induced Liver Injury Network (DILIN), et al. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141(5):1665–72.e1–9. doi:10.1053/j.gastro.2011.07.051.
Dalton HR, Fellows HJ, Stableforth W, Joseph M, Thurairajah PH, Warshow U, et al. The role of hepatitis E virus testing in drug-induced liver injury. Aliment Pharmacol Ther. 2007;26(10):1429–35.
Pérez-Gracia MT, García M, Suay B, Mateos-Lindemann ML. Current Knowledge on Hepatitis E. J Clin Transl Hepatol. 2015;3(2):117–26. doi:10.14218/JCTH.2015.00009.
Centers for Disease Control and Prevention. Hepatitis E FAQs for Health Professionals. http://www.cdc.gov/hepatitis/hev/hevfaq.htm. Accessed 27 Feb 2016.
Kullak-Ublick GA, Merz M, Griffel L, Kaplowitz N, Watkins PB. Liver safety assessment in special populations (hepatitis B, C, and oncology trials). Drug Saf. 2014;37(Suppl 1):S57–62. doi:10.1007/s40264-014-0186-3.
Chalhoub WM, Sliman KD, Arumuganathan M, Lewis JH. Drug-induced liver injury: what was new in 2013? Expert Opin Drug Metab Toxicol. 2014;10(7):959–80. doi:10.1517/17425255.2014.909408.
Andrade RJ, Ortega-Alonso A, Lucena MI. Drug-Induced Liver Injury Clinical Consortia: a global research response for a worldwide health challenge. Expert Opin Drug Metab Toxicol. 2016:1–5. PubMed PMID: 26820043 [Epub ahead of print].
Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, United States Drug Induced Liver Injury Network, et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN Prospective Study. Gastroenterology. 2015;148(7):1340–52.e7. doi:10.1053/j.gastro.2015.03.006.
Reuben A. Hy’s law. Hepatology. 2004;39(2):574–8.
Fontana RJ, Hayashi PH, Gu J, Reddy KR, Barnhart H, Watkins PB, DILIN Network, et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology. 2014;147(1):96–108.e4. doi:10.1053/j.gastro.2014.03.045.
Levine C, Trivedi A, Thung SN, Perumalswami PV. Severe ductopenia and cholestasis from levofloxacin drug-induced liver injury: a case report and review. Semin Liver Dis. 2014;34(2):246–51. doi:10.1055/s-0034-1375964.
Goldberg DS, Forde KA, Carbonari DM, Lewis JD, Leidl KB, Reddy KR, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology. 2015;148(7):1353–61.e3. doi:10.1053/j.gastro.2015.02.050.
Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575–86, viii. doi:10.1016/j.cld.2013.07.001.
Reuben A, Koch DG, Lee WM, Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52(6):2065–76. doi:10.1002/hep.23937.
Kulkarni S, Perez C, Pichardo C, Castillo L, Gagnon M, Beck-Sague C, et al. Use of Pediatric Health Information System database to study the trends in the incidence, management, etiology, and outcomes due to pediatric acute liver failure in the United States from 2008 to 2013. Pediatr Transplant. 2015;19(8):888–95. doi:10.1111/petr.12596.
Hao K, Yu Y, He C, Wang M, Wang S, Li X. RUCAM scale-based diagnosis, clinical features and prognosis of 140 cases of drug-induced liver injury. Zhonghua Gan Zang Bing Za Zhi. 2014;22(12):938–41. doi:10.3760/cma.j.issn.1007-3418.2014.12.012.
Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, DILIN Study Group, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32(1):55–68. doi:10.2165/00002018-200932010-00005.
Zhu Y, Li YG, Wang JB, Liu SH, Wang LF, Zhao YL, et al. Causes, features, and outcomes of drug-induced liver injury in 69 children from China. Gut Liver. 2015;9(4):525–33. doi:10.5009/gnl14184.
Douros A, Bronder E, Andersohn F, Klimpel A, Thomae M, Sarganas G, et al. Drug-induced liver injury: results from the hospital-based Berlin Case-Control Surveillance Study. Br J Clin Pharmacol. 2015;79(6):988–99. doi:10.1111/bcp.12565.
Galvin Z, McDonough A, Ryan J, Stewart S. Blood alanine aminotransferase levels >1,000 IU/l—causes and outcomes. Clin Med (Lond). 2015;15(3):244–7. doi:10.7861/clinmedicine.15-3-244.
Lammert C, Bjornsson E, Niklasson A, Chalasani N. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology. 2010;51(2):615–20. doi:10.1002/hep.23317.
Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI. Drug-induced liver injury: Interactions between drug properties and host factors. J Hepatol. 2015;63(2):503–14. doi:10.1016/j.jhep.2015.04.016.
Yu K, Geng X, Chen M, Zhang J, Wang B, Ilic K, Tong W. High daily dose and being a substrate of cytochrome P450 enzymes are two important predictors of drug-induced liver injury. Drug Metab Dispos. 2014;42(4):744–50. doi:10.1124/dmd.113.056267.
Chen M, Borlak J, Tong W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug-induced liver injury. Hepatology. 2013;58(1):388–96. doi:10.1002/hep.26208.
Grove JI, Aithal GP. Human leukocyte antigen genetic risk factors of drug-induced liver toxicology. Expert Opin Drug Metab Toxicol. 2015;11(3):395–409. doi:10.1517/17425255.2015.992414.
Jiang J, Zhang X, Huo R, Li X, Yang Y, Gai Z, et al. Association study of UGT1A9 promoter polymorphisms with DILI based on systematically regional variation screen in Chinese population. Pharmacogenomics J. 2015;15(4):326–31. doi:10.1038/tpj.2014.75.
Urban TJ, Daly AK, Aithal GP. Genetic basis of drug-induced liver injury: present and future. Semin Liver Dis. 2014;34(2):123–33. doi:10.1055/s-0034-1375954.
Overby CL, Hripcsak G, Shen Y. Estimating heritability of drug-induced liver injury from common variants and implications for future study designs. Sci Rep. 2014;21(4):5762. doi:10.1038/srep05762.
Aithal GP. Pharmacogenetic testing in idiosyncratic drug-induced liver injury: current role in clinical practice. Liver Int. 2015;35(7):1801–8. doi:10.1111/liv.12836.
Rodríguez-Sáinz C, Valor L, Hernández DC, Gil J, Carbone J, Pascual-Bernaldez M, et al. Flow cytometry analysis with a new FITC-conjugated monoclonal antibody-3E12 for HLA-B*57:01 rapid screening in prevention of abacavir hypersensitivity in HIV-1-infected patients. HIV Clin Trials. 2013;14(4):160–4. doi:10.1310/hct1404-160.
Matsumoto T, Ohno M, Azuma J. Future of pharmacogenetics-based therapy fortuberculosis. Pharmacogenomics. 2014;15(5):601–7. doi:10.2217/pgs.14.38.
Hirasawa M, Hagihara K, Okudaira N, Izumi T. The possible mechanism of idiosyncratic lapatinib-induced liver injury in patients carrying human leukocyteantigen-DRB1*07:01. PLoS One. 2015;10(6):e0130928. doi:10.1371/journal.pone.0130928.
Chen R, Zhang Y, Tang S, Lv X, Wu S, Sun F, et al. The association between HLA-DQB1 polymorphism and antituberculosis drug-induced liver injury: a Case-Control Study. J Clin Pharm Ther. 2015;40(1):110–5. doi:10.1111/jcpt.12211.
Stephens C, López-Nevot MÁ, Ruiz-Cabello F, Ulzurrun E, Soriano G, Romero-Gómez M, et al. HLA alleles influence the clinical signature of amoxicillin-clavulanate hepatotoxicity. PLoS One. 2013;8(7):e68111. doi:10.1371/journal.pone.0068111.
Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, DILIGEN Study, et al. International SAE Consortium. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41(7):816–9. doi:10.1038/ng.379.
Watkins PB, Lewis JH, Kaplowitz N, Alpers DH, Blais JD, Smotzer DM, et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015;38(11):1103–13. doi:10.1007/s40264-015-0327-3.
Russmann S, Niedrig DF, Budmiger M, Schmidt C, Stieger B, Hürlimann S, et al. Rivaroxaban postmarketing risk of liver injury. J Hepatol. 2014;61(2):293–300. doi:10.1016/j.jhep.2014.03.026.
Liakoni E, Rätz Bravo AE, Krähenbühl S. Hepatotoxicity of new oral anticoagulants (NOACs). Drug Saf. 2015;38(8):711–20. doi:10.1007/s40264-015-0317-5.
Raschi E, Poluzzi E, Koci A, Salvo F, Pariente A, Biselli M, et al. Liver injury with novel oral anticoagulants: assessing post-marketing reports in the US Food and Drug Administration adverse event reporting system. Br J Clin Pharmacol. 2015;80(2):285–93. doi:10.1111/bcp.12611.
Anastasia EJ, Rosenstein RS, Bergsman JA, Parra D. Use of apixaban after development of suspected rivaroxaban-induced hepatic steatosis; a case report. Blood Coagul Fibrinolysis. 2015;26(6):699–702. doi:10.1097/MBC.0000000000000363.
Hahn KJ, Morales SJ, Lewis JH. Enoxaparin-induced liver injury: case report and review of the literature and FDA Adverse Event Reporting System (FAERS). Drug Saf Case Rep. 2015;2:17.
Soriano V, Barreiro P, de Mendoza C, Peña JM. Hepatic decompensation with sofosbuvir plus simeprevir in a patient with Child-Pugh B compensated cirrhosis. Antivir Ther. 2015;. doi:10.3851/IMP2969.
Okajima A, Yamaguchi K, Taketani H, Hara T, Ishiba H, Seko Y, et al. Drug-induced liver injury in a chronic hepatitis C patient treated by peginterferon, ribavirin and simeprevir. Hepatol Res. 2015;45(10):E156–60. doi:10.1111/hepr.12477.
Igawa T, Fushimi S, Matsuo R, Ikeda F, Nouso K, Yoshino T, et al. Severe liver injury associated with simeprevir plus pegylated interferon/ribavirin therapy in a patient with treatment-naïve genotype 1b hepatitis C virus: a case report. Clin J Gastroenterol. 2014;7(5):465–70. doi:10.1007/s12328-014-0527-x.
Stine JG, Intagliata N, Shah NL, Argo CK, Caldwell SH, Lewis JH, et al. Hepatic decompensation likely attributable to simeprevir in patients with advanced cirrhosis. Dig Dis Sci. 2015;60(4):1031–5. doi:10.1007/s10620-014-3422-x.
Fujii Y, Uchida Y, Mochida S. Drug-induced immunoallergic hepatitis during combination therapy with daclatasvir and asunaprevir. Hepatology. 2015;61(1):400–1. doi:10.1002/hep.27559.
Shibata S, Umemura T, Komatsu M, Tanaka E. Severe hepatotoxicity associated with asunaprevir and daclatasvir in chronic hepatitis C. Hepatology. 2015. doi:10.1002/hep.28113 [Epub ahead of print].
FDA Drug Safety Communication: FDA warns of serious liver injury risk with hepatitis C treatments Viekira Pak and Technivie. 2015. http://www.fda.gov/Drugs/DrugSafety/ucm468634.htm. Accessed on 20 Dec 2015.
Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med. 2015;163(1):1–13. doi:10.7326/M15-0785.
U.S. Food and Drug Administration. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm483828.htm. Accessed 27 Feb 2016.
Hayes W, Tschumi S, Ling SC, Feber J, Kirschfink M, Licht C. Eculizumab hepatotoxicity in pediatric aHUS. Pediatr Nephrol. 2015;30(5):775–81. doi:10.1007/s00467-014-2990-5.
Ghabril M, Bonkovsky HL, Kum C, Davern T, Hayashi PH, Kleiner DE, US Drug-Induced Liver Injury Network, et al. Liver injury from tumor necrosis factor-α antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol. 2013;11(5):558–564.e3. doi:10.1016/j.cgh.2012.12.025.
Rodrigues S, Lopes S, Magro F, Cardoso H, Horta e Vale AM, Marques M, et al. Autoimmune hepatitis and anti-tumor necrosis factor alpha therapy: a single center report of 8 cases. World J Gastroenterol. 2015;21(24):7584–8. doi:10.3748/wjg.v21.i24.7584.
Shelton E, Chaudrey K, Sauk J, Khalili H, Masia R, Nguyen DD, et al. New onset idiosyncratic liver enzyme elevations with biological therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41(10):972–9. doi:10.1111/apt.13159.
French JB, Bonacini M, Ghabril M, Foureau D, Bonkovsky HL. Hepatotoxicity associated with the use of anti-TNF-α agents. Drug Saf. 2016;39(3):199–208. doi:10.1007/s40264-015-0366-9.
Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;4(13):211. doi:10.1186/s12916-015-0455-8.
Cheng R, Cooper A, Kench J, Watson G, Bye W, McNeil C, et al. Ipilimumab-induced toxicities and the gastroenterologist. J Gastroenterol Hepatol. 2015;30(4):657–66. doi:10.1111/jgh.12888.
Serper M, Wolf MS, Parikh NA, Tillman H, Lee WM, Ganger DR. Risk factors, clinical presentation, and outcomes in overdose with acetaminophen alone or with combination products: results from the acute liver failure study group. J Clin Gastroenterol. 2016;50(1):85–91. doi:10.1097/MCG.0000000000000378.
King JP, McCarthy DM, Serper M, Jacobson KL, Mullen RJ, Parker RM, et al. Variability in acetaminophen labeling practices: a missed opportunity to enhance patient safety. J Med Toxicol. 2015;11(4):410–4. doi:10.1007/s13181-015-0464-1.
Chan ST, Chan CK, Tse ML. Paracetamol overdose in Hong Kong: is the 150-treatment line good enough to cover patients with paracetamol-induced liver injury? Hong Kong Med J. 2015;21(5):389–93. doi:10.12809/hkmj144481.
McGovern AJ, Vitkovitsky IV, Jones DL, Mullins ME. Can AST/ALT ratio indicate recovery after acute paracetamol poisoning? Clin Toxicol (Phila). 2015;53(3):164–7. doi:10.3109/15563650.2015.1006399.
Hawton K, Bergen H, Simkin S, Dodd S, Pocock P, Bernal W, et al. Long term effect of reduced pack sizes of paracetamol on poisoning deaths and liver transplant activity in England and Wales: interrupted time series analyses. BMJ. 2013;7(346):f403. doi:10.1136/bmj.f403.
Gulmez SE, Larrey D, Pageaux GP, Bernuau J, Bissoli F, Horsmans Y, et al. Liver transplant associated with paracetamol overdose: results from the seven-country SALT study. Br J Clin Pharmacol. 2015;80(3):599–606. doi:10.1111/bcp.12635.
McGill MR, Jaeschke H. MicroRNAs as signaling mediators and biomarkers of drug- and chemical-induced liver injury. J Clin Med. 2015;4(5):1063–78. doi:10.3390/jcm4051063.
McGill MR, Jaeschke H. Mechanistic biomarkers in acetaminophen-induced hepatotoxicity and acute liver failure: from preclinical models to patients. Expert Opin Drug Metab Toxicol. 2014;10(7):1005–17. doi:10.1517/17425255.2014.920823.
Dear JW, Antoine DJ. Stratification of paracetamol overdose patients using new toxicity biomarkers: current candidates and future challenges. Expert Rev Clin Pharmacol. 2014;7(2):181–9. doi:10.1586/17512433.2014.880650.
Wang Y, Chen T, Tong W. miRNAs and their application in drug-induced liver injury. Biomark Med. 2014;8(2):161–72. doi:10.2217/bmm.13.147.
Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced liver injury at first presentation to hospital. Hepatology. 2013;58(2):777–87. doi:10.1002/hep.26294.
Yang X, Salminen WF, Shi Q, Greenhaw J, Gill PS, Bhattacharyya S, et al. Potential of extracellular microRNAs as biomarkers of acetaminophen toxicity in children. Toxicol Appl Pharmacol. 2015;284(2):180–7. doi:10.1016/j.taap.2015.02.013.
Vliegenthart AD, Shaffer JM, Clarke JI, Peeters LE, Caproali A, Bateman DN, et al. Comprehensive microRNA profiliing acetaminophen toxicity identifies novel ciruclating biomarkers for human liver and kidney injury. Sci Rep. 2015;5:15501. doi:10.1038/srep15501.
Thulin P, Nordahl G, Gry M, Yimer G, Aklillu E, Makonnen E, et al. Keratin-18 and microRNA-122 comlement alanine aminotransferase as novel safety biomarkers for drug-induced liver injury in two human cohorts. Liver Int. 2014;34(3):367–8. doi:10.1111/liv.12322.
Senior JR. New biomarkers for drug-induced liver injury: are they really better? What do they diagnose? Liver Int. 2014;34(3):325–7.
Stutchfield BM, Antoine DJ, Mackinnon AC, Gow DJ, Bain CC, Hawley CA, et al. CSF1 restores innate immunity after liver injury in mice and serum levels indicate outcomes of patients with acute liver failure. Gastroenterology. 2015;149(7):1896–1909.e14. doi:10.1053/j.gastro.2015.08.053.
Devarbhavi H, Singh R, Patil M, Sheth K, Adarsh CK, Balaraju G. Outcome and determinants of mortality in 269 patients with combination anti-tuberculosis drug-induced liver injury. J Gastroenterol Hepatol. 2013;28(1):161–7. doi:10.1111/j.1440-1746.2012.07279.x.
Hayashi PH, Fontana RJ, Chalasani NP, Stolz AA, Talwalkar JA, Navarro VJ US Drug-Induced Liver Injury Network Investigators, et al. Under-reporting and poor adherence to monitoring guidelines for severe cases of isoniazid hepatotoxicity. Clin Gastroenterol Hepatol. 2015;13(9):1676–82.e1. doi:10.1016/j.cgh.2015.02.024.
Martinez MA, Vuppalanchi R, Fontana RJ, Stolz A, Kleiner DE, Hayashi PH, et al. Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. 2015;13(2):369–376.e3. doi:10.1016/j.cgh.2014.07.054.
Alqahtani SA, Kleiner DE, Ghabril M, Gu J, Hoofnagle JH, Rockey DC, Drug-Induced Liver Injury Network (DILIN) Study Investigators. Identification and characterization of cefazolin-induced liver injury. Clin Gastroenterol Hepatol. 2015;13(7):1328–1336.e2. doi:10.1016/j.cgh.2014.11.036.
Dandakis D, Petrogiannopoulos C, Hartzoulakis G, Flevaris C, Lagoutari D, Drakogiogos G, et al. Cholestatic hepatitis associated with amoxicillin-clavulanic acid combination. A case report. Ann Gastroenterol. 2002;15(1):85–7.
Orman ES, Conjeevaram HS, Vuppalanchi R, Freston JW, Rochon J, Kleiner DE, DILIN Research Group, et al. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol. 2011;9(6):517–523.e3. doi:10.1016/j.cgh.2011.02.019.
Yeong TT, Lim KH, Goubet S, Parnell N, Verma S. Natural history and outcomes in drug-induced autoimmune hepatitis. Hepatol Res. 2015. doi:10.1111/hepr.12532.
Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51(6):2040–8. doi:10.1002/hep.23588.
Bessone F, Lucena MI, Roma MG, Stephens C, Medina-Cáliz I, Frider B, et al. Cyproterone acetate induces a wide spectrum of acute liver damage including corticosteroid-responsive hepatitis: report of 22 cases. Liver Int. 2015. doi:10.1111/liv.12899.
Friedrich ME, Akimova E, Huf W, Konstantinidis A, Papageorgiou K, Winkler D, et al. Drug-induced liver injury during antidepressant treatment: results of AMSP, a drug surveillance program. Int J Neuropsychopharmacol. 2015. doi:10.1093/ijnp/pyv126.
Gahr M, Zeiss R, Lang D, Connemann BJ, Schönfeldt-Lecuona C. Hepatotoxicity associated with agomelatine and other antidepressants: Disproportionality analysis using pooled pharmacovigilance data from the Uppsala Monitoring Centre. J Clin Pharmacol. 2015;55(7):768–73. doi:10.1002/jcph.475.
Davidov Y, Har-Noy O, Pappo O, Achiron A, Dolev M, Ben-Ari Z. Methylprednisolone-induced liver injury: case report and literature review. J Dig Dis. 2016;17(1):55–62. doi:10.1111/1751-2980.12306.
Caster O, Conforti A, Viola E, Edwards IR. Methylprednisolone-induced hepatotoxicity: experiences from global adverse drug reaction surveillance. Eur J Clin Pharmacol. 2014;70(4):501–3. doi:10.1007/s00228-013-1632-3.
Navarro VJ, Lucena MI. Hepatotoxicity induced by herbal and dietary supplements. Semin Liver Dis. 2014;34(2):172–93. doi:10.1055/s-0034-1375958.
Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89(6):851–65. doi:10.1007/s00204-015-1471-3.
Raschi E, De Ponti F. Drug- and herb-induced liver injury: progress, current challenges and emerging signals of post-marketing risk. World J Hepatol. 2015;7(13):1761–71. doi:10.4254/wjh.v7.i13.1761.
Stournaras E, Tziomalos K. Herbal medicine-related hepatotoxicity. World J Hepatol. 2015;7(19):2189–93. doi:10.4254/wjh.v7.i19.2189.
Teschke R, Wolff A, Frenze C, Schulze J. Review article: herbal hepatotoxicity—an update on traditional Chinese medicine preparations. Aliment Pharmacol Ther. 2014:40 (1);32–50. doi:10.1111/apt.12798.
Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399–408. doi:10.1002/hep.27317.
Lee WJ, Kim HW, Lee HY, Son CG. Systematic review on herb-induced liver injury in Korea. Food Chem Toxicol. 2015;84:47–54. doi:10.1016/j.fct.2015.06.004.
Hayashi PH, Barnhart HX, Fontana RJ, Chalasani N, Davern TJ, Talwalkar JA, et al. Reliability of causality assessment for drug, herbal and dietary supplement hepatotoxicity in the Drug-Induced Liver Injury Network (DILIN). Liver Int. 2015;35(5):1623–32. doi:10.1111/liv.12540.
Teschke R, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: challenges and pitfalls of causality assessment methods. World J Gastroenterol. 2013;19(19):2864–82. doi:10.3748/wjg.v19.i19.2864.
Roytman MM, Porzgen P, Lee CL, Huddleston L, Kuo TT, Bryant-Greenwood P, et al. Outbreak of severe hepatitis linked to weight-loss supplement OxyELITE Pro. Am J Gastroenterol. 2014;190:1296–8. doi:10.1038/ajg.2014.159.
Foley S, Butlin E, Shields W, Lacey B. Experience with OxyELITE pro and acute liver injury in active duty service members. Dig Dis Sci. 2014;59(12):3117–21. doi:10.1007/s10620-014-3221-4.
Teschke R, Schulze J, Eickhoff A, Wolff A, Frenzel C. Mysterious Hawaii liver disease case—naproxen overdose as cause rather than OxyElitePro. J Liver Clin Res. 2015;2(2):1013.
Teschke R, Schwarzenboeck A, Frenzel C, Schulze J, Eickhoff A, Wolff A. The mystery of the Hawaii liver disease cluster in summer 2013: A pragmatic and clinical approach to solve the problem. Ann Hepatol. 2015;15(1):91–109.
Klontz KC, DeBeck HJ, LeBlanc P, Mogen KM, Wolpert BJ, Sabo JL, et al. The role of adverse event reporting in the FDA response to multistate outbreak of liver disease associated with a dietary supplement. Public Health Rep. 2015;130(5):526–32.
Johnston DI, Chang A, Viray M, Chatham-Stephens K, He H, Taylor E. Hepatotoxicity associated with the dietary supplement OxyELITE Pro—Hawaii, 2013. Drug Test Anal. 2015. doi:10.1002/dta.1894.
Navarro VJ, Bonkovsky HL, Hwang SI, Vega M, Barnhart H, Serrano J. Catechins in dietary supplements and hepatotoxicity. Dig Dis Sci. 2013;58(9):2682–90. doi:10.1007/s10620-013-2687-9.
Fontana RJ, Ellerbe C, Durkalski VE, Rangnekar A, Reddy RK, Stravitz T, US Acute Liver Failure Study Group, et al. Two-year outcomes in initial survivors with acute liver failure: results from a prospective, multicentre study. Liver Int. 2015;35(2):370–80. doi:10.1111/liv.12632.
Fontana RJ, Hayashi PH, Barnhart PH, Kleiner DE, Reddy KR, Chalasan N, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110(10):1450–9. doi:10.1038/ajg.2015.283.
Aithal PG, Day CP. The natural history of histologically proved drug induced liver disease. Gut. 1999;44(5):731–5.
Andrade RJ, Lucena MI, Kaplowitz N, García-Muņoz B, Borraz Y, Pachkoria K, et al. Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44(6):1581–8.
Björnsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol. 2009;50(3):511–7. doi:10.1016/j.jhep.2008.
Robles-Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina-Cáliz I, González-Jimenez A, SLatinDILI Network; Safer and Faster Evidence-based Translation Consortium, et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014;147(1):109–118.e5. doi:10.1053/j.gastro.2014.03.050.
Lo Re V 3rd, Haynes K, Forde KA, Goldberg DS, Lewis JD, Carbonari DM, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of Hy’s law and a new prognostic model. Clin Gastroenterol Hepatol. 2015;13(13):2360–8. doi:10.1016/j.cgh.2015.06.020.
Jeong R, Lee YS, Sohn C, Jeon J, Ahn S, Lim KS. Model for end-stage liver disease score as a predictor of short-term outcome in patients with drug-induced liver injury. Scand J Gastroenterol. 2015;50(4):439–46. doi:10.3109/00365521.2014.958094.
Possamai LA, McPhail MJ, Khamri W, Wu B, Concas D, Harrison M, et al. The role of intestinal microbiota in murine models of acetaminophen-induced hepatotoxicity. Liver Int. 2015;35(3):764–73. doi:10.1111/liv.12689.
Lin IC, Yang HC, Strong C, Yang CW, Cho YT, Chen KL, et al. Liver injury in patients with DRESS: a clinical study of 72 cases. J Am Acad Dermatol. 2015;72(6):984–91. doi:10.1016/j.jaad.2015.02.1130.
Avancini J, Maragno L, Santi CG, Criado PR. Drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome: clinical features of 27 patients. Clin Exp Dermatol. 2015;40(8):851–9. doi:10.1111/ced.12682.
Devarbhavi H, Raj S, Aradya VH, Rangegowda VT, Veeranna GP, Singh R, et al. Drug-induced liver injury associated with Stevens-Johnson syndrome/toxic epidermal necrolysis: patient characteristics, causes and outcome in 36 cases. Hepatology. 2015. doi:10.1002/hep.28270.
Lee T, Lee YS, Yoon SY, Kim S, Bae YJ, Kwon HS, et al. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J Am Acad Dermatol. 2013;69(3):407–15. doi:10.1016/j.jaad.2013.03.024.
Foureau DM, Walling TL, Maddukuri V, Anderson W, Culbreath K, Kleiner DE, et al. Comparative analysis of portal hepatic infiltrating leucocytes in acute drug-induced liver injury, idiopathic autoimmune and viral hepatitis. Clin Exp Immunol. 2015;180(1):40–51. doi:10.1111/cei.12558.
Nooredinvand HA, Connell DW, Asgheddi M, Abdullah M, O’Donoghue M, Campbell L, et al. Viral hepatitis prevalence in patients with active and latent tuberculosis. World J Gastroenterol. 2015;21(29):8920–6. doi:10.3748/wjg.v21.i29.8920.
Lewis JH. Clinical perspective: statins and the liver–harmful or helpful? Dig Dis Sci. 2012;57(7):1754–63. doi:10.1007/s10620-012-2207-3.
Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, Phase 3 HoFH Lomitapide Study investigators, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381(9860):40–6. doi:10.1016/S0140-6736(12)61731-0.
Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res. 2012;53(12):2515–24. doi:10.1194/jlr.R026658 (Epub 2012 Jul 17. Review).
Bergeron N, Phan BA, Ding Y, Fong A, Krauss RM. Proprotein convertase subtilisin/kexin type 9 inhibition: a new therapeutic mechanism for reducing cardiovascular disease risk. Circulation. 2015;132(17):1648–66. doi:10.1161/CIRCULATIONAHA.115.016080.
Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, et al. RUTHERFORD-2 Investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):331–40. doi:10.1016/S0140-6736(14)61399-4.
Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, et al. TESLA Investigators. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):341–50. doi:10.1016/S0140-6736(14)61374-X.
Sabatine MS, Wasserman SM, Stein EA. PCSK9 Inhibitors and Cardiovascular Events. N Engl J Med. 2015;373(8):774–5.
Gupta S. LDL cholesterol, statins and PCSK 9 inhibitors. Indian Heart J. 2015;67(5):419–24. doi:10.1016/j.ihj.2015.05.020 (Epub 2015 Aug 5. Review).
Safe-T Consortium. http://www.imi-safe-t.eu. Accessed 27 Feb 2016.
International SAE Consortium. http://www.saeconsortium.org. Accessed 27 Feb 2016.
DILIsym. http://www.dilisym.com. Accessed 27 Feb 2016.
Stine JG, Lewis JH. Current and future directions in the treatment and prevention of drug-induced liver injury: a systematic review. Expert Rev Gastroenterol Hepatol. 2015;25:1–20.
Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Acute Liver Failure Study Group, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856–64, 864.e1. doi:10.1053/j.gastro.2009.06.006.
Squires RH, Dhawan A, Alonso E, Narkewicz MR, Shneider BL, Rodriguez-Baez N, Pediatric Acute Liver Failure Study Group, et al. Intravenous N-acetylcysteine in pediatric patients with nonacetaminophen acute liver failure: a placebo-controlled clinical trial. Hepatology. 2013;57(4):1542–9. doi:10.1002/hep.26001.
Chughlay MF, Kramer N, Spearman CW, Werfalli M, Cohen K. N-Acetylcysteine for non-paracetamol drug-induced liver injury: a systematic review. Br J Clin Pharmacol. 2016. doi:10.1111/bcp.12880.
Gu J, Tang SJ, Tan SY, Wu Q, Zhang X, Liu CX, et al. An open-label, randomized and multi-center clinical trial to evaluate the efficacy of silibinin in preventing drug-induced liver injury. Int J Clin Exp Med. 2015;8(3):4320–7.
Luangchosiri C, Thakkinstian A, Chitphuk S, Stitchantrakul W, Petraksa S, Sobhonslidsuk A. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Complement Altern Med. 2015;15(1):334. doi:10.1186/s12906-015-0861-7.
Tsipotis E, Shuja A, Jaber BL. albumin dialysis for liver failure: a systematic review. Adv Chronic Kidney Dis. 2015;22(5):382–90. doi:10.1053/j.ackd.2015.05.004.
Olin P, Hausken J, Foss A, Karlsen TH, Melum E, Haugaa H. Continuous molecular adsorbent recirculating system treatment in 69 patients listed for liver transplantation. Scand J Gastroenterol. 2015;50(9):1127–34. doi:10.3109/00365521.2015.1027262.
Maiwall R, Maras JS, Nayak SL, Sarin SK. Liver dialysis in acute-on-chronic liver failure: current and future perspectives. Hepatol Int. 2014;8(Suppl 2):505–13. doi:10.1007/s12072-014-9534-8.
Lee KC, Baker LA, Stanzani G, Alibhai H, Chang YM, Jimenez Palacios C, et al. Extracorporeal liver assist device to exchange albumin and remove endotoxin in acute liver failure: Results of a pivotal pre-clinical study. J Hepatol. 2015;63(3):634–42. doi:10.1016/j.jhep.2015.04.020.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Dr. Philip Sarges and Dr. Joshua Steinberg have no conflicts of interest that are directly relevant to the content of this study. Dr. James H. Lewis is a consultant to Otsuka America Pharmaceutical, Inc., Astra Zeneca, Sanofi, Lundbeck Pharmaceuticals, GlaxoSmithKline, and Takeda Pharmaceuticals; and is a member of speaker bureaus for Gilead Sciences and Bristol Myers Squibb.
Rights and permissions
About this article
Cite this article
Sarges, P., Steinberg, J.M. & Lewis, J.H. Drug-Induced Liver Injury: Highlights from a Review of the 2015 Literature. Drug Saf 39, 801–821 (2016). https://doi.org/10.1007/s40264-016-0427-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-016-0427-8