Abstract
Background and Objective
Most second and third generation antiseizure medications (ASMs) are associated with cognitive adverse events, which are a major concern for patients. However, the profile of cognitive adverse events differs between ASMs. This study investigated the effects of cenobamate on cognition in patients with drug-resistant epilepsy (DRE) within the Spanish Expanded Access Program (EAP).
Methods
This was a retrospective, observational study. Inclusion criteria were age ≥ 18 years, DRE with focal seizures, and availability of cognition assessments and EAP authorization. Data were sourced from the clinical records of patients who took part in the Spanish cenobamate EAP. Primary endpoints included cognition (based on 20 neuropsychological outcomes, including verbal and visuospatial episodic memory, verbal fluency, executive function, working memory, attention, and speed of processing), seizure frequency, and concomitant antiseizure medication (ASM) usage at 6 months.
Results
The study included 20 patients; 10 patients (50%) had daily seizures, 7 (35%) had weekly seizures and 3 (15%) had monthly seizures. The median number of prior antiseizure medications (ASMs) and concomitant ASMs were 10 and 3, respectively. Mean cenobamate doses were 12.5 mg/day at baseline and 191.2 mg/day at 6 months. There was a statistically significant improvement in cognitive scores between baseline and 6 months for two measures of verbal episodic memory (p = 0.0056 and p = 0.0013) and one measure of visuospatial episodic memory (p = 0.011), and a significant worsening in cognitive score for attention (p = 0.030). At 6 months, 14 patients (70%) had a ≥ 50% reduction in seizure frequency, 3 patients (15%) had a ≥ 90% reduction, and 1 patient (5%) was seizure free. There were significant decreases in the mean number of concomitant ASMs (p = 0.0009), the sum of the ratios of prescribing daily dose/daily defined dose (total ratio of DDD) for concomitant ASMs (p < 0.0001), and concomitant ASM drug load (p = 0.038) between baseline and 6 months. Total ratio of DDD was significantly lower at 6 months for perampanel (p = 0.0016), benzodiazepines (p = 0.035), and sodium channel blockers (p = 0.0005) compared with baseline. Based on analysis of covariance, cognitive tests related to verbal or visuospatial episodic memory (e.g., RT of FCSRT, or ROCFT), executive functions (e.g., TMT-B), and processing speed (some 5-Digit Test subtests) appeared to be closely related to the reduction in pharmacological burden rather than the improvement in seizure control.
Conclusions
Significant improvements in cognition, seizure frequency, and concomitant ASM usage were observed after the introduction of cenobamate in patients with DRE in a real-world setting. Covariance analysis supports the reduction in concomitant ASMs as the most important factor driving cognitive improvements with cenobamate. As this was an exploratory study with an uncontrolled, retrospective design and a low number of patients, further studies are required to confirm the findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Significant improvements in cognition, seizure frequency, and concomitant ASM usage were observed after introduction of cenobamate in patients with drug-resistant epilepsy in a real-world setting. |
Cognitive improvements with cenobamate were driven by a reduction in concomitant ASM usage. |
Reducing the dose of some ASMs may have a positive effect on cognition. |
1 Introduction
Antiseizure medications (ASMs) are the cornerstone of treatment for patients with epilepsy [1]. However, 30–40% of patients continue to experience seizures despite treatment with multiple ASMs [2,3,4], and ASMs are associated with significant side effects that impact patients’ quality of life [5].
Clinical trial data suggest that most second- and third-generation ASMs are associated with cognitive adverse events [6], which are a major concern for patients [7]. Patients receiving polytherapy and those with a history of behavioral and psychiatric comorbidities are most likely to experience cognitive adverse events [8, 9]. However, the profile of cognitive adverse events differs between ASMs [9, 10].
Cenobamate is a relatively new ASM approved in Europe as adjunctive treatment for refractory focal-onset seizures (FOS) [11] and in the USA for adult patients with FOS [12]. In two randomized controlled trials, cenobamate significantly reduced seizure frequency and was associated with high seizure freedom rates in adults with uncontrolled FOS treated with 1–3 ASMs [13, 14].
Preliminary real-world evidence suggests that adjunctive cenobamate does not increase cognitive adverse events in patients with drug-resistant focal epilepsy [15], in contrast with other ASMs [10]. For most patients, treatment with cenobamate resulted in stable or improved cognitive performance, although these conclusions are based on a single measure of cognitive performance, a low cenobamate medium dose (125 mg/day), and a short follow-up (3 months) [15].
The aim of the present study was to assess the effects of cenobamate on cognition using a battery of 20 neuropsychological tests in patients with refractory FOS taking part in a Spanish Early Access Program (EAP).
2 Methods
2.1 Study Design
This was a retrospective, observational, single-center study to assess the effects of cenobamate on cognition in a real-world setting. The study was conducted in the Epilepsy Unit of Hospital Regional Universitario de Málaga, Spain. The study protocol was approved by the hospital’s ethics committee and was in accordance with the code of ethics set out in the 1964 Declaration of Helsinki and its later amendments.
Patients with drug-resistant FOS who received cenobamate within the Epilepsy Unit of Hospital Regional Universitario of Málaga between January 2022 and July 2022 as part of a Spanish EAP were retrospectively screened for inclusion in the study.
Inclusion criteria were: (1) age ≥ 18 years; (2) diagnosis of drug-resistant epilepsy (DRE) with FOS; (3) inclusion in the Spanish cenobamate EAP; (4) standardized cognition assessments prior to (i.e., baseline) and 6 months after the start of treatment; and (5) written informed consent from the patient or their legal representative. EAP authorization was limited to highly drug-resistant patients; patients with severe hepatic impairment, end-stage renal disease or nonfocal seizures were excluded from EAP authorization. Patients were excluded if they were unable to undergo neuropsychological evaluation due to intellectual, sensory, or motor deficits.
The first 20 patients who met the inclusion criteria were included in the study, and there were no exclusions during the study period.
2.2 Data Collection
Data were collected from patients’ clinical records and stored according to usual clinical practice by participating physicians. The following data were collected at baseline: patient demographics, age at epilepsy onset, etiology, epileptogenic focus, baseline seizure frequency, prior epilepsy surgery, previous and concomitant ASM use, cenobamate dose, and cognition endpoints (see below). The following data were collected from clinical charts at 6 months: cenobamate dose, number of seizures, changes in concomitant ASM use, and cognition endpoints.
2.3 Cognition Tests
In all participants, a battery of 20 neuropsychological outcomes was used for cognitive evaluation at baseline and at 6 months. The time window between repeat neuropsychological assessments tests was at least 6 months, in line with ILAE guidelines which suggest a minimum of 6–9 months between tests to account for learning and repetition effects [16].
The Free and Cued Selective Reminding Test (FCSRT) [17] was used to evaluate verbal episodic memory and learning. Briefly, participants were shown a card containing four words and invited to recall the words freely, or if unsuccessful, following a cue. Three trials were conducted with a 20 s period of counting backwards between each. This procedure was repeated after a 30 min interval. Four outcomes were recorded: total free recall (TFR; sum of free recall), total recall (TR; sum of free recall + cued recall), delayed free recall (DFR; sum of delayed free recall), and delayed total recall (DTR; free delayed recall + cued delayed recall). Parallel tests were conducted to account for learning and repetition effects, as validated previously [18].
The Rey–Osterrieth Complex Figure Test (ROCFT) [17, 19] was used to evaluate visuospatial episodic memory, planning, and problem solving (executive functions). Briefly, participants were asked to reproduce a geometric figure to assess copying capacity and the time to completion was recorded. The picture was then removed, and participants were asked to reproduce the figure from memory. Participants were then distracted for 20 min and asked to repeat the figure again from memory. Copying capacity (ROCF-COP), memory (ROCF-MCP), and execution time (ROCF-T) were evaluated. Parallel tests were conducted to account for learning and repetition effects.
The Verbal Fluency Test and Executive Functions Verbal Fluency Test was used to evaluate verbal fluency, semantic memory, language, and executive function [20, 21]. Briefly, participants were asked to recite as many words as possible beginning with a “p” in 1 min (Fluency-Phonetics, FP). They were then asked to name as many animals as possible (Fluency-Semantics, FAN).
Frontal executive function was evaluated using INECO frontal screening (IFS), including a battery of subtests [22]: conflicting instructions; go–no go; backward digit span; verbal working memory (backward digit); spatial working memory (backward Corsi); proverb interpretation; and verbal inhibitory control (Hayling Test). This test battery obtains information on conceptualization, cognitive flexibility, motor programming, sensitivity to interference, verbal working memory, visual working memory, motor inhibitory control, and prehension behavior [22].
The forward and backward digit span test (WAIS-III) [23, 24] was used to evaluate working memory and attention. Briefly, participants were invited to repeat a series of numbers read out by an examiner, initially in the same order (DD) and then in reverse order (DI).
The Trail-Making Test (TMT) was used to assess attention, speed of visuomotor tracking, divided attention, mental flexibility, and motor function [24, 25]. Briefly, participants were invited to connect disordered numbers on a sheet of paper without lifting the pen from the paper (TMT-A). They were then invited to connect disordered letters and numbers alternately and in order as quickly as possible on a new sheet of paper (TMT-B).
The Five Digit Test was used to assess speed of processing, cognitive flexibility, sustained attention, automation, and inhibition [26, 27]. Briefly, participants were shown cards containing digits arranged in groups that were to be interpreted either from the value of the digit or their arrangement (e.g., five twos could be read as five or two depending on instructions from the examiner). Scores were divided into six categories (5A, alternate; 5C, counting; 5E, choice; 5F, flexibility; 5I, inhibition; 5L, reading).
For each test, reference values were derived from normative data from the Spanish NEURONORMA Project [17, 21, 24].
2.4 Statistical Analysis
Continuous variables were expressed as means (± standard deviation, SD) or medians (range) according to their distribution, and categorical variables as frequencies and percentages. The Student’s t-test was used to compare continuous variables between groups, as appropriate, and continuous related variables were analyzed using the Wilcoxon test. Reliable change indices were calculated as described by Brooks et al. [28] All statistical analyses were performed using IBM SPSS Statistics version 25.0. The threshold for statistical significance was 5% (p < 0.05).
3 Results
3.1 Patient Baseline Characteristics and Disposition
The first 20 patients who fulfilled the inclusion/exclusion criteria and received at least one dose of cenobamate in the Epilepsy Unit of Hospital Regional Universitario de Málaga were included in the study. The mean age of patients was 40.9 years (range 25–63 years); 55% were female. Mean (SD) age at epilepsy onset was 16.3 ± 11.9 years. Focal cortical dysplasia, mesial temporal sclerosis syndrome, and nonlesional focal epilepsy were the most common etiologies (five patients each; 25%) and the most common epileptogenic focus was left temporal (seven patients; 35%). Five patients (25%) had received prior epilepsy surgery (Table 1).
Patients had received a median of ten ASMs before baseline (Table 1). The median number of concomitant ASMs at baseline was three; 15/20 patients (75%) were receiving at least three ASMs and 5/20 (25%) were receiving at least four ASMs at baseline (Fig. 1). The most frequent mechanisms of action for concomitant ASMs at baseline were sodium channel blockers [SCBs; 18/20 patients (90%)], benzodiazepines [14/20 patients (70%)], and synaptic vesicle protein 2A (SV2A) modulators [9/20 patients (45%)].
3.2 Dosage
Cenobamate was initiated at 12.5 mg/day in all patients and titrated according to the label (i.e., increase every 2 weeks to 25, 50, 100, 150, and 200 mg/day) [11]. This start-low, go-slow titration approach was used to mitigate the risk of drug reaction with eosinophilia and systemic symptoms (DRESS). At 6 months, the mean cenobamate dose was 191.2 ± 56.9 mg (median 200 mg, range 50–250 mg).
3.3 Seizure Frequency
After 6 months of cenobamate treatment, 14 patients (70%) had a ≥ 50% reduction in seizure frequency compared with baseline, 3 patients (15%) had a ≥ 90% reduction, and 1 patient (5%) became seizure free. Of the six nonresponders, none had a change in seizure frequency (either improvement or worsening) with cenobamate. Nonresponders had a mean of 11.33 ± 3.77 ASMs before baseline, 3 ± 0.68 concomitant ASMs, and a final cenobamate dose of 175 ± 68.9 mg. Baseline characteristics were similar for responders and nonresponders (Online Resource 1).
3.4 Concomitant ASM Use During the Study Period
The sum of prescribed daily dose/defined daily dose ratios (total DDD ratio) of concomitant ASMs at baseline (not including cenobamate) was 3.6 ± 1.6 (Table 2); the total DDD ratio was highest for SCBs (2) and SV2As (1.8) (Fig. 2). The mean number of concomitant ASMs taken by patients at 6 months of cenobamate treatment was significantly lower than at baseline (p = 0.0009). Similarly, both the total DDD ratio for concomitant ASMs (p < 0.0001) and concomitant ASM drug load (p = 0.038) were significantly lower at 6 months compared with baseline (Table 2; Online Resource 2).
Change in sum of total DDD ratios between baseline and month 6 according to mechanism of action of concomitant ASMs. AMPAr, AMPA receptor antagonist (perampanel); benzo, benzodiazepines (clobazam, diazepam, phenobarbital); CCB, calcium channel blockers (valproic acid); DDD, daily defined dose; SCB, sodium channel blockers (carbamazepine, eslicarbazepine acetate, lacosamide, lamotrigine, zonisamide, topiramate); SV2A, synaptic vesicle protein 2A modulators (brivaracetam and levetiracetam)
Total DDD ratio was significantly lower at 6 months for AMPA receptor (AMPAr) antagonists (p = 0.0016), benzodiazepines (p = 0.035), and SCBs (p = 0.0005) compared with baseline (Fig. 2; Online Resource 2).
3.5 Cognitive Scores
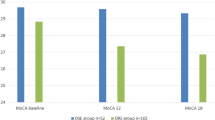
In the overall population, there was a statistically significant increase in cognitive scores between baseline and 6 months of cenobamate treatment for measures of verbal episodic memory (FCSRT TFR, p = 0.0056; FCSRT TR, p = 0.0013) and visuospatial episodic memory (ROCF-MCP, p = 0.011; Table 3; Fig. 3). For FCSRT TFR, 16/19 patients (84%) had below normal scores at baseline compared with 11/19 (58%) after 6 months of cenobamate (Online Resource 3). For FCSRT TR, 15/19 patients (79%) had below normal scores at baseline compared with 9/19 (47%) after 6 months of cenobamate (Online Resource 3). For ROCF-MCP, 10/19 patients (53%) had below normal scores at baseline compared with 7/20 (35%) after 6 months of cenobamate (Online Resource 3).
Change in cognitive parameter scores between baseline and 6 months of cenobamate treatment. Bars show the mean change from baseline in scalar scores for each test, except for the Five Digit test scores (5A, 5C, 5E, 5F, 5I, 5L), which are expressed as percentile differences. Positive mean changes indicate improvement; negative mean changes indicate worsening. 5A, alternate; 5C, counting; 5E, choice; 5F, flexibility; 5I, inhibition; 5L, reading; DD, Digit direct; DFR, delayed free recall; DI, Digit inverse: DTR delayed total recall, FAN fluency-semantics, FP fluency-phonometrics, IFS INECO frontal screening, ROCF Rey–Osterrieth Complex Figure, ROCF-COP ROCF-Copying, ROCF-MCP ROCF-Copy from memory, ROCF-T ROCF-Time, TFR total free recall, TMT Trail-Making Test, TMT-A TMT-attention, TMT-B TMT-processing, TR total recall. Bars indicate standard errors of mean values. *p < 0.05 versus baseline; **p < 0.01 versus baseline
On the basis of intraindividual analysis, FCSRT TFR scores improved in 11/19 patients (58%) between baseline and 6 months, FCSRT TR scores improved in 13/19 patients (68%), and ROCF-MCP scores improved in 14/20 patients (70%). When the more stringent RCI analysis was used to assess changes in cognitive scores between baseline and 6 months, 3/19 patients (16%) showed a reliable improvement in FCSRT TR score (Online Resource 4).
The cognitive score for attention showed a slight but significant worsening as measured by TMT-A (p = 0.030; Table 3; Fig. 3; Online Resource 3). Absolute TMT-A scores declined in 11/20 patients (55%) between baseline and 6 months, although this was not confirmed using RCI to assess reliable changes (Online Resource 4). All other cognitive scores were not significantly different between baseline and 6 months.
3.6 Outcomes According to Response to Treatment or DDD Ratios
In the 14 patients who achieved a ≥ 50% response to cenobamate, FCSRT TFR (p = 0.0015) and FCSRT TR (p = 0.0064) scores were significantly higher, and TMT-A score (p = 0.045) was significantly lower at 6 months compared with baseline. While there was still a trend toward increased ROCF-MCP score in ≥ 50% responders at 6 months, this was not statistically significant (p = 0.055) (Online Resource 5).
Analysis of covariance (ANCOVA) was performed to determine the contributions of response to treatment and total DDD ratio on cognitive improvement using change in score of the different neuropsychological tests as the dependent variable (Online Resource 6). Although the findings are mixed, tests related to episodic verbal or visuospatial memory (e.g., RT of FCSRT or ROCFT), executive functions (e.g., TMT-B), or even processing speed (some Five Digit Test subtests) appear to be closely related to the reduction in pharmacological burden rather than the improvement in seizure control.
4 Discussion
In this real-world analysis, we observed significant improvements in cognition endpoints related to memory in patients with highly refractory FOS following treatment with cenobamate. These effects were observed alongside reductions in seizure frequency and concomitant ASM usage.
Our results are consistent with preliminary data from a real-world study, which showed stable or improved cognitive performance in most patients with DRE following treatment with cenobamate [15]. Together, our data suggest that although CNS-related adverse events are frequently reported with cenobamate in both clinical studies [14, 29] and patient series [30,31,32], they do not appear to reduce cognitive outcomes in real-world practice. In the study by Schuetz et al., cognitive performance was assessed with a low median cenobamate dose (125 mg/day) over a short follow-up (3 months) using EpiTrack©, a simplified assessment of executive function and working memory [15]. In our study, we performed a battery of 20 neuropsychological tests to provide a thorough examination of cognition. Using a higher median cenobamate dose (200 mg) and longer follow-up (6 months) compared with the earlier study, we observed statistically significant improvements in two measures of verbal episodic memory and one measure of visuospatial episodic memory in patients with highly refractory FOS following treatment with cenobamate. We also observed a significant worsening in attention based on the TMT-A score.
In two recent prospective studies in 32 and 22 patients with DRE, no differences in cognitive, emotional, or quality of life (QoL) variables were observed after 3 and 6 months of CNB administration, respectively [33]. With regard to cognitive parameters, most patients remained stable during the course of these studies on the basis of the more stringent RCI analysis, as was also observed in our study. Larger prospective studies will be needed to fully establish the effects of cenobamate on different cognitive parameters.
An analysis of phase III add-on trials found that most second and third generation ASMs exhibit a clear dose response for cognitive adverse events [6]. Polytherapy, which is common among patients with DRE, is associated with an increase in cognitive adverse events [8], and a significant reduction in executive function has been demonstrated with each additional ASM added to polytherapy [34]. Comparing the effect of individual ASMs on cognitive performance is complex due to the varied and changing nature of cognitive impairments among patients, a lack of standardized assessment tools, and complex treatment regimens typically involving multiple ASMs [10]. Nevertheless, cognitive adverse events have been shown to differ between ASMs. A randomized trial of topiramate and tiagabine in patients with DRE demonstrated similar efficacy in reducing seizure frequency but significant differences in cognitive adverse events; while topiramate-treated patients showed deterioration of frontal lobe-associated functions, including verbal fluency, language comprehension, working memory, and visual block tapping, patients treated with tiagabine showed a deterioration in only one of three measures of verbal learning and memory [9]. Similarly, in patients with drug-resistant developmental and epileptic encephalopathies, levetiracetam and perampanel have been associated with aggressiveness and irritability, whereas topiramate and zonisamide have been linked to language, cognitive, and memory deficiencies [10]. Our finding that cenobamate improved memory scores and reduced antiseizure activity supports cenobamate as a valuable tool in the armory for patients with DRE requiring polytherapy.
In our study, 70% of patients achieved a ≥ 50% reduction in seizure frequency at 6 months. Our data are consistent with outcomes from the wider EAP cohort of 170 patients, which reported a ≥ 50% responder rate of 63% [35], but are higher than responder rates reported in cenobamate phase II studies [13, 14]. This could be explained by longer follow-up in our study and more flexible management within a clinical practice setting compared with the regulatory studies. A post hoc analysis of a phase III open-label study of cenobamate reported similar response rates to our study, with 72% of patients achieving a ≥ 50% reduction in seizure frequency, 40% achieving a ≥ 90% reduction, and 13% achieving seizure freedom [36].
Two possible explanations exist for our striking results on cognition. On the one hand, we observed a high rate of clinical response with cenobamate on the basis of a reduction in the number of seizures. Seizure frequency in patients with DRE is associated with higher cognitive impairment, so the improvement in cognition in our patients could be related to the good clinical response. On the other hand, our study also showed a significant reduction in concomitant ASM usage with cenobamate between baseline and 6 months, as measured either by the number of concomitant ASMs used, the sum of total DDD ratios for ASMs, or the ASM drug load. As polytherapy is associated with cognitive adverse events [8], the improvement in cognitive endpoints in our study could have been driven by reduced ASM usage in the presence of cenobamate. Our exploratory covariance analysis supports the reduction in concomitant ASMs as the most important factor driving cognitive improvements in our patients.
The reduced concomitant ASM usage observed in our study after cenobamate treatment confirms previous findings from regulatory and real-world studies [35, 37]. Notably, the reduction in total DDD ratios was significant for discrete ASM classes, including SCBs, benzodiazepines, and perampanel. This may partly be explained by similarities between the dual mechanism of action of cenobamate and the mechanism of actions of SCBs and benzodiazepines: cenobamate blocks persistent sodium currents by promoting the inactivated state of voltage-gated sodium channels and is a positive allosteric modulator of GABAA receptors [38]. Perampanel, unlike cenobamate, is known to antagonize AMPA receptors, although it is unclear whether this is the primary mechanism of action underlying its antiseizure activity. SCBs (including carbamazepine, topiramate, and zonisamide) [10, 39], benzodiazepines (including clobazam and phenobarbital) [10, 40], and perampanel [41] have all been associated with cognitive side effects in patients with epilepsy.
Finally, our data on the effects of cenobamate on processing speed using different tests appear to be contradictory. TMT-A Test scores indicated a worsening in processing speed with cenobamate, whereas Five Digit Test scores (which cover the same cognitive domain) did not. While there is no definitive explanation for this disparity, performance in the TMT-A Test has a greater reliance on motor skills compared with performance in the 5-Digit Test. Unsurprisingly, 5 of our 20 patients had motor deficits affecting the right hand, which would be expected to confound outcomes in the TMT-A Test. As the Five Digit Test does not involve motor skills, we consider it a purer test for determining processing speed than TMT-A.
This study has a number of limitations, including its retrospective design, the lack of a control group, and a low number of patients with neuropsychological test data. Therefore, the study should be regarded as exploratory, and additional evidence is required to confirm the findings. However, subjects were examined with a comprehensive battery of neuropsychological tests and were representative of the wider EAP population on the basis of seizure frequency and concomitant ASM usage data.
5 Conclusion
We observed significant improvements in cognition, seizure frequency, and concomitant ASM usage in patients with highly refractory FOS treated with cenobamate in a real-world setting, suggesting that it may be a valuable tool for add-on therapy in patients with DRE. Additional studies are needed to confirm these findings in larger patient series.
References
Sociedad Española de Neurología. Manual de Práctica Clínica en Epilepsia. Recomendaciones diagnostico-terapéuticas de la SEN [Internet]. 2019 [cited 2021 Nov 11]. http://epilepsia.sen.es/wp-content/uploads/2020/06/Recomendaciones-Epilepsia-SEN-2019.pdf
Chen Z, Brodie MJ, Liew D, et al. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279–86.
Golyala A, Kwan P. Drug development for refractory epilepsy: the past 25 years and beyond. Seizure. 2017;44:147–56.
Sultana B, Panzini MA, Veilleux Carpentier A, et al. Incidence and prevalence of drug-resistant epilepsy: a systematic review and meta-analysis. Neurology. 2021;96:805–17.
Gilliam F. Optimizing health outcomes in active epilepsy. Neurology. 2002;58:S9-20.
Sarkis RA, Goksen Y, Mu Y, et al. Cognitive and fatigue side effects of anti-epileptic drugs: an analysis of phase III add-on trials. J Neurol. 2018;265:2137–42.
Andrew T, Milinis K, Baker G, et al. Self-reported adverse effects of mono and polytherapy for epilepsy. Seizure. 2012;21:610–3.
Javed A, Cohen B, Detyniecki K, et al. Rates and predictors of patient-reported cognitive side effects of antiepileptic drugs: an extended follow-up. Seizure. 2015;29:34–40.
Fritz N, Glogau S, Hoffmann J, et al. Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy Behav. 2005;6:373–81.
Strzelczyk A, Schubert-Bast S. Psychobehavioural and cognitive adverse events of anti-seizure medications for the treatment of developmental and epileptic encephalopathies. CNS Drugs. 2022;36:1079–111.
ONTOZRY Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/ontozry-epar-product-information_en.pdf. Accessed Aug 2023.
XCOPRI Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212839s000lbl.pdf. Accessed Aug 2023.
Chung SS, French JA, Kowalski J, et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94(22):e2311–22.
Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38–48.
Schuetz E, Wagner K, Metternich B, et al. Effects of cenobamate on cognitive performance of epilepsy patients. Seizure. 2022;102:129–33.
Wilson SJ, Baxendale S, Barr W, et al. Indications and expectations for neuropsychological assessment in routine epilepsy care: report of the ILAE Neuropsychology Task Force, Diagnostic Methods Commission, 2013–2017. Epilepsia. 2015;56(5):674–81.
Peña-Casanova J, Gramunt-Fombuena N, Quiñones-Ubeda S, et al., NEURONORMA Study Team. Spanish Multicenter Normative Studies (NEURONORMA Project): norms for the Rey–Osterrieth complex figure (copy and memory), and free and cued selective reminding test. Arch Clin Neuropsychol. 2009;24(4):371–93.
Grau-Guinea L, Pérez Enríquez C, García-Escobar G, et al. Development, equivalence study, and normative data of version B of the Spanish-language Free and Cued Selective Reminding Test. Neurologia. 2021;36(5):353–60.
Rey A. Test de copia y de reproducción de memoria de figuras geométricas complejas. Madrid: TEA Ediciones, S.A; 2003.
Jaimes-Bautista AG, Rodríguez-Camacho M, Martínez-Juárez IE, et al. Análisis cuantitativo y cualitativo de la fluidez verbal semántica en pacientes con epilepsia del lóbulo temporal. Neurologia. 2020;35:1–9.
Peña-Casanova J, Quiñones-Ubeda S, Gramunt-Fombuena N, et al., NEURONORMA Study Team. Spanish Multicenter Normative Studies (NEURONORMA Project): norms for verbal fluency tests. Arch Clin Neuropsychol. 2009;24(4):395–411.
Torralva T, Roca M, Gleichgerrcht E, et al. INECO Frontal Screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia. J Int Neuropsychol Soc. 2009;15:777–86.
Wechsler D. Escala de Inteligencia de Wechsler para adultos-III. Manuel de aplicación y corrección. 3° Edición. Madrid: TEA Ediciones, S.A; 2001.
Peña-Casanova J, Quiñones-Ubeda S, Quintana-Aparicio M, et al., NEURONORMA Study Team. Spanish Multicenter Normative Studies (NEURONORMA Project): norms for verbal span, visuospatial span, letter and number sequencing, trail making test, and symbol digit modalities test. Arch Clin Neuropsychol. 2009;24(4):321–41.
Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc. 2006;1:2277.
Sedó MA. ‘5 digit test’: a multilinguistic non-reading alternative to the Stroop test. Rev Neurol. 2004;38:824–8.
Sedó MA. Manual del Test de los 5 dígitos. TEA Ediciones. 2009. https://web.teaediciones.com/FDT--TEST-DE-LOS-CINCO-DIGITOS.aspx. Accessed 18 Jan 2024.
Brooks BL, Holdnack JA, Iverson GL. To change is human: “abnormal” reliable change memory scores are common in healthy adults and older adults. Arch Clin Neuropsychol. 2016;31:1026–36.
Klein P, Aboumatar S, Brandt C, et al. Long-term efficacy and safety from an open-label extension of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2022;99(10):e989–98.
Makridis KL, Bast T, Prager C, et al. Real-world experience treating pediatric epilepsy patients with cenobamate. Front Neurol. 2022;13: 950171.
Varughese RT, Shah YD, Karkare S, et al. Adjunctive use of cenobamate for pediatric refractory focal-onset epilepsy: a single-center retrospective study. Epilepsy Behav. 2022;130: 108679.
Elliott T, Ridley-Pryor T, Gienapp AJ, et al. Initial real-world experience with cenobamate in adolescents and adults: a single center experience. Pediatr Neurol. 2022;129:19–23.
Catalán-Aguilar J, Hampel KG, Cano-López I, et al. Prospective study of cenobamate on cognition, affectivity, and quality of life in focal epilepsy. Epilepsia Open. 2023. https://doi.org/10.1002/epi4.12857. (Epub ahead of print).
Witt J-A, Elger CE, Helmstaedter C. Adverse cognitive effects of antiepileptic pharmacotherapy: each additional drug matters. Eur Neuropsychopharmacol. 2015;25:1954–9.
Villanueva V, Santos-Carrasco D, Cabezudo-García P, et al. Real-world safety and effectiveness of cenobamate in patients with focal onset seizures: outcomes from an expanded access program. Epilepsia Open. 2023. https://doi.org/10.1002/epi4.12757. (Online ahead of print).
Sperling MR, Abou-Khalil B, Aboumatar S, et al. Efficacy of cenobamate for uncontrolled focal seizures: post hoc analysis of a phase 3, multicenter, open-label study. Epilepsia. 2021;62(12):3005–15.
Rosenfeld WE, Nisman A, Ferrari L. Efficacy of adjunctive cenobamate based on number of concomitant antiseizure medications, seizure frequency, and epilepsy duration at baseline: a post-hoc analysis of a randomized clinical study. Epilepsy Res. 2021;172: 106592.
Barbieri MA, Perucca E, Spina E, et al. Cenobamate: a review of its pharmacological properties, clinical efficacy and tolerability profile in the treatment of epilepsy. CNS Neurol Disord Drug Targets. 2022. https://doi.org/10.2174/1871527321666220113110044. (Epub ahead of print).
Witt J-A, Helmstaedter C. Monitoring the cognitive effects of antiepileptic pharmacotherapy—approaching the individual patient. Epilepsy Behav. 2013;26:450–6.
Park S-P, Kwon S-H. Cognitive effects of antiepileptic drugs. J Clin Neurol. 2008;4:99–106.
Meador KJ, Yang H, Pina-Garza JE, et al. Cognitive effects of adjunctive perampanel for partial-onset seizures: a randomized trial. Epilepsia. 2016;57:243–5.
Acknowledgements
The authors acknowledge Karthinathan Thangavelu for assistance with statistical analysis and Ian Marshall (WriteMedical Ltd) for editorial assistance (including writing the draft manuscript, creating figures and tables, and journal styling).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for open access publishing: Universidad Málaga/CBUA. This study has been funded by Andalusian Network of Clinical and Translational Research in Neurology (Neuro-RECA) of the Ministry of Health and Consumer Affairs of Andalusia (code: RIC-0111-2019). Open access for this paper has been sponsored by the University of Malaga.
Conflict of Interest
PJSC has received consultant and/or speaker honoraria from Angelini Pharma, Bial, Eisai, GW Pharmaceuticals (now a part of Jazz Pharmaceuticals), Roche Pharmaceuticals, UCB Pharma, and Zogenix España. PCG has received consultant and/or speaker honoraria from Angelini-Pharma, Bial, Eisai, GW Pharmaceuticals (now a part of Jazz Pharmaceuticals), UCB Pharma, Exeltis, and Neuraxpharm. GGM has received consultant and/or speaker honoraria from Bial, Eisai, GW Pharmaceuticals (now a part of Jazz Pharmaceuticals), and UCB Pharma. JDP belongs to the Medical Department of Angelini pharmaceuticals. TRG has no disclosures.
Ethics Approval
We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The study protocol was approved by the hospital’s ethics committee and was in accordance with the code of ethics set out in the 1964 Declaration of Helsinki and its later amendments.
Consent to Participate
For all patients participating in the Spanish cenobamate EAP, written informed consent was obtained from the patient or their legal representative.
Consent for Publication
Not applicable.
Availability of Data and Material
Anonymized data not published within this article will be made available by request from any qualified investigator.
Code Availability
Not applicable.
Author’s Contributions
PJSC*: Major role in the acquisition of data, study concept or design, and analysis or interpretation of data. TRG*: Major role in the acquisition of data, study concept or design, and analysis or interpretation of data. PCG: Analysis or interpretation of data. GGM: Analysis or interpretation of data. JDP: Study concept or design. *PJSC and TRG contributed equally to this paper. All authors have read and approved the final submitted manuscript and agree to be accountable for the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Serrano-Castro, P.J., Ramírez-García, T., Cabezudo-Garcia, P. et al. Effect of Cenobamate on Cognition in Patients with Drug-Resistant Epilepsy with Focal Onset Seizures: An Exploratory Study. CNS Drugs 38, 141–151 (2024). https://doi.org/10.1007/s40263-024-01063-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-024-01063-6