Abstract
Introduction and Objective
Pazopanib is registered for metastatic renal cell carcinoma and soft-tissue sarcoma (STS). Its variable pharmacokinetic (PK) characteristics and narrow therapeutic range provide a strong rationale for therapeutic drug monitoring (TDM). Prior studies have defined target levels of drug exposure (≥ 20.5 mg/L) linked to prolonged progression-free survival (PFS), but the added value of using TDM remains unclear. This study investigates the effect of TDM of pazopanib in patients with STS on survival outcomes and dose-limiting toxicities (DLTs) and evaluates the feasibility of TDM-guided dosing.
Methods
A TDM-guided cohort was compared to a non-TDM-guided cohort for PFS, overall survival (OS) and DLTs. PK samples were available from all patients, though not acted upon in the non-TDM-guided cohort. We evaluated the feasibility of TDM by comparing the proportion of underdosed patients in our TDM cohort with data from previous publications.
Results
A total of 122 STS patients were included in the TDM-guided cohort (n = 95) and non-TDM-guided cohort (n = 27). The average exposure in the overall population was 30.5 mg/L and was similar in both groups. Median PFS and OS did not differ between the TDM-guided cohort and non-TDM-guided cohort (respectively 5.5 vs 4.4 months, p = 0.3, and 12.6 vs 10.1 months, p = 0.8). Slightly more patients in the non-TDM-guided cohort experienced DLTs (54%) compared to the TDM-guided cohort (44%). The proportion of underdosed patients (13.3%) was halved compared to historical data (26.7%).
Conclusion
TDM reduced the proportion of patients with subtherapeutic exposure levels by ~ 50%. Nonetheless, the added value of TDM for achieving target trough levels of ≥ 20.5 mg/L for pazopanib on survival outcomes could not be confirmed in STS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Therapeutic drug monitoring (TDM) reduced the proportion of patients with subtherapeutic pazopanib exposure levels by ~ 50%. |
Median progression-free survival and overall survival did not differ between a TDM-guided cohort and non-TDM-guided cohort including patients treated with pazopanib for soft-tissue sarcoma. |
1 Introduction
Pazopanib is a tyrosine kinase inhibitor (TKI) targeting vascular endothelial growth factor receptor (VEGFR)-1,2,3, platelet-derived growth factor receptor α and β (PDGFRα-β) and the stem cell receptor (c-KIT). Pazopanib is approved for the treatment of patients with metastatic renal cell carcinoma (mRCC) and second line metastatic soft-tissue sarcomas (STS). STS are a rare group of malignancies that arise in the bone or any soft tissue, including fat, muscles, blood vessels, nerves, and other connective tissues, with ~ 80 different histological subtypes [1, 2]. After failure of first-line chemotherapy, pazopanib has shown a benefit in median progression-free survival (mPFS) of approximately 3 months in patients with unresectable advanced/metastatic STS (excluding liposarcomas) compared to placebo [3]. Despite the positive outcomes in this phase 3 trial, limited sample sizes per subtype leave uncertainties regarding the varying sensitivities of each subtype to pazopanib.
Currently, pazopanib is given in a ‘one-size-fits-all’ once daily dose of 800 mg taken in a fasted state [4]. Pazopanib has challenging pharmacokinetic (PK) characteristics, with, e.g., saturated absorption and high inter- and intrapatient variability in PK [5, 6]. Combined with its narrow therapeutic window (exposure levels of 20.5–46 mg/L) and an observed better effect above a certain target, this provides a strong rationale for therapeutic drug monitoring (TDM), where the dose is individualized based on measured drug levels [5]. In previous studies, the exposure to pazopanib has been related to its efficacy and toxicity in patients with mRCC. Patients with pazopanib plasma trough concentrations (Ctrough) ≥ 20.5 mg/L showed significantly longer PFS compared to patients with a Ctrough < 20.5 mg/L (52.0 weeks vs 19.6 weeks, p = 0.004) [7]. This exposure threshold was later confirmed in a real-world RCC cohort [8]. The same threshold prevented progression when used in the adjuvant setting in a study of Sternberg et al. [9]. A similar trend was observed in patients with STS [8]. More recently, a Ctrough threshold of 27 mg/L at day 15 was proposed in this latter group of patients [10]. These data suggest that patients treated with pazopanib could benefit from a more personalized dosing approach.
In a previous publication, the first results of the Dutch Pharmacology Oncology Group-Therapeutic Drug Monitoring (DPOG-TDM) study have been reported [11]. This is an ongoing, prospective study in which the feasibility of individualized dosing for multiple oral targeted therapies has been established, including pazopanib [12]. The efficacy of TDM of pazopanib for patients with STS has never been studied up to this point. Therefore, the aim of this study was to evaluate the added value of TDM of pazopanib on clinical outcomes such as mPFS, median overall survival (mOS), median time on treatment (mTOT), and toxicity for patients with STS. Thereby, treatment outcomes of a TDM-guided cohort are compared with a non-TDM-guided cohort. In addition, the feasibility of TDM of pazopanib for both STS and mRCC is assessed in patients included in the DPOG-TDM study.
2 Methods
2.1 Patient Population and Data Collection
2.1.1 TDM-Guided Cohort Versus Non-TDM-Guided Cohort
All patients included in this study received pazopanib for STS and had at least one recorded pazopanib plasma concentration obtained in the first 90 days of therapy. Data from different cohorts were pooled and divided into a TDM-guided dosing cohort and a non-TDM-guided dosing cohort (Fig. 1). For the TDM-guided cohort, we used a combined dataset of patients from the DPOG-TDM study (clinical trials number NL-OMON21747 [https://trialsearch.who.int/]) and the TUNE implementation project (Dutch Cancer Society [KWF] project number 11575), supplemented with data collected at the Radboud University Medical Center (RUMC), Nijmegen (The Netherlands) [12, 13]. All patients in this cohort underwent TDM-guided dosing. If the plasma trough level was below 20.5 mg/L and the patient was already on 800 mg once daily, it was recommended to split the dose from 800 mg once daily to 400 mg twice daily. Some patients started pazopanib as 600 mg in a fed state, following the results of the DIET study, in which this dosing regimen resulted in a bioequivalent exposure to 800 mg in a fasted state [14]. In this case, the TDM intervention first involved a dose increase to 800 mg in a fed state, before splitting the dose to 400 mg twice daily. For the non-TDM-guided cohort, the STS cohort described by Verheijen et al. was complemented with data from patients when TDM-guided dosing was not yet standard practice at The Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital (NKI-AVL), Amsterdam (The Netherlands) [8]. PK data were collected for all patients in the non-TDM-guided cohort, but the dose was not adjusted based on exposure levels.
The following data were collected: patient demographics, STS subtype, previous treatment lines, starting dose, treatment duration, reason for discontinuation, date of radiological progression, date of death, calculated Ctrough, and Eastern Cooperative Oncology Group (ECOG) performance status. In addition, data on dose-limiting toxicities (DLTs) were collected.
2.1.2 Feasibility Analysis
In addition to the previously mentioned patients with STS from the DPOG-TDM study, those patients treated with pazopanib for mRCC in the DPOG-TDM study were also included for feasibility analysis. All patients underwent TDM-guided dosing and started pazopanib as 800 mg once daily in a fasted state.
2.2 Study Objectives
2.2.1 TDM-Guided Cohort Versus Non-TDM-Guided Cohort
First, the efficacy of TDM of pazopanib was evaluated by comparing mPFS, mOS, and mTOT for a TDM-guided cohort and a non-TDM-guided cohort. PFS was defined as the time between start of pazopanib and first reported objective tumor progression or death. Patients who stopped treatment due to other reasons were censored at the date pazopanib was discontinued, and patients who were still on treatment were censored at the last date of follow-up. OS was defined as the time between start of pazopanib and the date of death. Patients who were still alive at the last date of follow-up were also censored. TOT was defined as the time between start and stop date of pazopanib treatment. Second, the incidence of DLTs was compared between the TDM-guided cohort and non-TDM-guided cohort. For exploratory analysis, the PK exposure of patients with and without DLTs was compared, regardless of TDM. DLTs were defined as adverse events requiring a dose reduction, treatment discontinuation or a treatment interruption of at least 14 days.
2.2.2 Feasibility Analysis
The feasibility of TDM of pazopanib was assessed using patients included in the DPOG-TDM study, which included patients with both STS and mRCC. First, we calculated the percentage of successful TDM-guided interventions in the group of patients with one or more low PK samples. A TDM-guided intervention was defined as successful if the median Ctrough after the intervention was ≥ 20.5 mg/L, and the patient tolerated the new dose and/or dosing schedule for at least 4 weeks. Furthermore, the physician non-adherence rate (i.e., adherence to TDM recommendations by the treating physician) as well as the proportion of patients below the target (Ctrough < 20.5 mg/L) after 12 weeks were assessed. According to the protocol of the DPOG-TDM study, this proportion should be halved in comparison to historical data (26.7%) to achieve its primary endpoint. The latter was derived from a weighted average of reported values of four studies where no TDM-guided interventions were conducted [11].
2.3 Pharmacokinetic Sampling
PK samples were collected during routine outpatient clinic visits. Both date and time of the last dose and sampling time were recorded to estimate the Ctrough. Plasma concentrations were measured with a validated assay [15]. Samples were collected at predefined time points, around 4, 8, and 12 weeks after treatment initiation, followed by subsequent collections every 12 weeks thereafter. In the case of a low PK measurement, an intervention was advised [12]. For all patients, pazopanib trough levels were calculated or extrapolated using the following formula [16]:
Ctrough is the estimated minimum plasma concentration, Cmeasured is the measured plasma concentration, the dosing interval is the time between two consecutive administrations (i.e., 12 or 24 h depending on a once-daily or twice-daily schedule), TAD is the time after dose, and t½ is the average population elimination half-life of the drug (i.e., 31 h for pazopanib) [4]. PK samples collected before the time to reach the maximum concentration (Tmax) (TAD 0–3.5 h for pazopanib) may have led to underestimated Ctrough levels and were therefore excluded from the analysis [4]. The average exposure over the entire treatment duration was calculated by using the geometric mean (GM) Ctrough of all measured PK samples per patient. To provide a description of Ctrough values at a group level, we utilized the median of all GM.
2.4 Statistical Analyses
All statistical analyses were performed using R version 4.2.1 (R project, Vienna, Austria) [17]. Baseline characteristics for the TDM-guided cohort and non-TDM-guided cohort were compared using the chi-square test or t test, depending on the variable. Similarly, mPFS, mOS, and mTOT were compared between the TDM-guided cohort and non-TDM-guided cohort using Kaplan-Meier analyses. Differences between groups were tested using the log-rank test. In addition, a multivariate Cox proportional hazard regression analysis was performed. The following known prognostic covariates were included: the ECOG performance status, STS subtype (leiomyosarcoma and synovial sarcoma), and the number of prior lines of therapy [3]. Hazard ratios (HRs) with 95% confidence intervals (CIs) were reported. A p value < 0.05 was deemed statistically significant.
2.5 Ethical Regulations
The DPOG-TDM study and the TUNE study were approved by the institutional review boards of the NKI-AVL and the RUMC, respectively, as well as the collection of data from patients included in routine clinical care in the NKI-AVL. Approval from the board of directors of each individual hospital was obtained for all participating centers. All patients provided written informed consent. The local institutional review boards of both the NKI-AVL and the RUMC approved data collection and sharing.
3 Results
3.1 TDM-Guided Cohort Versus Non-TDM-Guided Cohort
3.1.1 Patient Characteristics
A total of 122 patients with STS who started pazopanib treatment between February 2013 and January 2023 were included for survival analysis, of which 95 and 27 patients, respectively, were enrolled in the TDM-guided cohort and non-TDM-guided cohort (Fig. 1). Overall, respectively, 52 and 70 patients were included during routine clinical care and from study cohorts (DPOG-TDM study and TUNE). The data cut-off was at 14 September 2023. All patients were diagnosed with STS, with leiomyosarcoma as the most prevalent subtype (38%). An overview of baseline characteristics is given in Table 1. Only the starting dose was significantly different between both groups, with 17% of patients starting pazopanib 600 mg with food in the TDM-guided cohort compared to no patients on this starting dose and regimen in the non-TDM-guided cohort. All other baseline characteristics, including pazopanib exposure during treatment, were similar between groups.
3.1.2 Pharmacokinetics
A total of 434 PK samples were available for STS patients in the TDM-guided cohort (n = 335) and non-TDM-guided cohort (n = 99). Overall, a median of three samples (range 1–10) were measured per patient (Table 1), which did not differ between groups. Moreover, the median calculated Ctrough in the overall population was 30.5 mg/L (interquartile range [IQR] 24.8–38.5) and was similar in the TDM-guided and non-TDM-guided cohorts (respectively, 30.6 mg/L [IQR 25.0–38.6] and 30.2 mg/L [IQR 24.4–35.1]).
3.1.3 Survival Analysis
Overall, mPFS was 5.1 months (95% CI 4.0–7.4) and did not differ between the TDM-guided cohort and non-TDM-guided cohort (5.5 months [95% CI 4.0–7.6] vs 4.4 months [95% CI 2.0–8.8], respectively; p = 0.3) (Fig. 2a). In multivariate analysis, the HR was 0.79 (95% CI 0.5–1.3, p = 0.3) for TDM (Table S1, electronic supplementary material). mOS in the entire cohort was 12.2 months (95% CI 11.3–15.7) and was similar in the TDM-guided cohort and non-TDM-guided cohort (12.6 months [95% CI 11.3–16.6] vs 10.1 months [95% CI 9.1–27.8], respectively; p = 0.8) (Fig. 2b). Multivariate analysis resulted in an HR of 0.95 (95% CI 0.6–1.6, p = 0.9) for TDM. For OS, both performance status and leiomyosarcoma appeared to be independent prognostic factors (Table S2, electronic supplementary material). STS subtype analyses were performed for both mPFS and mOS (Fig. S1a and S1b in the electronic supplementary material).
3.1.4 Time on Treatment
Of 122 included patients, 118 patients (97%) were not on treatment anymore at the time of data cut-off. The majority of patients stopped treatment due to progressive disease (77%), followed by toxicity (15%). The overall mTOT was 4.1 months (95% CI 3.2–5.6). When comparing the TDM-guided cohort with the non-TDM-guided cohort, no differences in mTOT were observed (3.7 [95% CI 2.2–8.9) vs 4.2 [95% CI 3.2–5.6], respectively; p = 0.9) (Fig. 3).
3.1.5 Toxicity Analysis
Overall, 63 patients experienced DLTs (52%), with a slightly higher incidence in the non-TDM-guided cohort (54%) compared to the TDM-guided cohort (44%). Median time to first DLT was 39 days (range 3–727 days). The most frequently reported DLTs included diarrhea, fatigue, hypertension, liver toxicity, mucositis, and nausea. The median exposure of patients with DLTs was 38.9 mg/L (IQR 31.1–49.7) in the period before the DLT, and decreased to 28.7 mg/L (IQR 23.7–38.1) after dose reduction. This latter value is similar to the exposure of patients without DLTs (27.9 mg/L [IQR 24.5–35.3]), although the doses differed between groups.
3.2 Feasibility Analysis
3.2.1 Patient Characteristics
A total of 106 pazopanib patients in ten hospitals in The Netherlands were enrolled in the DPOG-TDM study between June 2017 and November 2022. The cut-off date for the feasibility data was 11 July 2023. Subsequently, 98 patients had PK data available and were thus evaluable for feasibility analyses. Of those, 62 and 36 patients were treated for STS and mRCC, respectively.
3.2.2 Feasibility of TDM
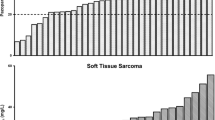
As shown in Fig. 4, almost two thirds (63%) of assessable patients had an exposure above the threshold of 20.5 mg/L during their entire treatment. The remaining 36 patients had at least one PK sample below this threshold. Of those, a TDM-guided intervention was not performed in 11 patients, mainly due to toxicity (45%), treatment discontinuation (27%), and physician’s non-adherence (18%). In the 25 patients in which a TDM-guided intervention was performed, this was successful in 17 (68%). In total, this means that a PK intervention was feasible in 47% (n = 17) of all patients with one or more low PK samples (n = 36). The main reason for a non-successful intervention was toxicity (50%). In the end, 81% of patients had adequate exposure. Subgroup analyses were performed for patients with mRCC and STS (Fig. S2a and S2b, in the electronic supplementary material), indicating comparable outcomes between both indications.
Furthermore, of 98 patients with PK data available, only 75 patients completed the PK measurements after 4, 8, and 12 weeks. The other 23 patients discontinued treatment prior to their second or third PK measurement due to either disease progression or toxicity. Considering all patients who completed the first three PK measurements, 13.3% exhibited an exposure below the target at this point. This proportion is twice as low compared to historical data (26.7%) [11]. Consequently, the primary endpoint for the pazopanib cohort in the DPOG-TDM study was achieved.
4 Discussion
In this observational study in metastatic STS patients treated with pazopanib, mPFS and mOS did not differ between the TDM-guided and non-TDM-guided cohorts. It appeared that although the pazopanib dose differed due to DLTs, the best tolerated exposure was similar in the DLT and non-DLT groups. With respect to feasibility analysis, TDM led to a halving of the number of underexposed patients.
It should be noted that in current practice, a Ctrough of 20.5 mg/L is used as a therapeutic target for pazopanib for both mRCC and STS. Although this threshold has been studied fairly well in patients with mRCC [7], only a trend towards a prolonged mPFS was observed in patients with STS in a publication of Verheijen et al. [8]. Later, an exploratory study proposed a novel therapeutic target of 27 mg/L for patients with STS, based on the Ctrough measured at day 15 after start of pazopanib [18]. In a larger confirmative observational study, the authors concluded that a Ctrough ≥ 27 mg/L measured at day 15 after start of pazopanib was independently associated with an improved 3-month mPFS in patients with STS [10]. Moreover, they utilized the 3-month PFS as the primary endpoint in accordance with the pivotal phase 3 trial, although it might be more worthwhile to assess any differences in mPFS without the limitation of the 3-month timeframe [3]. Despite the observed difference in mPFS, one could question the choice to only consider the Ctrough at day 15 rather than considering all available PK samples during a patient’s pazopanib treatment. Given that pazopanib exposure decreases in the first weeks, it is reasonable to assume that in the study of Minot-This et al. [10] the same target exposure was reached as earlier studied by Verheijen et al. (20.5 mg/L) [6]. After all, a 25% decrease from 27 mg/L corresponds to 20.5 mg/L. There is not enough data to suggest deviating from this threshold—at least not based on our data.
Given that no clear optimal threshold of pazopanib in STS patients has been defined, we considered performing these analyses using our cohort. However, this was not possible because selection bias was introduced through the use of TDM-guided dosing interventions. Clinicians based their decision for dose optimization on both the measured Ctrough levels and clinical assessments, including considerations of efficacy and toxicity, possibly influencing clinical outcomes. As TDM becomes gradually more common in daily clinical practice, either routinely or only on indication, it can be difficult to find a real-world cohort to perform these analyses. Therefore, one could argue that it is of great importance to already investigate the exposure–response and exposure–toxicity relation during earlier stages of drug development. This is in line with the aims of Project Optimus of the Food and Drug Administration; an initiative to reform dose optimization and the dose selection paradigm in oncology drug development [19].
The main strength of this study is the use of real-world data. Also, considering that STS is a rare type of cancer, we were still able to gather over 120 patients [1]. Nonetheless, although the use of real-world data is ideal to mirror the actual clinical practice, it is not without limitations. For example, the frequency of hospital visits for patients in the real-world setting may not be as consistent compared to clinical trials, where the timing of clinical assessments is strictly protocolized. Certainly, in a rare disease like STS, where mPFS on pazopanib is only ~ 3 months more than placebo, the timing of CT scanning influences detection of progressive disease, introducing evaluation-time bias. Clinicians might opt for an earlier or later CT scan not only due to practical considerations, such as holidays, but also due to factors like drug tolerability and patient complaints that could affect tumor progression detection. This issue persists when utilizing TOT as a substitute endpoint, as treatment discontinuation due to progressive disease typically occurs shortly (i.e., a couple of days) after its detection on a CT scan. mOS is therefore usually considered the gold-standard endpoint. Interpreting real-world data thus requires careful consideration of factors influencing clinical endpoints, and subsequently the effect of TDM on mPFS and mOS. Another limitation of using real-world data from different cohorts includes that studies are not performed in the exact same manner. For instance, patients could only be included in the DPOG-TDM study if they started at 800 mg once daily in a fasted state, whereas the TUNE study included patients regardless of their starting dose, similar to patients in the non-TDM-guided cohort. This could have introduced some bias, although this is thought to be limited as TDM was performed to guide patients towards a therapeutic exposure and dose was therefore adjusted per the individual patient’s need.
Although we were not able to demonstrate the added value of TDM in terms of survival, that does not necessarily imply that there is no beneficial effect of TDM guidance. It is important to note that in a non-TDM-guided cohort the proportion of patients below the threshold is only 26.7%, as previously described [11]. In combination with the fact that in approximately half of these patients it is not possible to increase the dose (i.e., due to toxicity), it is only a small proportion of patients that could possibly benefit from TDM and thus induce the difference in mPFS and mOS. To demonstrate such a small effect on clinical endpoints, a sample size of at least 250 patients per arm (i.e., 500 in total) would be needed. Unfortunately, for a rare disease like sarcoma, it is very difficult, or even unlikely, that such a large sample size could be collected.
5 Conclusion
The added value of TDM on survival outcomes of STS patients could not be confirmed due to the limited sample size. Importantly, based on our findings, we cannot confirm nor reject the earlier established pazopanib target of 20.5 mg/L. Instead, we highlight the importance of prospectively investigating thresholds for oral oncolytics in registration trials to optimize treatment outcomes. TDM appeared to be effective in halving the proportion of underdosed patients compared to historical data. A dose intervention was feasible and successful in almost 50% of the underdosed patients.
References
Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up(☆). Ann Oncol. 2021;32(11):1348–65.
von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, et al. Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(7):815–33.
van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–86.
European Medicines Agency. Summary of product characteristics Vortrient 200 and 400 mg film-coated tablets. 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/votrient. Cited 15 Aug 2023.
Westerdijk K, Desar IME, Steeghs N, van der Graaf WTA, van Erp NP, Dutch P, Oncology G. Imatinib, sunitinib and pazopanib: from flat-fixed dosing towards a pharmacokinetically guided personalized dose. Br J Clin Pharmacol. 2020;86(2):258–73.
Yu H, van Erp N, Bins S, Mathijssen RH, Schellens JH, Beijnen JH, et al. Development of a pharmacokinetic model to describe the complex pharmacokinetics of pazopanib in cancer patients. Clin Pharmacokinet. 2017;56(3):293–303.
Suttle AB, Ball HA, Molimard M, Hutson TE, Carpenter C, Rajagopalan D, et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer. 2014;111(10):1909–16.
Verheijen RB, Swart LE, Beijnen JH, Schellens JHM, Huitema ADR, Steeghs N. Exposure-survival analyses of pazopanib in renal cell carcinoma and soft tissue sarcoma patients: opportunities for dose optimization. Cancer Chemother Pharmacol. 2017;80(6):1171–8.
Sternberg CN, Donskov F, Haas NB, Doehn C, Russo P, Elmeliegy M, et al. Pazopanib exposure relationship with clinical efficacy and safety in the adjuvant treatment of advanced renal cell carcinoma. Clin Cancer Res. 2018;24(13):3005–13.
Minot-This MS, Boudou-Rouquette P, Jouinot A, de Percin S, Balakirouchenane D, Khoudour N, et al. Relation between plasma trough concentration of pazopanib and progression-free survival in metastatic soft tissue sarcoma patients. Pharmaceutics. 2022;14(6).
Groenland SL, van Eerden RAG, Westerdijk K, Meertens M, Koolen SLW, Moes D, et al. Therapeutic drug monitoring-based precision dosing of oral targeted therapies in oncology: a prospective multicenter study. Ann Oncol. 2022;10:1071–82.
Groenland SL, van Eerden RAG, Verheijen RB, Koolen SLW, Moes D, Desar IME, et al. Therapeutic drug monitoring of oral anticancer drugs: the Dutch Pharmacology Oncology Group-therapeutic drug monitoring protocol for a prospective study. Ther Drug Monit. 2019;41(5):561–7.
Dutch Cancer Society. Implementatie van geïndividualiseerd doseren van orale doelgerichte therapie bij de behandeling van kanker 2019. https://www.kwf.nl/onderzoek/onderzoeksdatabase/implementatie-van-geindividualiseerd-doseren-van-orale-doelgerichte. Cited 12 July 2023.
Lubberman FJE, Gelderblom H, Hamberg P, Vervenne WL, Mulder SF, Jansman FGA, et al. The effect of using pazopanib with food vs. fasted on pharmacokinetics, patient safety, and preference (DIET Study). Clin Pharmacol Ther. 2019;106(5):1076–82.
Giraud EL, Te Brake LMH, van den Hombergh ECA, Desar IME, Kweekel DM, van Erp NP. Results of the first international quality control programme for oral targeted oncolytics. Br J Clin Pharmacol. 2024;90(1):336–43.
Wang Y, Chia YL, Nedelman J, Schran H, Mahon FX, Molimard M. A therapeutic drug monitoring algorithm for refining the imatinib trough level obtained at different sampling times. Ther Drug Monit. 2009;31(5):579–84.
R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria 2021. https://www.r-project.org/.
Bellesoeur A, Boudou-Rouquette P, Thomas-Schoemann A, Joly C, Tlemsani C, Vidal M, et al. Individualized pazopanib dosing-letter. Clin Cancer Res. 2017;23(20):6377.
Food and Drug Administration. Project optimus—reforming the dose optimization and dose selection paradigm in oncology. 2023. https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus. Cited 1 Nov 2023.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
The TUNE project (Grant no. 11575) was funded by the Dutch Cancer Society (KWF Kankerbestrijding). In addition, the DPOG-TDM study was supported by unrestricted research grants from Ipsen, Merck, Novartis, Pfizer, and Roche. They had no involvement in any other aspect of this study. A research grant for the Dutch GIST Registry was received from Novartis, Pfizer, Bayerv and Deciphera. These funding resources were not involved in the conduct of this research.
Conflict of interest
MM, ELG, MBAvdK, KW, NADG, RFB, AR, ALTI, H-MO, AV, ML, PH, SLWK, DJARM, KEB, DJT, ADRH, and IMED declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. WTAvdG reports institutional research fees paid to the institute from Lilly and advisory compensation from Springworks, PTC Therapeutics, and Agenus, all outside the submitted work. RHJM reports research funding paid to the institute from Astellas, Bayer, Boehringer-Ingelheim, Cristal Therapeutics, Deuter Oncology, Nordic Pharma, Novartis, Pamgene, Pfizer, Roche, Sanofi, and Servier, all outside the submitted work. HG reports institutional research funding from Daiichi Sankyo, Deciphera Pharmaceuticals, Ipsen, and Novartis. ADRH is an Editorial Board member of Clinical Pharmacokinetics. ADRH was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. NPvE reports research funding paid to the institute from Astellas and Ipsen, all outside the submitted work. NS reports research grants paid to the institute from Abbvie, Actuate Therapeutics, Amgen, Array, Ascendis Pharma, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, BridgeBio, Bristol-Myers Squibb, Cantargia, CellCentric, Cogent Biosciences, Cresecendo Biologics, Cytovation, Deciphera, Dragonfly, Eli Lilly, Exelixis, Genentech, GlaxoSmithKline, IDRx, Immunocore, Incyte, InteRNA, Janssen, Kinnate Biopharma, Kling Biotherapeutics, Luszana, Merck, Merck Sharp & Dohme, Merus, Molecular Partners, Navire Pharma, Novartis, Numab Therapeutics, Pfizer, Relay Pharmaceuticals, Revolution Medicin, Roche, Sanofi, Seattle Genetics, Taiho, and Takeda, all outside the submitted work. NS provided consultation or attended advisory boards for Boehringer Ingelheim, Cogent Biosciences, Ellipses Pharma, Incyte, and Luszana.
Ethics approval
The DPOG-TDM study (METC17.0941; 07-08-2017) and the TUNE study (2019-5402; 09-05-2019) were approved by the institutional review boards of the NKI-AVL and the RUMC, respectively, as well as the collection of data from patients included in routine clinical care in the NKI-AVL. Approval from the board of directors of each individual hospital was obtained for all participating centers. The local institutional review boards of both the NKI-AVL and the RUMC approved data collection and sharing (14290_IRBd22-206; 25-01-2023).
Informed consent
All patients provided written informed consent.
Data availability
The data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
MM and ELG wrote the manuscript; MM, ELG, ADRH, NPvE, IMED, and NS designed the research; MM and ELG performed the research; MM and ELG analyzed the data; MBAvdK, KW, NADG, RFB, AR, ALTI, H-MO, AV, ML, PH, WTAvdG, HG, DJARM, KEB, DJT, SLWK, and RHJM were responsible for critical revision of the manuscript. All authors gave final approval of the version to be submitted.
Consent to participate
All patients provided written informed consent.
Consent for publication
All authors provide this consent.
Code Availability
The codes used for analyses in the current study are available from the corresponding author on reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Meertens, M., Giraud, E.L., van der Kleij, M.B.A. et al. Evaluating the Clinical Impact and Feasibility of Therapeutic Drug Monitoring of Pazopanib in a Real-World Soft-Tissue Sarcoma Cohort. Clin Pharmacokinet 63, 1045–1054 (2024). https://doi.org/10.1007/s40262-024-01399-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-024-01399-8