Abstract
Introduction
Cabozantinib is one of the preferred treatment options in the latest metastatic renal cell carcinoma (mRCC) guidelines. Cabozantinib is also associated with high drug expenses irrespective of the used dose, because a flat-prizing model has been implemented. In addition, concomitant intake with a high-fat meal increases its bioavailability on average by 57%. Combined with the long terminal half-life of cabozantinib (99 h), this creates possibilities to extend the dosing interval to reduce drug expenses whilst maintaining equivalent exposure.
Objectives
The primary objective was to evaluate the population pharmacokinetic (POPPK) model of cabozantinib developed for its registration using real-world patients’ therapeutic drug monitoring (TDM) data. The secondary objective was to design, simulate, and evaluate alternative dose regimens with the aim to reduce drug expenses whilst maintaining comparable exposure.
Methods
Retrospective TDM data from mRCC patients treated with cabozantinib were obtained. The data were evaluated using the published Food and Drug Administration (FDA) cabozantinib POPPK model, a two-compartment disposition model with a dual (fast and slow) lagged first-order absorption process derived from FDA registration documents, as a basis. Subsequently, simulations of alternative drug expenses saving regimens were evaluated.
Results
Twenty-seven mRCC patients with 75 pharmacokinetic observations were included. Patients were treated for a median of 75 days with a median dose of 40 mg. Model evaluation results showed that the cabozantinib TDM concentrations were adequately predicted by the published FDA cabozantinib POPPK model, except for a slightly higher clearance (CL) of 3.11 L/h compared to the reported value (2.23 L/h). The simulation study indicated that an alternative dose regimen that consists of taking 60 mg of cabozantinib for 2 days and then skipping 1 day results in comparable average exposure when compared with a 40 mg daily dose, both without food interaction, while saving 33.3% of the total drug expenses per month. The food effect of a high-fat meal was also taken into account when simulating other alternative dose regimens; 40 mg every 72 h combined with a high-fat meal resulted in comparable exposure when compared with a 20 mg daily dose fasted, while saving 66.7% in drug expenses.
Conclusions
In this study, the optimized cabozantinib POPPK model resulted in adequate prediction of real-world cabozantinib pharmacokinetic data. Alternative dosing regimens with and without using known food interactions were proposed that resulted in potential strategies to significantly reduce cabozantinib drug expenses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The optimized cabozantinib population pharmacokinetic (POPPK) model derived from the POPPK model from the registration study adequately predicts real-world cabozantinib pharmacokinetic data. |
A barrier for wider use of cabozantinib are the relatively high costs associated with its use, irrespective of the used dose since a flat-pricing model (same price for different strengths) is implemented. |
The developed alternative dosing regimens have the potential to save up to approximately 66.7% in drug expenses while maintaining adequate exposure. |
1 Introduction
Renal cell carcinoma (RCC) is one of the prevalent cancer types in Europe. Up to 30% of patients diagnosed with RCC present themselves with synchronous metastases, and recurrence is seen in 30% of patients after complete resection of the primary tumor [1]. The 5-year survival of early-stage RCC is 93%, while metastatic RCC (mRCC) patients have dismal 5-year survival rates of approximately 12%. The therapeutic options for the treatment of mRCC have significantly expanded in recent years, encompassing a diverse range of multi-targeted tyrosine kinase inhibitors (TKIs), immune checkpoint inhibitors (ICIs), and mammalian target of rapamycin (mTOR) inhibitors [2] . To date, the dual inhibitor of vascular endothelial growth factor (VEGF) receptor and MET proto-oncogene (MET) cabozantinib could improve progression-free survival (PFS)/overall survival (OS), and has been proven to be effective as single-agent therapy in patients that were previously treated with VEGF-targeted therapy [3]. In addition, cabozantinib significantly improved median PFS (hazard ratio (HR) 0.58) and OS (HR 0.66) when compared with everolimus in previously treated mRCC patients [4]. In the newest guidelines [5, 6], cabozantinib is recommended as one of the preferred therapies for mRCC patients with all risk stratifications.
Cabozantinib is approved for mRCC patients at an initial oral once daily (QD) dose of 60 mg or 40 mg combined with nivolumab. The approved dose is supported by several population pharmacokinetics (POPPK)/pharmacodynamics (PD) studies with data from clinical trials [7,8,9,10]. Exposure-response analysis in the registration file regarding PFS, objective response rate (ORR), and tumor growth inhibition showed that the average exposure of standard dosing with 60 mg had better outcomes compared with 40-mg and 20-mg doses. However, the real-world tolerability of cabozantinib is quite different from the clinical trials. Previous research [11] by members of our group showed that 77.8% of patients cannot tolerate the labeled 60-mg QD dose, which is similar to the findings in the registration study (79% dose reduction) [12]. The median best tolerated dose was 40 mg QD. In this report, no clear relationship between increased cabozantinib exposure and improved PFS was observed. Average cabozantinib exposure (average concentration at steady state (Cavg,ss) 572 ng/mL) was below the previously proposed target (Cavg,ss in clinical trials for 40-mg QD dosing, 750 ng/mL [13]) in 83% of patients. Another group [14] demonstrated a threshold of trough concentrations (Ctrough) of 536.8 ng/mL for efficacy and of 617.7 ng/mL for toxicity. Recently, Blanchet et al. [15] observed even a lower efficacy target of Ctrough > 336 ng/mL regarding PFS, but was not statistically associated with longer OS. To conclude, tolerability issues seem to reduce the maximum tolerated dose to 40 mg in the large majority of patients without compromising its efficacy. There is only limited pharmacokinetic (PK) information in real-world patient data that can be viewed as a first step towards characterization of the exposure-response of cabozantinib in real-world patient data.
Currently, three POPPK models for cabozantinib have been published. Two POPPK models of cabozantinib [8, 10] included both healthy volunteers and patients with different tumor types. Another model, based on mRCC patients only, was depicted in the Food and Drug Administration (FDA) registration file (now referred to as the FDA cabozantinib POPPK model) [13]. The POPPK of cabozantinib was, in all cases, described with a dual absorption model with linear elimination process [8, 10, 13]. Single doses of cabozantinib showed dose-proportional increases in mean plasma cabozantinib concentrations. It is worth noting that cabozantinib exhibits an extremely long terminal half-life, around 99 h, which leads to a negligible difference in cabozantinib exposure when extending the dosing interval.
A barrier for reimbursement of cabozantinib by healthcare insurance is the high drug expenses, regardless of monotherapy or when combined with immunotherapy. Cabozantinib drug expenses are estimated to be €6200 per patient per month in Europe, and a flat-pricing model is applicable for all available strengths (the 20-mg tablet, 40-mg tablet, and 60-mg tablet share exactly the same price and therefore have a different price per mg). Nevertheless, several studies on cost-effectiveness [16, 17] have found that cabozantinib was more effective than treatment with axitinib or everolimus in mRCC second-line treatment but was associated with higher total drug expenses. Previously, high-fat meals proved to increase cabozantinib exposure by about 57% on average [18], which represents a dose intensity of 20 mg (fasted) versus 31 mg (non-fasted). Combined with its long terminal half-life, this opens the way for potential drug expenses saving regimens while maintaining adequate efficacy, like investigators showed in the DIET study, where 25% of pazopanib expenses could be saved with lower doses taken together with food [19, 20].
The primary objective of this study was to evaluate the predictive value of the POPPK model of cabozantinib used for its registration using real-world patients’ therapeutic drug monitoring (TDM) data. The secondary objective was to design, simulate, and evaluate alternative dose regimens (equivalent average exposure in steady state within a 3-day interval) with the aim to reduce drug expenses (total expense within a month) using the known drug–high fat meal interaction.
2 Methods
2.1 General Study Design
First, included patients’ clinical data were retrieved, and a model evaluation with the previous published FDA cabozantinib POPPK model from the registration documents [13] was performed. Subsequently, less complex models were explored and a final POPPK model was established. Based on the final POPPK model, several simulation scenarios were performed with the best tolerated dose (40 mg QD) defined in our previous research [11] to find the regimen with the least drug expenses without significantly reducing Cavg,ss or area under the curve (AUC).
2.2 Patients and Data
This study was a single-center, retrospective, data analysis study of mRCC patients treated with cabozantinib between August 2018 and December 2021 at the Department of Oncology, Leiden University Medical Center (LUMC). Patients with at least one routinely planned TDM observation were included. Demographic (age, weight, height, sex) and laboratory data (liver and renal functions) at the start of cabozantinib treatment were retrieved from the electronical health records. Creatine clearance (CrCL) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation expressed as mL/min/1.73m2, which is normalized to a body surface area of 1.73 m2 [21]. For cabozantinib treatment, information on the start/finish date and time, dose adjustments, dose interruptions, and reason for discontinuation or adjustment, and TDM sample time and results were also collected.
This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the Institutional Review Board (IRB) at the Scientific Committee of Clinical Oncology, Medical Ethics Review Committee Leiden/Den Haag/Delft. As data from routine care were used, a waiver was granted for the requirement of informed consent by the IRB.
2.3 Bioanalytics of Cabozantinib Samples
A previously described sensitive and selective ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) assay was used to determine total cabozantinib concentrations in plasma. The method was validated for cabozantinib in the range 10–4000 ng/mL [22].
2.4 Cabozantinib Pharmacokinetic Modeling
2.4.1 Description of the Prior POPPK Model
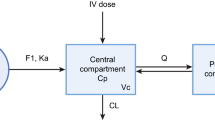
Details on the previously developed POPPK model, including the population, development, and evaluation, are provided in the FDA registration file of cabozantinib for the mRCC indication[13]. In short, the study was based on rich clinical PK data from one phase I study and one phase III study of 63 healthy participants and 325 mRCC patients treated with cabozantinib. A two-compartment disposition model with dual (fast and slow) lagged first-order absorption processes adequately characterized the concentration–time profile of cabozantinib in the above population, which is depicted in Fig. 1. Covariates that included in the FDA cabozantinib POPPK model were female gender (21% lower apparent plasma clearance [CL/F]) and Asian race (27% lower CL/F). This model is referred to as the “FDA cabozantinib POPPK model” in this article.
Schematic representation of the FDA cabozantinib POPPK model for cabozantinib [13] (Ka1 = absorption rate constant from depot 1, Ka2 = absorption rate constant from depot 2, ALAG1 = lag time of depot 1, ALAG2 = lag time of depot 2, CL/F = apparent plasma clearance, Q/F = apparent distribution rate constant between compartments)
2.4.2 External Model Evaluation of the FDA Cabozantinib POPPK Model Using TDM Data
The obtained PK data were initially evaluated using the FDA cabozantinib POPPK model. To this end, all parameters’ values were fixed to the values described in the registration file [13]. Due to the lack of ethnicity data in the current dataset, it was assumed that all patients were Caucasians since roughly 90% of the population in our University Medical Center is Caucasian. Other covariates, i.e., effect of sex on CL/F, in the FDA cabozantinib POPPK model were maintained in the model.
2.4.3 Further Model Development
On the basis of the FDA cabozantinib POPPK model, the model was adjusted where needed as follows: (1) simplification of the model structure, which included the possibility of removal of dose-dependent absorption rate constant (Ka) or correlation of random effects; (2) re-estimating PK parameters, which included CL/F, inter-individual variability (IIV) of CL/F, and residual error; and (3) due to the limited sample size of the real-world dataset, new covariates exploration was skipped and covariates in the FDA cabozantinib POPPK model were maintained.
2.4.4 Model Evaluation
2.4.4.1 External Evaluation Criteria
Individual predicted (IPRED) and observed concentrations (DV) over time and population predicted (PRED) and DV over time were plotted for each individual for evaluation of the FDA cabozantinib POPPK model fit. Goodness of fit (GOF) plots, including IPRED versus DV, PRED versus DV, conditional weighted residual error (CWRES, which is the weighted difference between the model prediction based on estimation approximation and the data [23]) versus PRED, and CWRES versus time after last dose (TAD), were also used to evaluate the performance of the FDA cabozantinib POPPK model.
Apart from the GOF plots, several model accuracy measurements assisted the evaluation. These measurements included mean absolute error (MAE), mean absolute percentage error (MAPE), mean square error (MSE), and root mean square error (RMSE). The equations are shown below (Eqs. 1–4), where Obsi represents the individual observation, IPREDi represents the individual model prediction, and n represents the number of observations/predictions.
2.5 Final Model Evaluation Criteria
Visual evaluation methods (GOF plots) were applied to evaluate the performance. The precision of the PK parameter estimates was expressed as relative standard error (RSE, %) and confidence intervals (CIs) of the estimated parameter(s). The RSE were directly computed by NONMEM, and a value < 30% for fixed effects and < 50% for random effects was considered acceptable [24]. Bootstrap and normalized prediction distribution errors (NPDE) were also performed to evaluate the stability and predictive ability of the model for internal model validation procedures [25, 26]. For bootstrap, 1000 bootstrap replicates were generated by resampling from the original dataset. Median parameter values and the 2.5th–97.5th percentiles from bootstrap estimates were compared with the final model estimates.
2.6 Simulations
Simulations to evaluate drug expenses saving regimens were divided into two steps. The first step was to compare the exposure of drug expenses saving regimens in the fasted state. The second step was to compare regimens in the fed state (high-fat meal) with the current recommended daily dose in the fasted state.
In previous real-world studies [11, 14, 15], the 40-mg QD regimen was defined as the best tolerated regimen, which was also used as the standard regimen in the simulation part. Based on the final developed model, multiple alternative dosing regimens, with/without high-fat meals, were simulated in order to identify regimens that provide comparable drug exposure to dosing with 40 mg QD but with lower drug expenses. In addition, the original FDA POPPK model code was used to simulate alternative dosing regimens. To simulate the impact of high-fat meals, it was assumed that the bioavailability of the drug will increase by 50% when taken with high-fat meals. This is based on a previous phase I food-effect study [18]. The study showed that when the cabozantinib capsule was administered with a high-fat, high-calorie meal in healthy participants, the maximum concentration (Cmax) and AUC values (AUC0–t and AUC0–inf) were increased by 41% and 57%, respectively [18]. The detailed simulated regimens are depicted in Fig. 2.
Assessed PK parameters included trough concentration in steady state (Cmin,ss), Cmax at steady state (Cmax,ss), Cavg,ss, and area under the concentration–time curve over a 72-h dosing interval (AUCss,72 h). AUCss,72 h was derived by adding an AUC compartment in the NONMEM control stream. The criteria of bioequivalence of a narrow therapeutic index [27] drug, which is within 90–111% of assessed PK parameters, was adopted to evaluate these proposed alternative regimens.
2.7 Software
POPPK modelling and simulations were performed with the non-linear mixed-effects modelling software NONMEM® version 7.4.4 (Icon Development Solutions, Ellicott City, MD, USA), using the Perl-speaks-NONMEM (PsN) Toolkit 4.8.1 and Pirana 2.9.7 as the modelling interface. The first-order conditional estimation with interaction (FOCE-I) method was used throughout. Data handling, visualization, and statistics were performed in R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Patients
Twenty-seven patients with 75 observations were included in this study. Patients’ characteristics and dosing information are summarized in Table 1. The mean age of included patients was 65 years old, and 70% were male. Mean baseline body mass index (BMI) was 24.7 kg/m2, mean baseline CrCL was 70 mL/min, and mean baseline alanine aminotransferase (ALAT) was 46 U/L. The patients that provided the data for the development of the FDA cabozantinib POPPK model (registration file) originated from one phase I study (n = 63, healthy volunteers) and one phase III study (n = 318, mRCC patients). When compared with the patients included in this study (Supplemental Table S1; see the electronic supplementary material), no obvious difference was observed. Concentration versus TAD profiles are shown in Supplemental Fig. S1. Most samples were obtained under 40-mg QD and 60-mg QD dosing, and extensive variability was observed among all dose levels. Median TAD was 25.30 h (1.25–267.15 h), 48% observations were trough samples, and the mean of trough samples were 594.5 ng/mL, with a range of 308–1134 ng/mL. Steady state was not assumed for the included patients. All administered doses were added to the dataset (for that, we used the ADDL and II columns in the NONMEM dataset).
3.2 External Model Evaluation of the FDA Cabozantinib POPPK Model
The model control stream of the FDA cabozantinib POPPK model was reproduced and is presented in the electronic supplementary material. Only IIV of fast absorption fraction (IIV_F1) was decreased from 0.385 to 0.05. Initially, the IIV_F1 was set to the value of the FDA registration file of 0.385. This setting resulted in terminated runs due to a negative F1 fraction, and it was impossible to continue using this value. Therefore, we decrease the variance and set it to a 20% variation of F1 (which improved both the object function value [OFV] and GOF plots) in both POSTHOC and following final model development. The diagnostic plots are shown in Supplemental Figure S2. The IPRED versus DV plot indicated an acceptable fit with an unbiased local regression (LOESS) line, while the PRED versus DV plot suggested a systematic overestimation of those given the 60-mg QD dose. No deviation was observed from the CWRES versus PRED and CWRES versus TAD plots, which indicated no potential misspecification of the model. Further model adjustment was needed to improve PRED to fit for the real-world population.
3.3 Further Model Development
3.3.1 Estimating Parameters of the Final Cabozantinib POPPK Model
Among the explored models (Supplementary Table S2 and Figure S3; see the electronic supplementary material), estimating CL/F and residual error while keeping the other parameters at the original values led to a significantly improved PRED versus DV (Fig. 3) and lowered OFV by 23. Estimating apparent central volume of distribution (Vc/F) and/or IIV on CL/F, as well as removing IIV correlation of CL/F and Vc/F, did not further improve the mode fit. Thus, the IIV on CL/F was kept at the value of the FDA cabozantinib POPPK model. To summarize, the changes of the final model were estimating CL/F, residual error, and decrease of IIV_F1 to 0.05.
The GOF plots of the model are shown in Fig. 3. The estimated CL/F was 3.11 L/h with an RSE of 5%, which was higher than the reported CL/F of 2.23 L/h in the FDA cabozantinib POPPK model (Table 2).
3.3.2 Internal Model Evaluation of the Final Model
Bootstrap results indicated a stable estimation of the typical value of CL/F with a median 3.11 (95% CI 2.73–3.58) L/h, which is the same as the NONMEM estimate (3.11 L/h). The plots of NPDE frequency distribution, NPDE distribution versus TAD, and NPDE distribution versus the log value of the concentrations did not show obvious trends or bias in the predictions by the final model, as shown in Supplemental Figure S4 (see the electronic supplementary material). The statistical test of NPDE normality indicated a normal distribution.
3.4 Simulations
In the first step for alternative drug expenses saving regimens, two regimens assuming fasted status were compared. As depicted in Fig. 4a, 40 mg QD has a stable concentration fluctuation during steady state. The regimen 60 mg QD given 2 days with 1 skip day had a higher peak concentration and lower Ctrough than 40 mg QD (still higher than the reference concentrations), as well as a larger fluctuation than 40 mg QD. For the AUC metrics, 40 mg QD and 60 mg QD given 2 days with 1 subsequent skip day had comparable exposure AUCss,72 h (relative change 0.09%), which is bioequivalent according to the criteria described in Sect. 2.5. Based on these metrics, 60 mg QD given 2 days with 1 subsequent skip day may be an option for potential drug expense savings.
a Simulations of patients taking 40 mg QD cabozantinib fasted versus taking 60 mg QD 2 days+1 day skip regimen fasted; b Simulations of patients taking 40 mg QD cabozantinib fasted versus taking 40 mg QD 2 days+1 day skip regimen fed; c Simulations of patients taking 20 mg QD cabozantinib fasted versus taking 40 mg q72h fed (Black line: median simulation curve; shaded area: 90% prediction interval (PI))
The second step was performed to utilize the food effect for further reduction of cabozantinib expenses. Patients who had a dose reduction from 60 mg QD to 40 mg QD may be eligible for a regimen that consists of taking cabozantinib 40 mg QD with a high-fat meal for 2 days and 1 skip day, which is depicted in Fig. 4b, resulting in a 33% reduction of expenses. Patients that are on a 20-mg QD regimen due to intolerable effects may be eligible for a regimen that consists of 40 mg combined with a high-fat meal every 3 days, resulting in a 67% reduction of expenses. The difference between 20 mg QD fasted and 40 mg every 72 h (q72h) fed is depicted in Fig. 4c. It is worth noting that in real clinical settings, patients taking 20 mg QD always have a lower percentile of CL/F, and the simulation plot of Fig. 4c was intended to show the comparison of exposure between standard and drug expenses saving regimens, which did not represent the real exposure level of 20-mg QD patients. A summary of the exposure metrics of the 1000 simulations is shown in Table 3. Comparable Cmin,ss and AUCss,72 h were achieved among all comparison groups, respectively. All regimens had bioequivalent clinical outcome-related exposure metrics for Cavg,ss (within 90–111%) compared with standard daily fasted dosing. In addition, for comparison purposes, the original FDA POPPK model code was used to simulate alternative regimens. A summary of these exposure metrics of the 1000 simulations is shown in Supplementary Table S3 (see the electronic supplementary material). Also here, comparable Cmin,ss and AUCss,72 h were achieved among all comparison groups, respectively. All regimens had bioequivalent clinical outcome-related exposure metrics for Cavg,ss (within 90–111%) compared with standard daily fasted dosing.
4 Discussion
Although cabozantinib has been available for approximately 7 years, clinical real-world knowledge of its PK and PD is still limited. Furthermore, its use is associated with a high financial burden for society as a whole. To the best of our knowledge, this is the first study to evaluate the FDA cabozantinib POPPK model in a real-world setting. In the first part, the FDA cabozantinib POPPK model [13] in the registration file was evaluated with an external real-world mRCC cohort. The evaluation indicated an adequate fit of the FDA cabozantinib POPPK model with the real-world data (Supplementary Figure S2; see the electronic supplementary material), with only some underprediction of the 60-mg QD dose. After estimating CL/F while fixing all other parameters, the model fit was improved significantly and qualified for simulation of alternative dose regimens. By using the population approach, also non-steady state data and randomly sampled plasma concentrations could be included to evaluate the performance of the FDA PK model file. In addition, the use of the PK model for evaluation of cost reduction strategies can also give insights into increased peak concentrations and help to design confirmatory prospective studies. In the simulation part, a drug expenses saving regimen in the fasted state was developed. A 33% reduction of drug expenses could be achieved with a dose regimen that consists of 60 mg QD given for 2 subsequent days followed by 1 day without cabozantinib, when compared with a dose regimen of 40 mg QD fasted, while maintaining equivalent drug exposure (AUCss,72 h, Cmin,ss, Cavg,ss) in steady state. Since Cavg,ss is used as an exposure parameter in registration trials for outcomes and toxicity [7, 9, 28] and the proposed alternative regimen had similar target achievement (Cavg,ss relative change < 10%), it is highly likely that both efficacy and toxicity will be comparable according to the bioequivalent criteria for both normal drugs (80–125%) and narrow therapeutic index drugs (90–110%) [27]. When taking the food effect into consideration, 40 mg q72h with high-fat meal regimen could result in a 67% reduction of drug expenses when compared with 20 mg QD fasted (AUCss,72 h relative change +3.16%).
The estimated CL/F of the final model was 3.11 L/h, which was higher than in the FDA cabozantinib POPPK model (2.23 L/h). Except for the FDA registration file, there were only two POPPK models of cabozantinib published [8, 10] until the most recent. The first published cabozantinib POPPK study [8] included data from nine clinical studies (three phase I, two phase II, and four phase III) for a total of 8072 cabozantinib concentration records from 1534 individuals. A two-compartment model with first-order elimination and a dual absorption (first-order + zero-order) process adequately described the observed cabozantinib PK data. The CL/F in this study was 2.478 L/h. While splitting the group by tumor types, CL/F for mRCC patients was 2.2 L/h, which was similar to the FDA cabozantinib POPPK model. Shortly after, an updated integrated POPPK model including hepatocellular carcinoma patients was established, and CL/F was estimated to be 2.48 L/h. One explanation for the different CL/F between trials and the real-world study could be that the dataset from real-world patients consisted of only mRCC patients, while in the clinical trials, the model [13] was built based on both healthy volunteers and mRCC patients. In addition, in clinical trials, sampling time and strategies were strictly designed. During routine clinic care, TDM samples were collected sparsely and randomly around Ctrough, which could affect the results of the model parameter estimation. Another possible reason could be the different demographics of the FDA cabozantinib POPPK model’s patients and real-world data, since in the FDA cabozantinib POPPK model, data from both healthy volunteers and included mRCC patients were pooled for analysis, and clinical performance status of real-world patients was worse compared with the patients in registration studies.
Previous published case studies [29] have demonstrated how PK modeling complemented traditional pharmacoeconomic analyses by identifying the impact of specific patient subgroups, dose, dosing schedules, and adherence on cost-effectiveness during clinical development. In this study, a general drug expenses saving regimen (using the drug for 2 days and then skip 1 day) was proposed with comparable exposure (Cmin,ss, Cavg,ss, AUCss,72 h), which resulted in reducing directly an amount of €2067 per individual per month. The only concern could be the lower exposure on the skip day when compared with the standard QD dosing (relative change −11.65%). However, taking into consideration that cabozantinib has an extremely long elimination half-life (~ 99 h) [3], the effect of this slightly lower exposure on the skip day on the efficacy should be negligible. Moreover, Cmax and AUC0–inf could be increased by a high-fat meal by 40.5% and 57.0%, respectively [18]. Therefore, administration in a fasting state (at least 2 h after and 1 h before a meal) is recommended in the drug label [3]. The simulation results of taking advantage of the food effect showed that an amount of up to €4133 per month per individual could be reduced, with comparable exposure metrics. This simulation attempts to provide a unique opportunity regarding cost-effectiveness from PK/PD considerations [29], not only during drug development but also in real-world practice. Another reference curve of the 95% percentile simulated exposure of cabozantinib label dose (60 mg QD) was added to the simulation plots (Supplemental Figure S5), which indicated potential safety of all proposed alternative regimens.
Another important aspect that should be emphasized is, at least in oncology, the reimbursement is not dependent on the dose only, but also on the indication, clinical activity (improvement) compared to standard of care, and market size. In a previous, similar study of ceritinib, ASCEND-8 [30], a low-fat meal with 450 mg enhanced gastrointestinal tolerability versus 750 mg fasted in patients with ALK-positive Non-Small Cell Lung Cancer (NSCLC) while maintaining similar exposure. This study led to the label change of the manufacturer eventually. However, the current study result could be used as a template for other highly expensive drugs with a flat-prizing model. Ideally, the root cause for high drug costs is tackled, but this requires a worldwide fundamental system change. Until then, the alternative treatment regimens as proposed by us can be used to reduce drug expenses associated with cabozantinib treatment.
One could argue that concomitant intake with a high-fat meal could be inconvenient for some of the patients, specifically in the morning, leading to reduced adherence. Although cabozantinib in principle could also be taken shortly before dinner, a light breakfast was anticipated to be more convenient for most patients. Therefore, a prospective study investigating the effect of a light breakfast on the PK of cabozantinib was recently designed and is currently recruiting patients, which potentially creates additional options for drug expenses saving regimens (NCT05263245). Concomitant food intake could potentially increase variability in exposure. However, in addition, TDM could be implemented to minimize the risk of both under- and overexposure. The costs of a single cabozantinib TDM service (will not exceed 250 euros in total per sample) are much less than the potential savings of a regimen of 60 mg QD for 2 days followed by 1 day off. In addition, according to a previous study investigating the cost-effectiveness of TDM with another TKI (imatinib), a 7% increase in quality-adjusted life years was achieved with imatinib TDM compared with imatinib alone, which proved that TDM will not add extra economic burden [31]. Therefore, it is likely that TDM services in addition to the proposed regimens will be cost efficient. Another concern could be the medication compliance in oncology practice. The proposed complex dose skip with high-fat diet regimens for the targeted therapy would be quite challenging for patients to implement or remember [29]. Using medication boxes could be a relatively easy solution for this. It may require additional written and oral patient education and counselling information [32] to get optimal adherence.
Our study had some limitations. Due to the retrospective nature of the TDM data, some inconsistencies might have occurred in the actual time of the last dose intake. However, this heterogeneity is unavoidable in real-world studies and representative for patients with mRCC in real clinical practice. The current POPPK modeling and simulation study of cabozantinib is the first study to evaluate the registration POPPK model in routine care. In addition, the observed cabozantinib concentrations were comparable to several previously reported real-world analyses [14, 21], which all had a median Cmin,ss of around 600 ng/mL. Furthermore, the number of patients in this analysis was relatively small to build a POPPK model by ourselves. Therefore, the published cabozantinib POPPK model was adopted with some modifications. More parameters could be estimated if an increased number of samples become available, as well as the identification of significant covariates. The food-effect reference that was used was from the cabozantinib capsule formulation rather than the tablet formulations [18]. The systemic exposure following two formulations are similar, not bioequivalent, however [13]. Therefore, evaluation of the proposed drug expenses saving regimens with/without food effect should be performed in future prospective clinical studies.
5 Conclusion
In this model-based study with real-world patient data, a POPPK model that was based on patients included in studies for registration of cabozantinib resulted generally in an adequate prediction for the real-world cabozantinib TDM data. Alternative dosing regimens and options for increasing exposure utilizing the high-fat meal drug–food interaction were proposed that can drastically reduce cabozantinib drug expenses at similar exposure of dosing regimens that have proven to be effective and tolerable.
References
Attalla K, Weng S, Voss MH, Hakimi AA. Epidemiology, risk assessment, and biomarkers for patients with advanced renal cell carcinoma. Urol Clin N Am. 2020;47(3):293–303. https://doi.org/10.1016/j.ucl.2020.04.002.
Hahn AW, Klaassen Z, Agarwal N, et al. First-line treatment of metastatic renal cell carcinoma: a systematic review and network meta-analysis. Eur Urol Oncol. 2019;2(6):708–15. https://doi.org/10.1016/j.euo.2019.09.002.
Abdelaziz A, Vaishampayan U. Cabozantinib for the treatment of kidney cancer. Expert Rev Anticancer Ther. 2017;17(7):577–84. https://doi.org/10.1080/14737140.2017.1344553.
Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–23. https://doi.org/10.1056/NEJMoa1510016.
Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(5):706–20. https://doi.org/10.1093/annonc/mdz056.
Network NCC. NCCN Guideline Kidney Cancer V1 2021. 2021.
Lacy S, Nielsen J, Yang B, Miles D, Nguyen L, Hutmacher M. Population exposure-response analysis of cabozantinib efficacy and safety endpoints in patients with renal cell carcinoma. Cancer Chemother Pharmacol. 2018;81(6):1061–70. https://doi.org/10.1007/s00280-018-3579-7.
Lacy S, Yang B, Nielsen J, Miles D, Nguyen L, Hutmacher M. A population pharmacokinetic model of cabozantinib in healthy volunteers and patients with various cancer types. Cancer Chemother Pharmacol. 2018;81(6):1071–82. https://doi.org/10.1007/s00280-018-3581-0.
Miles DR, Wada DR, Jumbe NL, Lacy SA, Nguyen LT. Population pharmacokinetic/pharmacodynamic modeling of tumor growth kinetics in medullary thyroid cancer patients receiving cabozantinib. Anticancer Drugs. 2016;27(4):328–41. https://doi.org/10.1097/cad.0000000000000330.
Nguyen L, Chapel S, Tran BD, Lacy S. Updated population pharmacokinetic model of cabozantinib integrating various cancer types including hepatocellular carcinoma. J Clin Pharmacol. 2019;59(11):1551–61. https://doi.org/10.1002/jcph.1467.
Krens SD, van Erp NP, Groenland SL, et al. Exposure–response analyses of cabozantinib in patients with metastatic renal cell cancer. BMC Cancer. 2022;22(1):228. https://doi.org/10.1186/s12885-022-09338-1.
Jänne PA, Kim G, Shaw AT, Sridhara R, Pazdur R, McKee AE. Dose finding of small-molecule oncology drugs: optimization throughout the development life cycle. Clin Cancer Res. 2016;22(11):2613–7. https://doi.org/10.1158/1078-0432.Ccr-15-2643.
CDER. CLINICAL PHARMACOLOGY AND BIOPHARMACEUTICS REVIEW(S) OF CABOMETYX. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208692Orig1s000TOC.cfm
Cerbone L, Combarel D, Geraud A, et al. Association of cabozantinib pharmacokinetics, progression and toxicity in metastatic renal cell carcinoma patients: results from a pharmacokinetics/pharmacodynamics study. ESMO Open. 2021;6(6): 100312. https://doi.org/10.1016/j.esmoop.2021.100312.
Blanchet B, Xu-Vuilard A, Jouinot A, et al. Exposure-response relationship of cabozantinib in patients with metastatic renal cell carcinoma treated in routine care. Brit J Cancer. 2024. https://doi.org/10.1038/s41416-024-02585-y.
Meng J, Lister J, Vataire AL, Casciano R, Dinet J. Cost-effectiveness comparison of cabozantinib with everolimus, axitinib, and nivolumab in the treatment of advanced renal cell carcinoma following the failure of prior therapy in England. Clinicoecon Outcomes Res. 2018;10:243–50. https://doi.org/10.2147/ceor.S159833.
Sieg M, Hartmann M, Settmacher U, Arefian H. Comparative cost-effectiveness of cabozantinib as second-line therapy for patients with advanced hepatocellular carcinoma in Germany and the United States. BMC Gastroenterol. 2020;20(1):120. https://doi.org/10.1186/s12876-020-01241-y.
Nguyen L, Holland J, Mamelok R, et al. Evaluation of the effect of food and gastric pH on the single-dose pharmacokinetics of cabozantinib in healthy adult subjects. J Clin Pharmacol. 2015;55(11):1293–302. https://doi.org/10.1002/jcph.526.
Lubberman FJE, Gelderblom H, Hamberg P, et al. The effect of using pazopanib with food vs fasted on pharmacokinetics, patient safety, and preference (DIET study). Clin Pharmacol Therap. 2019;106(5):1076–82. https://doi.org/10.1002/cpt.1515.
Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, Hakala TR. The effect of food on cyclosporine absorption. Transplantation. 1985;40(2):174–6. https://doi.org/10.1097/00007890-198508000-00013.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
Krens SD, van der Meulen E, Jansman FGA, Burger DM, van Erp NP. Quantification of cobimetinib, cabozantinib, dabrafenib, niraparib, olaparib, vemurafenib, regorafenib and its metabolite regorafenib M2 in human plasma by UPLC-MS/MS. Biomed Chromatogr BMC. 2020;34(3): e4758. https://doi.org/10.1002/bmc.4758.
Hooker AC, Staatz CE, Karlsson MO. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res. 2007;24(12):2187–97. https://doi.org/10.1007/s11095-007-9361-x.
Alvarez JC, Moine P, Davido B, et al. Population pharmacokinetics of lopinavir/ritonavir in Covid-19 patients. Eur J Clin Pharmacol. 2021;77(3):389–97. https://doi.org/10.1007/s00228-020-03020-w.
Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90(2):154–66. https://doi.org/10.1016/j.cmpb.2007.12.002.
Thai HT, Mentré F, Holford NH, Veyrat-Follet C, Comets E. Evaluation of bootstrap methods for estimating uncertainty of parameters in nonlinear mixed-effects models: a simulation study in population pharmacokinetics. J Pharmacokinet Pharmacodyn. 2014;41(1):15–33. https://doi.org/10.1007/s10928-013-9343-z.
Jiang W, Makhlouf F, Schuirmann DJ, et al. A bioequivalence approach for generic narrow therapeutic index drugs: evaluation of the reference-scaled approach and variability comparison criterion. Aaps j. 2015;17(4):891–901. https://doi.org/10.1208/s12248-015-9753-5.
Castellano D, Pablo Maroto J, Benzaghou F, et al. Exposure-response modeling of cabozantinib in patients with renal cell carcinoma: Implications for patient care. Cancer Treat Rev. 2020;89: 102062. https://doi.org/10.1016/j.ctrv.2020.102062.
Srinivasan M, White A, Chaturvedula A, et al. Incorporating pharmacometrics into pharmacoeconomic models: applications from drug development. Pharmacoeconomics. 2020;38(10):1031–42. https://doi.org/10.1007/s40273-020-00944-0.
Cho BC, Kim D-W, Bearz A, et al. ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC). J Thorac Oncol. 2017;12(9):1357–67. https://doi.org/10.1016/j.jtho.2017.07.005.
Conti RM, Padula WV, Becker RV, Salamone S. The cost-effectiveness of therapeutic drug monitoring for the prescription drug-based treatment of chronic myeloid leukemia. J Manag Care Spec Pharm. 2021;27(8):1077–85. https://doi.org/10.18553/jmcp.2021.27.8.1077.
Felton MA, van Londen GJ, Marcum ZA. Medication adherence to oral cancer therapy: The promising role of the pharmacist. J Oncol Pharm Pract. 2016;22(2):378–81. https://doi.org/10.1177/1078155214559114.
Acknowledgements
The authors would like to appreciate Kaj van Schie for contributing to part of the data collection and Tan Zhang for code review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The work of Zhiyuan Tan was supported by the China Scholarship Council (202108310028).
Conflict of interest
No disclosures are applicable for this work. None of the other authors have any conflicts to declare.
Ethical approval
This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the Institutional Review Board (IRB) at the Scientific Committee of the Department of Medical Oncology, Medical Ethics Review Committee Leiden/Den Haag/Delft. As retrospective data from routine clinical care were used, a waiver was granted for the requirement of informed consent by the IRB.
Consent to participate
This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the Institution Review Board (IRB) at Scientific Committee of Clinical Oncology, Medical Ethics Review Committee Leiden/Den Haag/Delft. As data from routine care were used, a waiver was granted for the requirement of informed consent by IRB.
Consent for publication
This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the Institution Review Board (IRB) at Scientific Committee of Clinical Oncology, Medical Ethics Review Committee Leiden/Den Haag/Delft. As data from routine care were used, a waiver was granted for the requirement of informed consent by IRB.
Availability of data and material
Data are available from the corresponding author upon reasonable request.
Code availability
NONMEM codes are available in the supplements.
Author’s Contributions
ZT, SV, AY, CAJK and DJARM contributed to the research design, performed research, analyzed data, and wrote the manuscript and AR, AJG, TH treated patients and reviewed and revised the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tan, Z., Völler, S., Yin, A. et al. Population Pharmacokinetics of Cabozantinib in Metastatic Renal Cell Carcinoma Patients: Towards Drug Expenses Saving Regimens. Clin Pharmacokinet (2024). https://doi.org/10.1007/s40262-024-01379-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s40262-024-01379-y