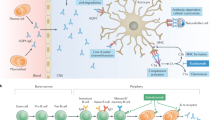
Abstract
Inebilizumab (Uplizna®) is a recently approved monoclonal antibody for use in adults with neuromyelitis optica spectrum disorder (NMOSD) who are anti-aquaporin-4 (AQP4) antibody seropositive. Inebilizumab targets the B cell antigen CD19 and effectively depletes circulating B cells, thus suppressing inflammatory NMOSD attacks that are potentially disabling or life-threatening. It is approved as an intravenous infusion in several countries. In the pivotal phase 2/3 N-MOmentum trial, inebilizumab reduced the risk of NMOSD attacks compared with placebo, including in AQP4-antibody seropositive patients. Inebilizumab also significantly reduced the risk of disability score worsening, the number of NMOSD-related hospitalisations and MRI lesion count, but had no significant effect on low-contrast binocular vision. The treatment effect on relapse risk and disability scores was sustained in inebilizumab-treated patients for ≥ 4 years during the open-label extension. Inebilizumab was generally well tolerated, with the most common adverse events being urinary tract infection and arthralgia. Thus, inebilizumab is an effective treatment option for adults with AQP4-antibody seropositive NMOSD.
Plain Language Summary
Neuromyelitis optica spectrum disorder (NMOSD) is a chronic condition denoted by relapsing autoimmune attacks affecting the central nervous system, which may lead to accruing disability or death. It is frequently associated with anti-aquaporin-4 (AQP4) autoantibodies. In recent years, three new monoclonal antibody therapies have gained regulatory approval for the treatment of NMOSD. Inebilizumab (Uplizna®), a monoclonal antibody that targets B cells, is approved for use in AQP4-antibody seropositive adults as an intravenous infusion. Inebilizumab was effective at preventing NMOSD relapse compared with placebo in a pivotal phase 2/3 trial. It also prevented worsening of disability scores, and decreased the number of NMOSD-related hospitalisations and MRI lesions, but did not significantly improve low-contrast binocular vision. The clinical benefit of inebilizumab was maintained long-term (≥ 4 years in the open-label extension). Inebilizumab was generally well tolerated, with most adverse events being mild to moderate in severity. The most common adverse events were urinary tract infection and joint pain. Inebilizumab provides an effective option for preventing NMOSD attacks in adults who are AQP4-antibody seropositive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.20506548. |
Monoclonal antibody that depletes a broad range of B cells, including plasmablasts and some plasma cells |
Effectively prevents NMOSD relapses, including in AQP4-antibody seropositive patients |
Clinical benefit is sustained over long-term treatment (≥ 4 years) |
Generally well tolerated; the most common adverse events were urinary tract infection and arthralgia |
1 Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a chronic disorder of the CNS characterized by relapsing inflammatory autoimmune attacks affecting the spinal cord, optic nerve, brain and/or brainstem [1]. Once considered a subclass of multiple sclerosis (MS), NMOSD is now recognised as a distinct disease. Recovery following NMOSD attacks is often incomplete, leading to accruing disability, including paralysis and blindness, and potentially death. Severe disability can occur rapidly; thus, preventing cumulative NMOSD attacks is of vital importance [1].
NMOSD can be defined and stratified by the presence/absence of anti-aquaporin-4 (AQP4) immunoglobulin (Ig) G autoantibodies in the serum [2]. This highly specific biomarker is detected in ≈ 76% of patients diagnosed with NMOSD. Although the exact aetiology of NMOSD is yet to be defined, evidence suggests that AQP4-antibodies (which target the most prevalent astrocyte water channel in the CNS) are likely pathogenic [2]. These autoantibodies initiate the complement cascade, thereby causing astrocyte injury and recruitment of inflammatory cells to the site [3]. Furthermore, type 1 interferon, which can be elevated in NMOSD, may cause some B cells to express interleukin (IL)-6 and promote the proliferation of inflammatory T cells, further driving CNS tissue damage [4]. This has led to investigations focused on components of these inflammatory pathways as therapeutic targets [1].
Treatment of NMOSD often includes intravenous corticosteroids with concurrent or rescue plasmapheresis to treat acute inflammatory attacks [5]. Early initiation of immunosuppressive maintenance therapy is recommended to prevent relapses [5]. Immunosuppression is required long term, with a high risk of relapse upon discontinuation of therapy [6]. Preceding the approval of a monoclonal antibody therapy, no approved preventative treatments for NMOSD attacks were available. Drugs such as rituximab (a monoclonal antibody that binds B cell antigen CD20) and azathioprine have been commonly used off-label as first-line maintenance therapies for NMOSD [1, 5, 7]. However, many patients experience breakthrough attacks, with relapse-free rates between 30 and 80% [1]. Treatment options with robust evidence of efficacy were urgently needed.
In recent years, three new monoclonal antibody therapies have gained regulatory approval for the treatment of NMOSD: inebilizumab (targeting CD19), eculizumab (targeting complement protein C5) and satralizumab (targeting IL-6 receptor) [7]. Inebilizumab (Uplizna®) is approved in several countries, including the USA [8] and EU [9] where inebilizumab is approved in adults with AQP4-antibody seropositive NMOSD. This article summarises the key pharmacological properties of inebilizumab and reviews its efficacy and tolerability in the treatment of NMOSD.
2 Pharmacodynamic Properties
Inebilizumab is a humanised, afucosylated IgG1κ monoclonal antibody that targets the B cell-specific antigen CD19 [10]. The exact mechanism behind the treatment effect of inebilizumab in NMOSD is yet to be defined. However, it is presumed to involve binding to the B cell, resulting in antibody-dependent, cell-mediated cytotoxicity. CD19 is expressed on a wider range of B cells than CD20. Unlike CD20, which is also present on a small subset of T cells [11], CD19 is specific for cells of the B cell lineage, including precursor B cells, plasmablasts and a subset of plasma cells [10]. Plasma cells are the primary source of autoantibodies such as AQP4 antibodies. Some B cells also produce IL-6, which is elevated in patients with NMOSD [4]. Thus, B cell depletion is expected to suppress the inflammatory attacks characteristic of NMOSD [10].
Dose-escalating phase 1 trials of intravenous inebilizumab in patients with systemic sclerosis (single dose; 0.1–10 mg/kg) and relapsing MS (two doses of 30, 100 or 600 mg 2 weeks apart) demonstrated that all doses tested rapidly decreased B cell levels, as measured by circulating CD20+ cells as a proxy for CD19+ B cells [12, 13]. Higher doses were associated with a greater proportion of patients achieving B cell depletion (i.e. a ≥ 90% reduction in B cell level from baseline) and more sustained depletion [12, 13].
In the phase 2/3 N-MOmentum study (Sect. 4), patients with NMOSD who received intravenous inebilizumab (two doses of 300 mg 2 weeks apart) experienced a rapid decrease in CD20+ B cell levels to < 10% of baseline within 4 weeks (p < 0.0001) [14]. This was maintained for the duration of the randomized controlled period (RCP; 6.5 months). In the open-label extension (OLE; Sect. 4.1), B cell levels remained low in inebilizumab-treated patients for up to 4 years with repeated dosing every 6 months [15]. Patients with a greater extent of B cell depletion (≤ 4 cells/μl) with inebilizumab had a moderately lower annualised attack rate (AAR) and fewer new or enlarging T2 lesions than those with a B cell count of > 4 cells/μl; the AAR and the number of new or enlarging T2 lesions observed in both subgroups were lower with inebilizumab than with placebo [16].
Immunogenicity is a possible issue with protein-based therapies. Anti-drug antibodies (ADAs) emerged during N-MOmentum in five (3%) inebilizumab-treated patients and four (7%) placebo-treated patients [14]. At the end of the OLE, the overall incidence of ADAs was 7.1% [9]. This did not affect the pharmacodynamic or pharmacokinetic profile of the drug. Vaccine-generated antibody concentrations (as assessed by anti-tetanus IgG levels) were not affected by inebilizumab [14].
3 Pharmacokinetic Properties
In general, intravenous inebilizumab has a pharmacokinetic profile typical of monoclonal IgG antibodies [17]. In N-MOmentum (Sect. 4), the median time to maximum plasma concentration was 0.07 days after the first dose of inebilizumab [14]. Population pharmacokinetic analyses, including N-MOmentum [14] and two smaller studies in patients with systemic sclerosis [13] and relapsing MS [12], showed the volume of distribution in the central and peripheral compartments was 2.95 and 2.57 L [17].
The pharmacokinetics of inebilizumab follow a two-compartment model with parallel first-order and time-dependent nonlinear elimination pathways [17]. Inebilizumab is predominately eliminated by proteolytic enzymes via the reticuloendothelial system. The estimated typical clearance is 188 ml/day, similar to the clearance of endogenous IgG. At the therapeutic dose of inebilizumab, the nonlinear elimination pathway is saturated. This is suggestive of receptor-mediated clearance, with inebilizumab clearance decreasing over time as the pool of CD19+ cells is depleted [17]. The mean elimination half-life of inebilizumab is 18 days [14, 17].
Inebilizumab is not cleared by the liver or kidneys [9]. While no formal studies have been performed in patients with impaired kidney or hepatic function [8, 9], there was no association of differential inebilizumab clearance with liver or kidney function biomarkers in population pharmacokinetic modelling [17]. Pharmacokinetic parameters were similar in patients across baseline disease characteristics, age, sex and race [17]. Concomitant drugs frequently used by patients with NMOSD (e.g. paracetamol, diphenhydramine, prednisolone and methylprednisolone) had no effect on inebilizumab clearance [17]. As inebilizumab is not metabolised or cleared by cytochrome P450 enzymes, efflux pumps or protein binding processes, there is a low risk of potential interaction with other drugs [8, 9, 17].
4 Therapeutic Efficacy of Inebilizumab
The efficacy of inebilizumab in NMOSD has been evaluated in the pivotal N-MOmentum study, a randomized, double-blind, placebo-controlled, multinational phase 2/3 trial [14]. The study enrolled patients aged ≥ 18 years diagnosed with NMOSD, an Expanded Disability Status Scale (EDSS) score of ≤ 8.0, and having ≥ one documented NMOSD attack needing rescue therapy within the year before screening or ≥ two attacks needing rescue therapy within the 2 years prior to screening. Rescue therapy was defined as intravenous corticosteroids, intravenous Ig, plasma exchange, or any combination of these. N-MOmentum enrolled both AQP4-antibody seropositive (n = 213) and seronegative (n = 17) patients. AQP4-antibody seronegative patients were required to meet additional criteria to avoid misdiagnosis of NMOSD [18].
A total of 231 eligible patients were randomized 3:1 into the inebilizumab or placebo treatment arms [14]. Patients received 300 mg of inebilizumab (n = 174) or placebo (n = 56) intravenously on days 1 and 15 (600 mg in total). To reduce the risk of an NMSOD attack immediately after the first dose of inebilizumab, all patients were treated with oral corticosteroids (20 mg/day prednisone or equivalent) between days 1–14, and tapered until day 21. Following the RCP, all patients were able to enrol in an OLE (Sect. 4.1) [14].
Demographic and clinical characteristics were generally balanced between the inebilizumab and placebo treatment arms, including age (mean 43.0 vs 42.6 years), disease duration (mean 2.4 vs 2.8 years), number of gadolinium-enhancing lesions (mean 1.2 vs 0.9) and EDSS score (mean 3.8 vs 4.2) [14]. The majority of patients were female (91% with inebilizumab vs 89% with placebo), had received previous treatment (99% vs 98%), and had received previous maintenance therapy (66% vs 68%). Baseline characteristics were similar in the AQP4-antibody seropositive subgroup and the overall population [14].
The primary endpoint was time to the onset of an NMOSD attack, defined as the presence of a new symptom or worsening of an existing symptom related to NMOSD that met at least one of the protocol-defined criteria for an attack, as determined by an adjudication committee [14, 19, 20]. Enrollment was halted early (on recommendation of the data-monitoring committee) due to clear evidence of efficacy with a conditional power of > 99% [14].
Inebilizumab significantly increased the time to onset of an NMOSD attack compared with placebo [14]. Significantly fewer patients in the inebilizumab group experienced an NMOSD attack during the RCP compared with those in the placebo group (Table 1). The number-needed-to-treat (NNT) to prevent an attack was 3.73 (95% CI 3.06–5.66). Inebilizumab was similarly efficacious in the AQP4-antibody seropositive subgroup (Table 1); the NNT was 3.23 (95% CI 2.72–4.54). However, the efficacy of inebilizumab could not be demonstrated in the AQP4-antibody seronegative subgroup, due to the small number of seronegative patients enrolled (13 in the inebilizumab group and 4 in the placebo group). In this subgroup, three NMOSD attacks occurred in the inebilizumab group, while no attacks occurred in the placebo group [14].
Subgroup analysis showed that the treatment effect of inebilizumab was consistent regardless of the location of attack (i.e. optic nerve or spinal cord; there were too few brainstem attacks to allow assessment of this location), race or ethnicity, body mass index, and whether only attacks adjudicated by unanimous decision or all investigator-suspected attacks were considered [14]. Further pre-planned sensitivity analyses demonstrated that inebilizumab consistently reduced the likelihood of an NMOSD-related attack regardless of who reported the attack or how it was evaluated, and across a range of demographic and baseline characteristics (all with an HR ≤ 0.4 and p < 0.05) [21]. The efficacy of inebilizumab in patients who had previously received rituximab was also similar to that in patients who had not previously received rituximab [22]. Seven patients in N-MOmentum had previously experienced a relapse while receiving rituximab; none of these patients experienced an attack while receiving inebilizumab during the RCP or OLE [22].
Four key secondary endpoints were analysed in N-MOmentum [14, 20]. Inebilizumab stabilised EDSS scores, with significantly fewer patients in the inebilizumab group having a worsened EDSS score than those in the placebo group (Table 1) [14]. The NNT to prevent one case of EDSS score worsening was 6 [23]. Subgroup analysis confirmed that the effect of inebilizumab on lowering the risk of EDSS score worsening was consistent regardless of baseline EDSS score, disease duration and number of previous attacks [23]. Post hoc analysis showed that inebilizumab reduced the risk of 3-month EDSS-confirmed disability progression at any point during the RCP, though the time-to-event design of the trial limits the interpretation of these findings [23]. Inebilizumab also significantly reduced the cumulative active MRI lesion count and the number of NMOSD-related hospitalisations (Table 1) [14]. There was no treatment effect on the low-contrast visual acuity binocular score compared with placebo (Table 1). However, in a post hoc sensitivity analysis, there were fewer incidences of optic neuritis in the inebilizumab group than the placebo group (HR 0.288; 95% CI 0.120–0.694 in the intention-to-treat population) [14].
Serum glial fibrillary acidic protein (sGFAP) may be a useful biomarker for NMOSD disease activity, with higher levels correlated with greater attack risk and severity [24]. In patients who did not experience an attack during N-MOmentum, inebilizumab was associated with reductions from baseline in sGFAP levels. In patients who did experience an attack, inebilizumab was associated with smaller attack-related increases in sGFAP and lower sGFAP concentrations compared with placebo [24].
4.1 Long-Term Efficacy
The long-term efficacy and safety of inebilizumab was monitored in 216 patients from N-MOmentum who entered the OLE, during which they received 300 mg of inebilizumab every 6 months (after an initial 300 mg on days 1 and 15 if the patient had originally been randomized to placebo) [25]. The mean duration of inebilizumab treatment was 3.2 years (maximum 5.5 years). Supporting the efficacy results reported in the initial RCP, 87.7% of patients who continued inebilizumab and 83.4% of patients who switched from placebo to inebilizumab were attack-free during the OLE. The overall AAR for both the RCP and OLE was 0.092 attacks/person-year [25]. A post hoc analysis of AARs in AQP4-antibody seronegative patients during the RCP and OLE suggested some benefit of inebilizumab in this subgroup [26].
Of the 213 AQP4-antibody seropositive patients enrolled in N-MOmentum, 201 continued to the OLE [15]. In a post hoc analysis of 75 AQP4-antibody seropositive patients who received inebilizumab for ≥ 4 years, 18 adjudicated attacks occurred in 13 patients, with four patients experiencing multiple attacks. Twelve attacks (67%) occurred within the first year of treatment and six attacks (33%) occurred in years 2–4. Five attacks (28%) were major in severity (all except one occurred in year 1) and 11 (61%) were minor; the severity of two attacks was not captured. The AAR was 0.052 attacks/person-year (95% CI 0.029–0.092). Attack-free probability was 87% after 1 year and was stable through to year 4. EDSS scores were also stable throughout this period [15].
5 Tolerability of Inebilizumab
Inebilizumab was generally well tolerated in adults with NMOSD in N-MOmentum and its OLE [14, 15, 25]. Safety analysis was conducted in the as-treated population [14]. The rate of adverse events (AEs) was similar in the inebilizumab and placebo groups during the 28-week RCP. In the inebilizumab group, AEs (not including events related to an adjudicated NMOSD attack) occurred in 125 (72%) patients, compared with 41 (73%) in the placebo group. The most common AEs (incidence ≥ 10%) associated with inebilizumab and occurring at a greater frequency than with placebo were urinary tract infection (UTI; 11% vs 9%) and arthralgia (10% vs 4%) [14].
Most other AEs occurred at similar frequencies in the inebilizumab and placebo treatment arms [14]. These included infusion-related reactions (IRRs; 9% with inebilizumab vs 11% with placebo), which were all mild to moderate in severity. Other common AEs (incidence ≥ 5% with inebilizumab) included headache (7% vs 7% with placebo), back pain (7% vs 4%), nasopharyngitis (7% vs 11%) and diarrhoea (5% vs 5%). Eight (5%) patients in the inebilizumab group experienced a serious AE, compared with five (9%) in the placebo group. No patients experienced more than one serious AE. No deaths occurred in the RCP of N-MOmentum [14].
The rate of AEs in the AQP4-antibody seropositive group was similar to that in the overall as-treated population [14]. The AE profiles in subgroups of African American patients [27] and patients with prior rituximab treatment were also similar to that in the overall population [22].
No new safety or tolerability concerns emerged after long-term treatment with inebilizumab [15, 25]. In the OLE, 89 (39.6%) patients reported an AE, with the most common being UTI (26.2%), nasopharyngitis (20.9%) and arthralgia (17.3%) [25]. IRRs with inebilizumab occurred in 28 (12.9%) patients. The overall rate of IRRs throughout the study was 11.1 events per 100 person-years. Three deaths occurred during the OLE; the possibility that one was treatment related could not be ruled out [14, 25].
Among AQP4-antibody seropositive patients who had been treated with inebilizumab for ≥ 4 years, 70 (90%) of 75 patients reported an AE and 29 (39%) patients experienced a treatment-related AE [15]. Seven (9%) patients experienced a serious AE; in two patients, these were considered to be treatment-related [15].
Immunosuppressive therapy may increase the risk of malignancy [9]; infection, including progressive multifocal leukoencephalopathy (PML) and reactivation of hepatitis B, tuberculosis (TB) [8, 9] and hepatitis C virus [9]; and late-onset neutropenia [9]. No cases of malignancy or PML have been reported so far in inebilizumab-treated patients [14, 15], although PML was included as a differential diagnosis in the death of one patient [14]. The rate of infection among patients who had received inebilizumab for ≥ 4 years was 71.4 events per 100 person-years (occurring in 79% of patients) [15]. Neither the rate nor severity of infection increased over time and most infections were mild or moderate in severity [15, 25]. Vigilance around infections may be particularly necessary in patients with prior rituximab treatment [22]. A post hoc analysis revealed that almost all patients (94%) with prior rituximab treatment experienced an infection(s) during inebilizumab treatment, although the actual number of patients was small (n = 17) [22].
In N-MOmentum, lymphocyte counts and IgG concentrations were lower in the inebilizumab group than in the placebo group, likely due to the depletion of B cells (Sect. 2) [14]. Hypogammaglobulinaemia during B cell-depleting therapy has previously been associated with higher risk of infection in NMOSD [28]. In AQP4-antibody seropositive patients treated with inebilizumab for ≥ 4 years, 46.7% of patients had normal IgG levels (≥ 700 mg/dL), 29.3% had mild hypogammaglobulinaemia (500 to < 700 mg/dL), 20% had moderate hypogammaglobulinaemia (300 to < 500 mg/dL) and 4% had severe hypogammaglobulinaemia (< 300 mg/dL) [15]. Low IgG titres were not associated with severe infection, although the number of patients was small [15].
6 Dosage and Administration
Inebilizumab is approved in the USA [8] and EU [9] for the treatment of NMOSD in AQP4-antibody seropositive adults. The recommended treatment schedule is two initial intravenous infusions of inebilizumab (300 mg each) given 2 weeks apart to deplete B cells, followed by a single infusion (300 mg) every 6 months, beginning from the first dose. The infusion is titrated to completion (≈ 90 min) [8, 9].
Before initiating inebilizumab, patients should be screened for hepatitis B virus, quantitative serum Igs, TB and, in the EU, hepatitis C virus, B cell count and complete blood count [8, 9]. Inebilizumab is contraindicated in patients with active hepatitis B infection, active or untreated latent TB, and those who have previously experienced a life-threatening IRR to inebilizumab. In the EU, inebilizumab is also contraindicated in patients who are severely immunocompromised or have a severe active infection, history of PML, active malignancy or hypersensitivity to any substance in the infusion [9]. Serum Ig levels should be monitored during inebilizumab treatment [8, 9]. If low Ig levels are associated with serious opportunistic or recurrent infections, discontinuation of inebilizumab should be considered [8, 9].
Prior to every dose of inebilizumab, an active infection should be ruled out and patients should be premedicated with a corticosteroid, an antihistamine, and an antipyretic to reduce the risk of an IRR [8, 9]. Patients should be monitored for IRRs during and for ≥ one hour after inebilizumab infusion. In the case of active infection, treatment should be delayed until the infection is resolved [8, 9]. During N-MOmentum, all patients were treated with oral corticosteroids at the start of inebilizumab treatment (Sect. 4) [14]. Local prescribing information should be consulted for further details surrounding premedication, dosage and administration, contraindications, warnings and precautions and use in special populations.
7 Current Status of Inebilizumab in the Management of NMOSD
Inebilizumab gained approval for the treatment of NMOSD in AQP4-antibody seropositive adults based on results from the pivotal N-MOmentum trial (Sect. 4) [14]. It is the only drug approved for NMOSD that binds to CD19, which targets a broader range of B cells (including plasmablasts and some plasma cells) than anti-CD20 antibodies [10]. Inebilizumab significantly reduced the risk of NMOSD attacks compared with placebo during the RCP in N-MOmentum (Sect. 4). This benefit was seen across all patient subgroups analysed, including AQP4-antibody seropositive patients. The efficacy of inebilizumab could not be statistically evaluated in AQP4-antibody seronegative patients due to the low number of seronegative patients enrolled, although AARs may suggest some benefit of inebilizumab in this subgroup (Sect. 4.1). Inebilizumab may have a protective effect even in patients who did experience an NMOSD attack (Sect. 4) [24].
Secondary outcomes from N-MOmentum show that, relative to placebo, inebilizumab also prevented worsening in disability scores (measured by EDSS) and reduced the cumulative active MRI lesion count and the number of NMOSD-related hospitalisations (Sect. 4). However, inebilizumab did not significantly improve low-contrast binocular vision (Sect. 4), suggesting that it may not reduce the severity of optic neuritis attacks or promote recovery [14]. The apparent lack of treatment effect may also be due to study design (measurement of binocular vs monocular low-contrast visual acuity) and the low incidence of optic neuritis attacks [14].
The effect of inebilizumab on NMOSD attack risk was sustained throughout the OLE (Sect. 4.1). Furthermore, in AQP4-antibody seropositive patients who had received inebilizumab for ≥ 4 years, most (67%) NMOSD attacks occurred in the first year of treatment (Sect. 4.1). This suggests that inebilizumab may have enhanced efficacy after longer-term treatment [15]. The mechanisms that underlie this are unknown, but may be related to the extent of B cell depletion after continued treatment [15]. EDSS scores also remained stable over ≥ 4 years (Sect. 4.1), suggesting that inebilizumab may prevent disability worsening over the longer term [15].
Inebilizumab was generally well tolerated, with most AEs being mild or moderate in severity (Sect. 5). No new safety concerns emerged after long-term treatment. The most common AEs were UTI, arthralgia, IRRs, headache, back pain, nasopharyngitis and diarrhoea. B cell-depleting therapies have been associated with an increased risk of hypogammaglobulinaemia, malignancies and infections, including PML. To mitigate this, patients should be monitored for IRRs during and after inebilizumab infusion, and serum Ig levels should be monitored throughout inebilizumab treatment (Sect. 6).
Eculizumab [29, 30] and satralizumab [31, 32] have also been approved as monotherapies for NMOSD in AQP4-antibody seropositive adults. To date, there have been no head-to-head studies comparing inebilizumab with these therapies. Direct comparison of clinical trial results is difficult due to major differences in study designs and patient characteristics. Nonetheless, a recent network meta-analysis suggested that eculizumab may be more efficacious than inebilizumab (HR 0.11, 95% credible interval 0.02–0.68) and satralizumab (0.10, 0.01–0.65) as a monotherapy for preventing relapse in patients with AQP4-antibody seropositive NMOSD [33]. These findings are limited by differences in patient inclusion criteria, attack definition and adjudication across trials, and the small number of patients who received eculizumab monotherapy (n = 21), which led to the need to use an imputed hazard ratio in this meta-analysis [34, 35]. Potential drawbacks of eculizumab include cost, frequency of administration (every 2 weeks) and a black box warning of the risk of life-threatening or fatal meningococcal infection [1, 7, 29, 30].
Inebilizumab is the only NMOSD treatment shown to have a statistically significant effect on disability worsening as measured by EDSS score (Sect. 4). Phase 3 trials of eculizumab [36] and satralizumab [37] also measured EDSS scores as secondary outcomes but did not find a statistically significant effect of drug treatment, which may have been due to lack of statistical power or differences in study design [1]. Inebilizumab [8, 9] also has the advantage of requiring less frequent administration than eculizumab (every 2 weeks) [29, 30] and satralizumab (every 4 weeks) [31, 32]. Satralizumab, a subcutaneous injection, can be self-administered [31, 32] and may be preferred by some patients.
Overall, inebilizumab [14], eculizumab [36] and satralizumab [37] have all shown a proven benefit in preventing NMOSD relapses and are useful options that can be considered as first- or subsequent-line treatment in AQP4-antibody seropositive patients [7]. Efficacy, safety, cost, accessibility, convenience, previous treatment history and other factors may all impact the choice of therapy [1, 7].
Real-world data on the use of inebilizumab would be of interest [1]. Additionally, the long-term effects of inebilizumab and other treatments on disability progression in NMOSD and long-term safety warrant further investigation. Pharmacoeconomic analyses may also be of benefit. Although AQP4-antibody seropositive patients now have several treatment options, there remain unmet needs in the treatment of AQP4-antibody seronegative patients [7], incomplete recovery from NMOSD attacks [7] and management of NMOSD symptoms such as chronic pain [38, 39].
In conclusion, current evidence indicates that inebilizumab is an effective treatment option for preventing potentially debilitating NMOSD relapses in adults who are AQP4-antibody seropositive.
Data Selection Inebilizumab: 191 records identified
Duplicates removed | 55 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 66 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 31 |
Cited efficacy/tolerability articles | 13 |
Cited articles not efficacy/tolerability | 26 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were inebilizumab, Uplizna, MEDI 551, neuromyelitis optica spectrum disorder, NMOSD. Records were limited to those in English language. Searches last updated 17 Aug 2022 | |
Change history
03 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40263-022-00957-7
References
Pittock SJ, Zekeridou A, Weinshenker BG. Hope for patients with neuromyelitis optica spectrum disorders—from mechanisms to trials. Nat Rev Neurol. 2021;17(12):759–73.
Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177.
Li J, Bazzi SA, Schmitz F, et al. Molecular level characterization of circulating aquaporin-4 antibodies in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2021;8(5): e1034.
Agasing AM, Wu Q, Khatri B, et al. Transcriptomics and proteomics reveal a cooperation between interferon and T-helper 17 cells in neuromyelitis optica. Nat Commun. 2020;11(1):2856.
Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol. 2014;261(1):1–16.
Kim SH, Jang H, Park NY, et al. Discontinuation of immunosuppressive therapy in patients with neuromyelitis optica spectrum disorder with aquaporin-4 antibodies. Neurol Neuroimmunol Neuroinflamm. 2021;8(2): e947.
Levy M, Fujihara K, Palace J. New therapies for neuromyelitis optica spectrum disorder. Lancet Neurol. 2021;20(1):60–7.
Horizon Therapeutics USA Inc. UPLIZNA (inebilizumab-cdon) injection, for intravenous use [US prescribing information]. 2021. https://dailymed.nlm.nih.gov/dailymed/. Accessed 17 Aug 2022.
Viela Bio B.V. Uplizna : EPAR - product information. 2022. https://www.ema.europa.eu/. Accessed 17 Aug 2022.
Chen D, Gallagher S, Monson NL, et al. Inebilizumab, a B cell-depleting anti-CD19 antibody for the treatment of autoimmune neurological diseases: insights from preclinical studies. J Clin Med. 2016;5:12.
Palanichamy A, Jahn S, Nickles D, et al. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J Immunol. 2014;193(2):580–6.
Agius MA, Klodowska-Duda G, Maciejowski M, et al. Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study. Mult Scler. 2019;25(2):235–45.
Schiopu E, Chatterjee S, Hsu V, et al. Safety and tolerability of an anti-CD19 monoclonal antibody, MEDI-551, in subjects with systemic sclerosis: a phase I, randomized, placebo-controlled, escalating single-dose study. Arthritis Res Ther. 2016;18(131):1–14.
Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394(10206):1352–63.
Rensel M, Zabeti A, Mealy MA, et al. Long-term efficacy and safety of inebilizumab in neuromyelitis optica spectrum disorder: analysis of aquaporin-4-immunoglobulin G-seropositive participants taking inebilizumab for ⩾4 years in the N-MOmentum trial. Mult Scler. 2021;28(6):925–32.
Bennett JL, Aktas O, Smith M, et al. Extent of B-cell depletion is associated with disease activity reduction in neuromyelitis optica spectrum disorder: results from the N-MOmentum study [abstract no. P028]. Mult Scler. 2021;27(Suppl 2):151–2.
Yan L, Kimko H, Wang B, et al. Population pharmacokinetic modeling of inebilizumab in subjects with neuromyelitis optica spectrum disorders, systemic sclerosis, or relapsing multiple sclerosis. Clin Pharmacokinet. 2021;61:387–400.
Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66(10):1485–9.
Cree BAC, Bennett JL, Sheehan M, et al. Placebo-controlled study in neuromyelitis optica—ethical and design considerations. Mult Scler. 2016;22(7):862–72.
Patra K, Cree BAC, Katz E, et al. Statistical considerations for an adaptive design for a serious rare disease. Ther Innov Regul Sci. 2016;50(3):375–84.
Cree BAC, Bennett JL, Kim HJ, et al. Sensitivity analysis of the primary endpoint from the N-MOmentum study of inebilizumab in NMOSD. Mult Scler. 2021;27(13):2052–61.
Flanagan EP, Levy M, Katz E, et al. Inebilizumab for treatment of neuromyelitis optica spectrum disorder in patients with prior rituximab use from the N-MOmentum study. Mult Scler Relat Disord. 2022;57(103352):1–6.
Marignier R, Bennett JL, Kim HJ, et al. Disability outcomes in the N-MOmentum trial of inebilizumab in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2021;8(3):1–15.
Aktas O, Smith MA, Rees WA, et al. Serum glial fibrillary acidic protein: a neuromyelitis optica spectrum disorder biomarker. Ann Neurol. 2021;89(5):895–910.
Cree BAC, Bennett JL, Weinshenker BG, et al. Safety and efficacy of inebilizumab in NMOSD over a mean treatment duration of 3.2 years: end of study data from the N-MOmentum trial [abstract no. P037]. Mult Scler. 2021;27(Suppl 2):158–60.
Marignier R, Pittock SJ, Paul F, et al. AQP4-IgG-seronegative patient outcomes in the N-MOmentum trial of inebilizumab in neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. 2022;57:103356.
Bernitsas E, Cimbora D, Dinh Q, et al. Safety, efficacy and pharmacokinetics of inebilizumab in African Americans with neuromyelitis optica spectrum disorder [abstract no P284 and Poster]. In: Americas Committee for Treatment and Research in Multiple Sclerosis 2022 Forum. 2022.
Avouac A, Maarouf A, Stellmann JP, et al. Rituximab-induced hypogammaglobulinemia and infections in AQP4 and MOG antibody-associated diseases. Neurol Neuroimmunol Neuroinflamm. 2021;8(3):e977.
Alexion Pharmaceuticals Inc. SOLIRIS (eculizumab) injection, for intravenous use [US prescribing information]. 2022. https://dailymed.nlm.nih.gov/dailymed/. Accessed 17 Aug 2022.
Alexion Europe SAS. Soliris : EPAR—product information. 2021. https://www.ema.europa.eu/. Accessed 17 Aug 2022.
Genentech Inc. ENSPRYNG (satralizumab-mwge) injection, for subcutaneous use [US prescribing information]. 2020. https://dailymed.nlm.nih.gov/dailymed/. Accessed 17 Aug 2022.
Roche Registration GmbH. Enspryng: EPAR—product information. 2021. https://www.ema.europa.eu/. Accessed 17 Aug 2022.
Wingerchuk DM, Zhang I, Kielhorn A, et al. Network meta-analysis of Food and Drug Administration-approved treatment options for adults with aquaporin-4 immunoglobulin G-positive neuromyelitis optica spectrum disorder. Neurol Ther. 2022;11(1):123–35.
Cree BAC, Greenberg B, Cameron C, et al. Letter to the editor regarding “Network meta-analysis of Food and Drug Administration-approved treatment options for adults with aquaporin-4 immunoglobulin G-positive neuromyelitis optica spectrum disorder.” Neurol Ther. 2022. https://doi.org/10.1007/s40120-022-00376-2.
Wingerchuk DM, Zhang I, Kielhorn A, et al. A response to: Letter to the editor regarding “Network meta-analysis of Food and Drug Administration-approved treatment options for adults with aquaporin-4 immunoglobulin G-positive neuromyelitis optica spectrum disorder.” Neurol Ther. 2022. https://doi.org/10.1007/s40120-022-00378-0.
Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614–25.
Traboulsee A, Greenberg BM, Bennett JL, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2020;19(5):402–12.
Asseyer S, Cooper G, Paul F. Pain in NMOSD and MOGAD: a systematic literature review of pathophysiology, symptoms, and current treatment strategies. Front Neurol. 2020;11:778.
Ayzenberg I, Richter D, Henke E, et al. Pain, depression, and quality of life in neuromyelitis optica spectrum disorder: a cross-sectional study of 166 AQP4 antibody-seropositive patients. Neurol Neuroimmunol Neuroinflamm. 2021;8(3):e985.
Acknowledgements
During the peer review process, the manufacturer of inebilizumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Tina Nie and Hannah Blair are salaried employees of Adis International Ltd/Springer Nature, and declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: A. Dinoto, Neurology Unit, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy; T. Misu, Department of Neurology, Tohoku University Hospital, Sendai, Japan; F. Paul, NeuroCure Clinical Research Center, Charité–Universitätsmedizin Berlin, Berlin, Germany; W. Qiu, Department of Neurology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China.
The original online version of this article was revised due to a retrospective Open Access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nie, T., Blair, H.A. Inebilizumab: A Review in Neuromyelitis Optica Spectrum Disorder. CNS Drugs 36, 1133–1141 (2022). https://doi.org/10.1007/s40263-022-00949-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-022-00949-7