Abstract
Background
Glatiramer acetate (GA) 20 mg/day (GA20) is associated with immediate post-injection reactions (PIRs). For convenience of use, approved GA 40 mg three times weekly (GA40) delivers a similar weekly dose. The dose and concentration of a single GA40 injection are, however, twice as high as for GA20, and post-injection adverse events may differ. Cases of atypical PIRs to GA40 prompted us to systematically monitor such events.
Objective
The aim was to characterize atypical PIRs in multiple sclerosis (MS) patients treated with GA40.
Methods
Clinical practice data were prospectively collected in consecutive relapsing–remitting MS patients. Descriptive statistics for categorical and continuous variables, Mann–Whitney and Chi-squared tests for baseline comparisons, and Cox regression models for association of variables to first atypical PIRs were applied.
Results
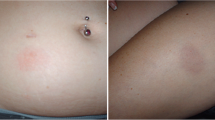
Forty-six out of 173 patients (26.6%) given GA40 experienced any PIRs. Of those, 38 (22.0%) had atypical, 14 (8.1%) had combined typical and atypical, and 26 (15.0%) had recurrent atypical PIRs, most frequently shivering (13.3%) and nausea/vomiting (8.1%). Compared to typical PIRs, onset of atypical PIRs was significantly delayed (median 30 vs 1 min, p < 0.0001), and their median duration longer (median 120 vs 6 min, p = 0.00013). Previous exposure to GA20 was associated with a lower risk of atypical PIRs [hazard ratio (HR) = 0.35, 95% confidence interval (CI) 0.17–0.72, p = 0.0039]. Patients experiencing PIRs with GA20 were at elevated risk for atypical PIRs with GA40 (HR = 5.75, 95% CI 1.66–19.94, p = 0.0059).
Conclusions
Atypical PIRs with GA40, especially gastrointestinal symptoms and/or fever/shivering, had a delayed onset and occurred in a significant proportion of our patients. Their real prevalence should be assessed in appropriately designed studies accounting for nocebo responses. Initial dose titration might reduce PIR frequency.

Similar content being viewed by others
References
Scott LJ. Glatiramer acetate: a review of its use in patients with relapsing-remitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. CNS Drugs. 2013;27(11):971–88. https://doi.org/10.1007/s40263-013-0117-3.
Bornstein MB, Miller A, Slagle S, Weitzman M, Crystal H, Drexler E, et al. A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis. N Engl J Med. 1987;317(7):408–14. https://doi.org/10.1056/nejm198708133170703.
Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45(7):1268–76.
Caporro M, Disanto G, Gobbi C, Zecca C. Two decades of subcutaneous glatiramer acetate injection: current role of the standard dose, and new high-dose low-frequency glatiramer acetate in relapsing-remitting multiple sclerosis treatment. Patient Preference Adherence. 2014;8:1123–34. https://doi.org/10.2147/ppa.s68698.
Teva Neuroscience Inc. COPAXONE (glatiramer acetate) solution for subcutaneous injection: USA prescribing information 2016. https://www.copaxone.com/Resources/pdfs/PrescribingInformation.pdf. Accessed 20 July 2017.
Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R. Three times weekly glatiramer acetate in relapsing-remitting multiple sclerosis. Ann Neurol. 2013;73(6):705–13. https://doi.org/10.1002/ana.23938.
Teva Neuroscience Inc. Teva announces U.S. FDA approval of three-times-a-week COPAXONE® (glatiramer acetate injection) 40 mg/mL. 2014. http://www.ir.tevapharm.com/mobile.view?c=73925&v=202&d=3&id=aHR0cDovL2FwaS50ZW5rd2l6YXJkLmNvbS9maWxpbmcueG1sP2lwYWdlPTkzNDQ3OTQmRFNFUT0yJlNFUT0mU1FERVNDPVNFQ1RJT05fRVhISUJJVCZleHA9JnN1YnNpZD01Nw%3D%3D. Accessed 20 July 2017.
Comi G, Cohen JA, Arnold DL, Wynn D, Filippi M. Phase III dose-comparison study of glatiramer acetate for multiple sclerosis. Ann Neurol. 2011;69(1):75–82. https://doi.org/10.1002/ana.22316.
Khan O, Rieckmann P, Boyko A, Selmaj K, Ashtamker N, Davis M, et al. Efficacy and safety of a three-times weekly dosing regimen of glatiramer acetate in relapsing-remitting multiple sclerosis patients: 3-year results of the Glatiramer Acetate Low-frequency Administration (GALA) open-label extension study (P7.273). Neurology. 2015;84(14 Supplement):P7–273.
Khan O, Rieckmann P, Boyko A, Selmaj K, Ashtamker N, Davis M, et al. Efficacy and safety of a 3 times weekly dosing regimen of glatiramer acetate in relapsing-remitting multiple sclerosis patients: 3-year results of the Glatiramer Acetate Low-frequency Administration (GALA) open-label extension study. Eur J Neurol. 2015;22:711.
Khan O, Rieckmann P, Boyko A, Selmaj K, Ashtamker N, Davis MD et al. Efficacy and safety of a three-times-weekly dosing regimen of glatiramer acetate in relapsing-remitting multiple sclerosis patients: 3-year results of the Glatiramer Acetate Low-Frequency Administration open-label extension study. Multiple sclerosis (Houndmills, Basingstoke, England). 2017;23(6):818–29. https://doi.org/10.1177/1352458516664033.
Kolodny S, Khan O, Rieckmann P, Davis MD, Ashtamker N, Steinerman JR et al. Efficacy and safety of a three-times weekly dosing regimen of glatiramer acetate in relapsing-remitting multiple sclerosis patients: 3-year results of the Glatiramer Acetate Low-Frequency Administration (GALA) open-label extension study. Multiple sclerosis (Houndmills, Basingstoke, England). 2016;22(3):417. https://doi.org/10.1177/1352458516629327.
Lobato-Berezo A, Martínez-Pérez M, Imbernón-Moya A. MA G-V. Nicolau syndrome after glatiramer acetate injection. Med Clin (Barc). 2015;145(12):e41.
MHRA. Public assessment report: Copaxone 40 mg/ml solution for injection, pre-filled syringe (glatiramer acetate). Procedure No: UK/H/0453/004/DC. 2014. http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con521776.pdf). Accessed 20 July 2017.
Mott S, Peña Z, Spain R, White K, Ehs B. Nicolau syndrome and localized panniculitis: a report of dual diagnoses with an emphasis on morphea profunda-like changes following injection with glatiramer acetate. J Cutan Pathol. 2016;43(11):1056–61. https://doi.org/10.1111/cup.12791.
Wolinsky J, Borresen T, Dietrich D, Gilder B, Sidi Y, Knappertz V, et al. GLACIER: an open-label, randomized, multicenter study to assess safety and tolerability of glatiramer acetate 40 mg/1 mL 3-times weekly versus 20 mg/1 mL Daily in patients with relapsing-remitting multiple sclerosis (S31.002). Neurology. 2014;82(10 Supplement):370–6.
Wolinsky JS, Borresen TE, Dietrich DW, Wynn D, Sidi Y, Steinerman JR, et al. GLACIER: an open-label, randomized, multicenter study to assess the safety and tolerability of glatiramer acetate 40 mg three-times weekly versus 20 mg daily in patients with relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2015;4(4):370–6. https://doi.org/10.1016/j.msard.2015.06.005.
Wynn D, Kolodny S, Rubinchick S, Steinerman J, Knappertz V, Wolinsky J. Patient experience with glatiramer acetate 40 mg/1 mL three-times weekly treatment for relapsing-remitting multiple sclerosis: results from the GLACIER extension study (P7.218). Neurology. 2015;84(14 Supplement):P7–218.
National Institute of Health. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03 NHI Publication No. 09-5410. June 2010. 2010. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 20 July 2017.
US Food and Drug administration. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. 2007. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf. Accessed 20 July 2017.
McKeage K. Glatiramer acetate 40 mg/mL in relapsing-remitting multiple sclerosis: a review. CNS Drugs. 2015;29(5):425–32. https://doi.org/10.1007/s40263-015-0245-z.
Comi G, Martinelli V, Rodegher M, Moiola L, Bajenaru O, Carra A et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2009;374(9700):1503–11. https://doi.org/10.1016/s0140-6736(09)61259-9.
Papadopoulos D, Mitsikostas DD. Nocebo effects in multiple sclerosis trials: a meta-analysis. Mult Scler (Houndmills, Basingstoke, England). 2010;16(7):816–28. https://doi.org/10.1177/1352458510370793.
Acknowledgments
We thank all patients who consented to be enrolled in the research and Simona Curci, Istituto Neurologico Carlo Besta, for helping with manuscript finalization and submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All patients provided written informed consent for reuse of their clinical data. For this survey, no approval from an ethics committee was necessary.
Funding
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
Zecca C.: The Department of Neurology, Regional Hospital Lugano (EOC), Lugano, Switzerland receives financial support from Biogen, Genzyme, Merck, Novartis, Roche, Teva. Gobbi C.: The Department of Neurology, Regional Hospital Lugano (EOC), Lugano, Switzerland receives financial support from Biogen, Genzyme, Merck, Novartis, Roche, Teva. Antozzi C.G. received funding for travelling for congress attendance from Biogen, Merck, and Teva. Torri Clerici V. acted as an Advisory Board member for Biogen, Merck, Novartis, and Genzyme and received funding for travel from Biogen, Merck, Bayer, Genzyme, and Almirall and honoraria for speaking or writing from Merck, Teva, Genzyme, and Almirall. She received support for a research project from Almirall and is involved as principal investigator in clinical trials for Novartis and FISM. Confalonieri P.A. acted as an Advisory Board member for Biogen and Novartis; received funding for travel from Biogen, Merck, Teva, and Novartis; and received honoraria for speaking or writing from Biogen and Novartis. He received support for research projects from Novartis and Merck and is involved as principal investigator or co-investigator in clinical trials for Teva, Novartis, Biogen and Merck. Mantegazza R.E. acted as an Advisory Board member for Biomarin, and received funding for travel or speaker honoraria from Sanofi-Aventis, Biomarin, Grifols, Teva, Bayer, Alexion, and Argenx. He is involved as principal investigator in clinical trials for Alexion, Merck Serono, Hoffman-La Roche, Teva, Besta-Azienda Ospedaliera San Gerardo, Biogen, Biomarin, Almirall, Novartis, Genzyme Corporation, and Catalyst. Rossi S. acted as an Advisory Board member for Biogen, Bayer Schering, Merck, Teva, Novartis, Genzyme, and Mylan; received travel funding from Biogen, Merck, Teva, Novartis, Bayer Schering, Genzyme, and Almirall; and received honoraria for consultancy, speaking or writing from Biogen, Merck, Teva, Novartis, Bayer Schering, Genzyme, and Roche. She received support for research projects from Teva, Merck and Bayer Schering and is involved as principal investigator in clinical trials for Teva, Novartis, Biogen, and Roche. Bellavia G., Brambilla L., Gutierrez L.P., Gerardi C., Fiori A.M., Bernardini L.R., Disanto G., Petrini L., Perugini J., Bellino A., and Camera G. declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zecca, C., Bellavia, G., Brambilla, L. et al. Atypical Post-Injection Reactions with Delayed Onset Following Glatiramer Acetate 40 mg: Need for Titration?. CNS Drugs 32, 653–660 (2018). https://doi.org/10.1007/s40263-018-0529-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-018-0529-1