Abstract
Background
Intranasal and buccal midazolam have recently emerged as possible alternatives to intravenous or rectal diazepam or intravenous lorazepam in the treatment of early status epilepticus (SE). However, to date no randomized controlled trial (RCT) has directly compared intranasal midazolam with buccal midazolam.
Objective
The aim of this study was to indirectly compare intranasal midazolam with buccal midazolam in the treatment of early SE using common reference-based indirect comparison meta-analyses.
Methods
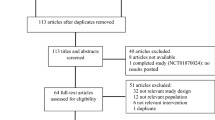
RCTs comparing intranasal or buccal midazolam versus either intravenous or rectal diazepam for early SE were systematically searched. Random-effects Mantel–Haenszel meta-analyses were performed to obtain odds ratios (ORs) for the efficacy and safety of intranasal or buccal midazolam versus either intravenous or rectal diazepam. Adjusted indirect comparisons were then made between intranasal and buccal midazolam using the obtained results.
Results
Fifteen studies, with a total of 1662 seizures in 1331 patients (some studies included patients with more than one episode of SE) were included; 1303 patients were younger than 16 years. Indirect comparisons showed no difference between intranasal and buccal midazolam for seizure cessation (OR 0.98, 95 % CI 0.32–3.01, comparator: intravenous diazepam; OR 0.87, 95 % CI 0.46–1.64, comparator: rectal diazepam). For serious adverse effects, we found a large width and asymmetrical distribution of confidence intervals around the obtained OR of 2.81 (95 % CI 0.39–20.12; comparator: rectal diazepam). No data were available for OR using intravenous diazepam as the comparator.
Conclusions
Indirect comparisons suggest that intranasal and buccal midazolam share similar efficacy in the treatment of early SE in children. Intranasal midazolam should be used with caution and under clinical monitoring of vital functions. RCTs directly comparing intranasal midazolam with buccal midazolam are required to confirm these findings.



Similar content being viewed by others
References
DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995;12:316–25.
Cascino GD. Generalized convulsive status epilepticus. Mayo Clin Proc. 1996;71:787–92.
Berg AT, Shinnar S, Levy SR, Testa FM. Status epilepticus in children with newly diagnosed epilepsy. Ann Neurol. 1999;45:618–23.
Berg AT, Shinnar S, Testa FM, et al. Status epilepticus after the initial diagnosis of epilepsy in children. Neurology. 2004;63:1027–34.
Silbergleit R, Lowenstein D, Durkalski V, Conwit R, Neurological Emergency Treatment Trials (NETT) Investigators. RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia. 2011;52(Suppl 8):45–7.
Silbergleit R, Lowenstein D, Durkalski V, Conwit R, NETT Investigators. Lessons from the RAMPART study—and which is the best route of administration of benzodiazepines in status epilepticus. Epilepsia. 2013;54(Suppl 6):74–7.
Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia. 1999;40:120–2.
Wermeling DP, Miller JL, Archer SM, Manaligod JM, Rudy AC. Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous, and intramuscular administration. J Clin Pharmacol. 2001;41:1225–31.
Brigo F, Nardone R, Tezzon F, Trinka E. Nonintravenous midazolam versus intravenous or rectal diazepam for the treatment of early status epilepticus: a systematic review with meta-analysis. Epilepsy Behav. 2015;49:325–336. doi:10.1016/j.yebeh.2015.02.030.
Prasad M, Krishnan PR, Sequeira R, Al-Roomi K. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev. 2014;9:CD003723.
Brigo F. New anti-epileptic drugs: overcoming the limits of randomised controlled trials. Int J Evid Based Healthc. 2011;9:440–3.
Brigo F, Igwe SC, Nardone R, Tezzon F, Bongiovanni LG, Trinka E. A common reference-based indirect comparison meta-analysis of intravenous valproate versus intravenous phenobarbitone for convulsive status epilepticus. Epileptic Disord. 2013;15:314–23.
Smith CT, Marson AG, Chadwick DW, Williamson PR. Multiple treatment comparisons in epilepsy monotherapy trials. Trials. 2007;5(8):34.
ICWG. Report of the Indirect Comparisons Working Group to the Pharmaceutical Benefits Advisory Committee: assessing indirect comparisons. Canberra (ACT): Australian Government Department of Health and Aging; 2009. http://www.pbs.gov.au/industry/useful-resources/PBAC_feedback_files/ICWG%20Report%20FINAL2.pdf. Accessed 16 Apr 2015.
Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472–5.
Higgins JPT, Green S, editors. Handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011.
Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21:1575–600.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Emerson JD. Combining estimates of the odds ratio: the state of the art. Stat Methods Med Res. 1994;3:157–78.
Glenny AM, Altman DG, Song F, et al. International Stroke Trial Collaborative Group. Indirect comparisons of competing interventions. Health Technol Assess. 2005;9:1–134 (iii–iv).
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis or randomized controlled trials. J Clin Epidemiol. 1997;50:683–91.
Otoul C, Arrigo C, van Rijckevorsel K, French JA. Meta-analysis and indirect comparisons of levetiracetam with other second-generation antiepileptic drugs in partial epilepsy. Clin Neuropharmacol. 2005;28:72–8.
Zaccara G, Giovannelli F, Maratea D, Fadda V, Verrotti A. Neurological adverse events of new generation sodium blocker antiepileptic drugs. Meta-analysis of randomized, double-blinded studies with eslicarbazepine acetate, lacosamide and oxcarbazepine. Seizure. 2013;22:528–36.
Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet. 1999;353:623–6.
Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321:83–6.
Fişgin T, Gurer Y, Teziç T, Senbil N, Zorlu P, Okuyaz C, et al. Effects of intranasal midazolam and rectal diazepam on acute convulsions in children: prospective randomized study. J Child Neurol. 2002;17:123–6.
Mahmoudian T, Zadeh MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav. 2004;5:253–5.
Baysun S, Aydin OF, Atmaca E, Gürer YK. A comparison of buccal midazolam and rectal diazepam for the acute treatment of seizures. Clin Pediatr (Phila). 2005;44:771–6.
McIntyre J, Robertson S, Norris E, Appleton R, Whitehouse WP, Phillips B, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366:205–10.
Bhattacharyya M, Kalra V, Gulati S. Intranasal midazolam vs rectal diazepam in acute childhood seizures. Pediatr Neurol. 2006;34:355–9.
Mpimbaza A, Ndeezi G, Staedke S, Rosenthal PJ, Byarugaba J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics. 2008;121:e58–64.
Talukdar B, Chakrabarty B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: a randomized controlled trial. Brain Dev. 2009;31:744–9.
Ashrafi MR, Khosroshahi N, Karimi P, Malamiri RA, Bavarian B, Zarch AV, et al. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. Eur J Paediatr Neurol. 2010;14:434–8.
de Haan GJ, van der Geest P, Doelman G, Bertram E, Edelbroek P. A comparison of midazolam nasal spray and diazepam rectal solution for the residential treatment of seizure exacerbations. Epilepsia. 2010;51:478–82.
Holsti M, Dudley N, Schunk J, Adelgais K, Greenberg R, Olsen C, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Arch Pediatr Adolesc Med. 2010;164:747–53.
Javadzadeh M, Sheibani K, Hashemieh M, Saneifard H. Intranasal midazolam compared with intravenous diazepam in patients suffering from acute seizure: a randomized clinical trial. Iran J Pediatr. 2012;22:1–8.
Tonekaboni SH, Shamsabadi FM, Anvari SS, Mazrooei A, Ghofrani M. A comparison of buccal midazolam and intravenous diazepam for the acute treatment of seizures in children. Iran J Pediatr. 2012;22:303–8.
Thakker A, Shanbag P. A randomized controlled trial of intranasal-midazolam versus intravenous-diazepam for acute childhood seizures. J Neurol. 2013;260:470–4.
Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet. 2002;359:696–700.
Ravani P, Parfrey PS, Curtis B, Barrett BJ. Clinical research of kidney diseases 1: researchable questions and valid answers. Nephrol Dial Transplant. 2007;22:3681–90.
Brigo F, Storti M, Del Felice A, Fiaschi A, Bongiovanni LG. IV valproate in generalized convulsive status epilepticus: a systematic review. Eur J Neurol. 2012;19:1180–91.
Guyatt G, Jaeschke R, Heddle N, Cook D, Shannon H, Walter S. Basic statistics for clinicians: 1. Hypothesis testing. CMAJ. 1995;152:27–32.
McAlister FA, Laupacis A, Wells GA, Sackett DL. Users’ Guides to the Medical Literature: XIX. Applying clinical trial results B. Guidelines for determining whether a drug is exerting (more than) a class effect. JAMA. 1999;282(14):1371–7.
Wilson MT, Macleod S, O’Regan ME. Nasal/buccal midazolam use in the community. Arch Dis Child. 2004;89:50–1.
Klimach VJ, Epic Clinical Network. The community use of rescue medication for prolonged epileptic seizures in children. Seizure. 2009;18:343–6.
Armijo JA, Herranz JL, Pena Pardo MA, Adín J. Intranasal and buccal midazolam in the treatment of acute seizures. Rev Neurol. 2004;38:458–68.
Mula M. The safety and tolerability of intranasal midazolam in epilepsy. Expert Rev Neurother. 2014;14:735–40.
Aggarwal R, Cardozo A, Homer JJ. The assessment of topical nasal drug distribution. Clin Otolaryngol Allied Sci. 2004;29:201–5.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097.
Acknowledgments
We are indebted to Annalisa Cuzzoni (Medical Library of Bolzano) for developing and implementing the search strategies, and we thank Piero Bonzano and Vincenzo De Simone (Accord Healthcare), as well as Alberto Liuti (ViroPharma), for seeking information on unpublished randomized controlled trials on buccal midazolam or studies in progress.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received in relation to the preparation of this article.
Conflicts of interest
Francesco Brigo has received speakers’ honoraria from UCB Pharma.
Eugen Trinka has acted as a paid consultant to Bial, Biogen Idec, Eisai, Ever Neuropharma, Medtronics, Takeda, Upsher-Smith, and UCB; has received speakers’ honoraria from Bial, Boehringer, Eisai, GL Lannacher, and UCB Pharma; and has received research funding from Biogen Idec, Merck, Novartis, Red Bull, UCB Pharma, the European Union, FWF (Österreichischer Fond zur Wissenschaftsförderung), and Bundesministerium für Wissenschaft und Forschung. Raffaele Nardone and Frediano Tezzon declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brigo, F., Nardone, R., Tezzon, F. et al. A Common Reference-Based Indirect Comparison Meta-Analysis of Buccal versus Intranasal Midazolam for Early Status Epilepticus. CNS Drugs 29, 741–757 (2015). https://doi.org/10.1007/s40263-015-0271-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-015-0271-x