Abstract
Background and Objective
Managing drug-food interactions is essential for optimizing the effectiveness and safety profile of quinolones. Following PRISMA guidelines, we systematically reviewed the influence of dietary interventions on the bioavailability of 22 quinolones.
Methods
All studies describing or investigating the impact of food, beverages, antacids, and mineral supplements on pharmacokinetic parameters or pharmacokinetic/pharmacodynamic indices of orally taken quinolones were considered for inclusion. We excluded reviews, in vitro and in silico studies, studies performed on animals, and those involving alcohol. We performed the search in Medline (via PubMed), Embase, and Cochrane Library, covering reports from database inception to December 2022. We used the following tools to assess the risk of bias: version 2 of the Cochrane risk-of-bias tool for parallel trials, the Cochrane risk-of-bias tool for cross-over studies, and the NIH quality assessment tool for before-after studies. We performed quantitative analyses for each quinolone if two or more food-effect studies with specified and comparable study designs were available. If meta-analyses were not applicable, we qualitatively summarized the results.
Results
We included 109 studies from 101 reports. Meta-analyses were conducted for 12 antibiotics and qualitative synthesis was employed for the remaining drugs. Of the studies, 60.5% were open-label, cross-over, as recommended by FDA. We judged 46% of studies as having a high risk of bias and only 4% of having a low risk of bias. Among 19 quinolones with available food impact data, 14 (74%) had potentially clinically important interactions. For nalidixic acid, oxolinic acid, and tosufloxacin, food exerted a high positive impact on bioavailability (AUC or Cmax increased by > 45%), whereas, for all the remaining drugs, postprandial absorption was lower. The most significant negative influence of food (AUC or Cmax decreased by > 40%) occurred for delafloxacin capsules and norfloxacin, whereas the moderate influence (AUC or Cmax decreased by 30–40%) occurred for nemonoxacin and rufloxacin. All 14 analysed quinolones showed a substantial reduction in bioavailability when co-administered with antacids and mineral supplements, except for calcium preparations. The impact of beverages was evaluated for 10 quinolones, with 50% experiencing significantly reduced absorption in the presence of milk (the highest negative impact for ciprofloxacin). Moreover, both ciprofloxacin and levofloxacin demonstrated compromised bioavailability when consumed with orange juice, particularly calcium-fortified.
Discussion
Several factors may influence interactions, including the physicochemical characteristics of quinolones, the type of intervention, drug formulation, and the patient’s health status. We assessed the quality of evidence as low due to the poor actuality of included studies, their methodological diversity, and uneven data availability for individual drugs.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Clinically important interactions with food occurred for 74 % of quinolones. |
Tested quinolones had lower bioavailability with antacids/non-calcium mineral supplements. |
Absorption of 50 % of investigated quinolones was significantly reduced with milk. |
Quinolone properties, formulation, intervention type, and patient health impact interactions. |
1 Introduction
1.1 Background
Quinolones are an essential class of antibacterial agents due to their potency, broad spectrum of activity against Gram-positive and Gram-negative bacteria, high oral bioavailability (from 70 to even 99%), and excellent tissue penetration [1]. In 2017, quinolones represented 9.5% of antibiotic consumption in the European Union and European Economic Area, with ciprofloxacin, levofloxacin, and norfloxacin as the most commonly prescribed drugs [2].
Quinolones inhibit essential bacterial enzymes involved in DNA formation: topoisomerases type II and IV and DNA gyrase [3]. Their use is restricted to well-defined indications, such as urinary tract infections, gastroenteritis, pneumonia, and gonococcal infections [3]. Still, the development of quinolones-resistant bacteria is an alarming and growing phenomenon. Resistance may arise, e.g., from mutations in target enzymes (topoisomerases II or IV and gyrase), reduced quinolone uptake by bacteria, and active transport of quinolones out of the bacterial cell [4].
One strategy to combat antimicrobial resistance involves optimizing antibiotic usage [5]. Specific indices that establish connections between antibiotic pharmacokinetic (PK) exposure and pharmacodynamic (PD) effectiveness have been identified. The action of quinolone antibiotics depends on their concentration – with a higher drug concentration being more critical for bacteria eradication than the duration of bacterial exposure [6]. Therefore, the most relevant PK/PD indices for this class of chemotherapeutics are the Cmax/MIC ratio (the maximum concentration relative to the minimum inhibitory concentration) and the AUC/MIC ratio (the area under the curve relative to the minimum inhibitory concentration) [6]. The threshold AUC/MIC value for the optimal clinical outcome differs depending on the drug and the source. In initial reports evaluating the relationship between PK/PD indices and clinical results, AUC/MIC values of ≥125 for ciprofloxacin and ≥ 34 for levofloxacin were significantly linked to clinical and microbiological recovery [6]. Subsequent investigations reported higher values required to achieve similar outcomes: AUC/MIC ≥ 250 for ciprofloxacin and ≥ 87 for levofloxacin [6]. Regarding the Cmax/MIC ratio, values ≥ 10–12 are believed to ensure the optimal effectiveness of quinolone treatment [6].
The way in which medications are administered concerning food presents a pivotal factor that can either positively or negatively impact drug absorption, the efficacy of the therapeutic regimen, and patient safety. Drug-food interactions may enhance or modify drug bioavailability, diminish or amplify the therapeutic effect, and contribute to an escalation or reduction in the frequency and severity of adverse drug reactions. Consequently, using chemotherapeutics correctly in relation to food can significantly improve treatment and prevent the development of antibiotic-resistant strains.
1.2 Existing Evidence
We identified two systematic reviews that explore the interactions between antibiotics and food or beverages. The first review, conducted in 2018 by Pino-Marin et al., examined drug-food and drug-drug interactions. However, there is room for improvement, as the authors included only 42 studies and utilized rather broad keywords such as ‘antimicrobial agents’ and ‘food-drug interactions’ [7]. Additionally, only five of the studies included in this review pertained to quinolones: three general reviews [8,9,10], one report referring to levofloxacin [11], and one to gatifloxacin (which is withdrawn from the global market) [12]. The second systematic review, carried out in 2020 by Mergenhagen et al., focused on interactions of antibiotics with alcohol and presented comprehensive and up-to-date evidence on this specific subject [13]. Consequently, we decided not to incorporate studies assessing the impact of alcohol into our analysis.
When considering the available evidence, there is a requirement for a comprehensive and up-to-date systematic review encompassing the impact of food, beverages, antacids, and mineral supplements, specifically on quinolone antibiotics.
1.3 Objectives
This systematic review was part of a broader investigation focusing on the interactions between antibiotics and food [14]. Its primary goals included examining the effects of food, beverages, antacids, and mineral supplements on the PK parameters and PK/PD indices of quinolone antibiotics. Additionally, it aimed to pinpoint the drug-food interactions with clinical relevance and to offer practical recommendations for administering quinolones with food, beverages, antacids, and mineral supplements.
2 Methods
While conducting and documenting this review, we followed the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The review protocol was registered in the Open Science Framework (OSF) Registries in November 2022 (https://doi.org/10.17605/OSF.IO/YE2W7). For the complete protocol, refer to Online Resource 1.
2.1 Eligibility Criteria
2.1.1 Types of Studies
For inclusion, we considered all studies that either described or examined the effects of food, beverages, antacids, and mineral supplements on the PK parameters and/or PK/PD indices of orally administered quinolone antibiotics. We imposed no restrictions based on study type, language, publication year, or participant count. However, we excluded reviews, in vitro and in silico investigations, and studies conducted on animals.
2.1.2 Types of Participants
We made no limitations with regard to characteristics of the study participants (e.g., gender, race, age, health condition).
2.1.3 Types of Interventions
As the experimental intervention, we defined the intake of oral quinolone with or after a meal (such as high-fat, low-fat, high-protein, etc.), beverage (e.g., juice, milk, coffee), antacid, or mineral supplement.
For meals, antacids, and mineral supplements, the control intervention was the administration of oral quinolones in a fasting state (before a meal, antacid, or mineral supplement), whereas for beverages—the intake of quinolone with water or on an empty stomach (before a liquid).
No limitations were imposed with regard to the formulation or dose of orally administered quinolones, the type of meals, antacids, or mineral supplements. However, we excluded studies that investigated interactions with alcohol.
2.1.4 Types of Outcomes
With regard to PK parameters, primary outcomes included the pre- and post-meal values of AUC (area under the plasma drug concentration-time curve, representing drug exposure), Cmax (maximum/peak serum drug concentration), and tmax (time to reach Cmax, reflecting the rate of drug absorption).
Additionally, we sought studies reporting post-meal alterations in PK/PD indices to connect antibiotic PK exposure with antibacterial PD response. Since quinolones primarily exhibit concentration-dependent bactericidal action, the most pertinent indices were Cmax/MIC and AUC/MIC.
Our secondary outcomes included pre- and post-meal values of other relevant PK parameters, if available, such as t½ (half-life), CL (clearance), Vd (volume of distribution),% of urinary recovery, etc.
2.2 Information Sources and Search Strategy
In November and December 2022, we searched three databases: Medline (accessible through PubMed), Embase, and the Cochrane Library, encompassing reports from database inception up to the date of our search. We found additional reports by examining the summaries of product characteristics (SmPCs) of quinolones available in the global market and scrutinizing the reference lists of studies we had previously identified. Furthermore, we found additional records classified as grey literature through a search on Google Scholar.
In the course of our search, we utilized the following keywords and phrases: quinolone chemotherapeutics names combined with “food”, “food-drug interaction”, “drug-food interaction,” “fed,” “fasted,” “fasting,” “postprandial,” “meal,” “breakfast,” “dietary supplement,” “antacids,” “milk,” “coffee,” “coca”, “cola”, “coke”, “beverage”, and “juice”, Whenever feasible, we incorporated MeSH terms and Emtree terms. In PubMed, Embase, and the Cochrane Library, our keyword search was limited to titles and abstracts, while in Google Scholar, it was restricted to titles alone. For a comprehensive description of our search strategy, refer to Online Resource 2.
2.3 Selection Process
We exported the search results from the databases and imported them into the Rayyan software, where the selection process took place. Initially, two authors, AW and PP, independently evaluated the titles and abstracts of each search record, selecting those that met the criteria for inclusion in the systematic review. In the subsequent phase, the authors retrieved and carefully reviewed the full texts of the chosen articles (if accessible), determining their inclusion. In cases of disagreement, we resolved the issues through discussion and consensus or, when needed, by seeking input from a third review author, PZ.
2.4 Data Collection Process
Two authors, AW and PP, independently gathered data from the included studies in an Excel spreadsheet. We collected the following information regarding:
-
study characteristics—first author, year of publication, study design, study language;
-
study participants—number of participants, participants’ health status, age, gender, race;
-
study intervention—quinolone name, dose and formulation, type of meal, beverage, antacid or mineral supplement, quantitative meal composition (caloric load, percentage, or weight amount of fat, carbohydrates, and protein), qualitative meal composition, dose and formulation of antacid or mineral supplement, time between drug intake and consumption of a meal, beverage, antacid or mineral supplement;
-
study outcomes—pre- and postprandial values and % postprandial change of PK parameters and/or PK/PD indices (listed in “Types of outcomes” section).
The data collection process was overseen by PZ, who addressed any discrepancies.
2.5 Assessment of Risk of Bias in Individual Studies
AW and PP independently assessed the quality of each study included in the systematic review. Various tools were employed to evaluate the risk of bias, depending on the study type. These tools included version 2 of the Cochrane risk-of-bias tool for parallel trials (RoB 2) [15], the Cochrane risk-of-bias tool for cross-over studies [16], and the NIH quality assessment tool for before-after (pre-post) studies [17]. Any disparities in the assessments were deliberated to reach a consensus.
2.6 Data Synthesis
We conducted quantitative analyses for each quinolone only when there were two or more food-effect studies with specified and comparable study designs; parallel and cross-over studies, as well as randomized and non-randomized studies, were not combined in the same meta-analysis.
We used the Review Manager (RevMan) software, Version 5.4.1, The Cochrane Collaboration, 2020, to complete the meta-analyses. Anticipating significant heterogeneity among the studies, we applied the random effects model and the inverse variance method to calculate study weights. The outcomes of the meta-analyses were presented visually in forest plots.
For drugs where meta-analyses were not feasible due to the absence of PK data or the unknown or variable study designs, we summarized the findings from the available studies and discussed the results.
2.6.1 The Effect Measures
The effect measures were the ratio of means (with vs without dietary intervention) for AUC (measured in µg·h/mL) and Cmax (measured in µg/mL), and mean difference (between the administration with and without dietary intervention) for tmax (measured in hours). We adopted a 90% confidence interval, as recommended by the US Food and Drug Administration (FDA) for bioequivalence studies. In cases where the reported values used different units than specified above, we converted them accordingly.
When effect measures were presented as geometric means with confidence intervals or the coefficient of variation, we transformed them into arithmetic means and standard deviations following the method outlined by Higgins et al [18]. In instances where median values and either a range or an interquartile range were provided, we employed the approach suggested by Wan et al [19] to estimate the arithmetic mean and standard deviation (SD).
2.6.2 Assessment of Heterogeneity
To assess and quantify the heterogeneity among the studies incorporated into the meta-analyses, we computed I2 statistics and conducted the Chi-squared test. An I2 value less than 25%, coupled with a Chi-squared test p-value exceeding 0.1, denoted low heterogeneity. When I2 fell between 25 and 75%, it signified moderate heterogeneity, whereas an I2 higher than 75% and a p value below 0.1 indicated high heterogeneity [20].
2.6.3 Additional Analyses
In situations of moderate or high heterogeneity, we carried out subgroup analyses, with grouping variables such as meal type, drug formulation, drug dose, participants’ health status, or the risk of bias within individual studies. The specific grouping variables varied across different meta-analyses, depending on the characteristics of the studies under consideration. Per the Cochrane Handbook for Systematic Reviews of Interventions guidance, subgroup analyses are typically warranted when at least ten studies are included in a meta-analysis. However, given the limited number of available studies, we conducted subgroup analyses if at least two were within each subgroup.
To judge the reliability of the combined results, we performed sensitivity analyses involving a shift from the random-effects model to the fixed-effects model.
In cases where meta-analyses encompassed more than ten studies, we explored the potential for publication bias by creating and interpreting funnel plots.
2.7 Judgement of Clinical Relevance
For AUC and Cmax, we employed the FDA criteria for bioequivalence in assessing the clinical relevance of interactions with dietary interventions. Our judgment of the interactions was as follows:
-
Clinically important—if results were statistically significant and the ratio of means (with vs without dietary intervention) was outside the range of 0.8–1.25.
-
Possibly clinically important—when the ratio of means was within the range of 0.8–1.25, but the lower or upper limit of the confidence interval fell outside the range.
-
Probably not clinically important—if both the ratio of means and confidence interval limits were within the bioequivalence range.
-
Not clinically important—when the analyses yielded statistically non-significant results.
For tmax, in cases where results of analyses were statistically significant, we considered interaction with dietary interventions to be clinically important if the mean difference between fed versus fasted exceeded 1 hour.
Furthermore, interactions deemed clinically important were categorized as having:
-
High impact—if the average AUC or Cmax after the dietary intervention was lower by more than 40% or higher by more than 45%, and the results of studies were consistent.
-
Moderate impact—when the average AUC or Cmax after the dietary intervention decreased by 30–40% or increased by 35–45%, and/or the results of studies were conflicting.
-
Low impact—when the average AUC or Cmax after the dietary intervention decreased by 20–30% or increased by 25–35%, and/or the results of studies were conflicting.
3 Results
3.1 Eligible Studies
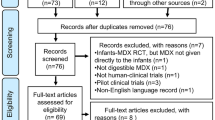
In the extensive database search, we identified 8849 records, comprising 3876 from Medline (via PubMed), 4575 from Embase, and 398 from the Cochrane Library. Automated tools were employed (Mendeley and Rayyan) to eliminate 4878 duplicates and we manually removed additional 139 duplicate records. Subsequently, the titles and abstracts of the remaining 3825 papers were thoroughly screened. Among these, 3679 studies did not align with our research question or met the exclusion criteria. Of the remaining 146 studies, we could not retrieve two reports [21, 22] as they lacked full text, and the abstracts did not provide sufficient information. After assessing the eligibility of the 144 remaining reports, 48 were excluded. The inter-rater agreement was nearly perfect, with 99.66% concordance (Cohen’s kappa: 0.932). For the list of excluded studies and the reasons for their exclusion, refer to Online Resource 3.
We also uncovered 242 records through alternative information sources, including Google Scholar (225), SmPCs (12), conference reports (3), and citation searching (2). Within this group of sources, we retrieved five specific records, and subsequently, after assessing the eligibility, we included them in the systematic review.
Finally, our systematic review incorporated 109 studies derived from 101 distinct reports. Figure 1 illustrates the flowchart outlining the search strategy.
3.2 Study Characteristics
The FDA recommends using cross-over study designs to evaluate the effect of food, and most studies (60.5%) in our systematic review followed this recommendation [23]. The remaining studies were longitudinal (21.1%), parallel (9.2%), or case studies (1.8%). In a small proportion (7.3%) of studies, the information provided did not specify the exact design. Additionally, for only 55% of the included studies, the authors declared that the study was randomized. Table 1 lists the studies included in our systematic review, while Online Resource 4 provides detailed study characteristics. Studies were presented in separate tables based on their investigation into the impact of (1) food, (2) antacids or mineral supplements, and (3) beverages. Studies were grouped by drug and organized according to the type of intervention and drug formulation. Consequently, some studies may appear in the tables more than once if they tested different formulations or interventions for the same drug or assessed the impact of food on the PK parameters of multiple quinolones.
3.3 Risk of Bias Assessment
Ninety-nine studies were identified with clearly defined study designs that were eligible for a quality evaluation. The risk of bias assessments conducted by AW and PP yielded comparable outcomes. Although there was a variance in the assessment of one domain for 20 studies, the conclusions were consistent for all studies. We categorized 46% of the studies as having poor quality (a high risk of bias), while only 4% were of good quality (a low risk of bias). The remaining studies fell into the category of fair quality (representing a moderate risk of bias). For detailed results of the risk of bias assessment, refer to Online Resource 5.
3.4 Quantitative Synthesis
Of the 20 examined quinolones, 12 met the criteria for inclusion in the quantitative synthesis. The drugs and studies excluded from the meta-analyses and the reasons for their exclusion are listed in Online Resource 6. We carried out 85 meta-analyses, including 12 for beverages, 38 for antacids or mineral supplements, and 35 for food. Table 2 summarizes the outcomes from the individual quinolones’ meta-analyses and the judgment of clinical relevance. The forest plots for each synthesis are presented in Online Resource 7.
3.4.1 Subgroup Analyses
In 46 (54%) of the meta-analyses, we observed moderate or high levels of heterogeneity. In response to this, subgroup analyses were carried out wherever feasible. Among the various grouping variables, we found that the type of antacid/mineral supplement and drug formulation were the only factors with the potential to explain the heterogeneity. Table 3 provides only the statistically significant findings from the subgroup analyses, with the judgment of clinical relevance. The forest plots for all conducted analyses are available in Online Resource 7.
3.4.2 Sensitivity Analyses
In 77 meta-analyses, altering the statistical model from the random effects model to the fixed effects model yielded no substantial qualitative differences in the overall effect. Notably, the mean difference confidence intervals tended to be narrower in the fixed effects models. However, the change in the magnitude of the overall effect (as indicated by the Z value) was inconsistent, and no constant increase or decrease was observed in the Z value for the fixed effects models compared to the random effects models.
For the remaining 8 meta-analyses, we detected significant disparities between the random and fixed-effects models, as detailed in Table 4 (for the forest plots of these meta-analyses, please refer to Online Resource 7). In all instances, using the random effects model yielded non-statistically significant differences after the intervention. Conversely, switching to a fixed effects model resulted in statistically significant differences, signifying variable impacts of the intervention, depending on the specific drug.
We noted significantly reduced heterogeneity or a higher overall effect in five meta-analyses when one of the studies was excluded. These exclusions have been described in Table 2.
3.4.3 Publication Bias
The minimum level of 11 studies was not reached in any meta-analysis; hence, we did not assess the publication bias.
3.5 Qualitative Synthesis
In the case of 13 quinolones, meta-analyses could not be conducted for at least one of the interventions. The primary reason was the lack of adequate studies with similar designs. Quinolones excluded from the quantitative synthesis are listed in Online Resource 6. For these particular drugs, Table 5 summarizes the existing evidence with the judgment of clinical relevance. A more detailed description of the studies is available in Online Resource 4.
3.6 Summary of Results
3.6.1 Clinically Important Interactions
Figures 2 and 3 present a summary of results obtained through quantitative and qualitative analyses. Among the 19 quinolones for which data on the impact of food were available, we identified clinically important or possibly clinically important interactions with food for 14 (74%) of them. Except for nalidixic acid, oxolinic acid, and tosufloxacin, for which administration with meals exerted a high positive impact (AUC or Cmax increased by more than 45%), all the remaining interactions reduced drug absorption. The most significant negative influence of food (AUC or Cmax decreased by more than 40%) occurred for delafloxacin capsules and norfloxacin, whereas the moderate one (AUC or Cmax decreased by 30–40%) for nemonoxacin and rufloxacin (Fig. 3).
The impact of antacids and mineral supplements was examined for 14 quinolones, and clinically important or possibly clinically important decreases in bioavailability were observed for all. The magnitude of the effect depended on the type of antacid/mineral supplement: aluminium and aluminium–magnesium preparations had the highest negative influence on the bioavailability of quinolones, whereas calcium supplements—had the lowest (Fig. 3).
The data concerning the impact of beverages were limited to 10 quinolones, and the absorption of 5 (namely ciprofloxacin, levofloxacin, norfloxacin, pazufloxacin, and prulifloxacin) was significantly diminished when consumed with milk. The highest negative impact of milk occurred for norfloxacin (Fig. 3).
3.6.2 Differences from the Recommendations in SmPCs
Figure 2 presents numerous disparities between the administration guidelines outlined in the SmPCs of quinolones and our findings. For ciprofloxacin tablets, gemifloxacin, levofloxacin tablets, and lomefloxacin, we observed a low but possibly clinically important negative impact of food on bioavailability. In contrast, according to SmPCs, all these drugs can be taken regardless of meals. In contrast, SmPCs of enoxacin, nalidixic acid tablets, prulifloxacin, and sitafloxacin, recommend to administer medications on an empty stomach, whereas our analysis indicates that the impact of food on these quinolones absorption is probably clinically irrelevant.
Regarding the influence of antacids/mineral supplements, our results generally align with the recommendations given in SmPCs; however, we detailed them considering the type of supplement (for more details, see Figure 3 and “Type of dietary intervention” subsection).
We identified limited information in the SmPCs regarding the optimal administration of quinolone antibiotics in relation to beverages. Our study has addressed some of the existing knowledge gaps in this area. Nevertheless, data on the potential impact of beverages on absorption are still lacking for half of quinolones.
4 Discussion
4.1 Factors Affecting the Interactions
Our review findings indicate a significant and varied influence of food, antacids, mineral supplements, and beverages on the PK parameters of quinolones. Through quantitative and qualitative analyses, we delve into several potential factors that could underlie the variable effects of the tested interventions.
4.1.1 Physicochemical Drugs Properties
Table 6 provides the physicochemical properties of quinolones concerning their interaction with food. Quinolones are generally poorly soluble in water compounds, having logarithm of the partition coefficient (log P) values falling within the range from more than 0 to less than 3 (indicating moderate lipophilicity). Quinolones exhibit amphoteric properties due to the presence of both basic (tertiary nitrogen) and acidic (carboxylic acid) groups [24]. Quinolone derivatives are generally characterized by good bioavailability, which may vary between and within generations. Within the second generation, their representatives possess moderate (norfloxacin: 30–50%) and high bioavailability (ciprofloxacin: 70–85%), while in the case of the third and fourth generation, their bioavailability is practically quantitative (levofloxacin: 99–100% and moxifloxacin: 90%) [25, 26]. Fluoroquinolone absorption occurs through a passive transport mechanism across cell membranes. Research on fluoroquinolones’ passive diffusion indicates that they form molecular agglomerates (“stacks”), reducing their electrostatic potential and facilitating the passage of the neutral form through cell membranes [27]. Regarding the Biopharmaceutical Classification System (BCS), which considers water solubility and intestinal permeability, we found representatives from all four BCS classes. However, it is worth noting that the BCS class was unspecified for many quinolones.
Drugs that belong to the BCS class II are characterized by low solubility in water and high intestinal permeability. Food typically enhances their absorption, such as by promoting the dissolution of lipophilic drugs [28]. We observed a positive impact of food (increased drug absorption or urinary recovery) for nalidixic acid that belongs to the BCS class II, oxolinic acid (with not specified BCS class), and tosufloxacin—classified either as BCS class II or IV antibiotic. Log P values of all 3 drugs (nalidixic acid—1.24, oxolinic acid—1.35, and tosufloxacin—2.74) indicate moderate lipophilicity, generally considered the most suitable for passive diffusion.
In the case of BCS class IV drugs, both solubility and permeability are low, and the influence of food can differ individually. This was observed in quinolones, where we found a neutral food effect for enoxacin, but for cinoxacin, ciprofloxacin, delafloxacin, fleroxacin, and norfloxacin, absorption was reduced when taken with a meal.
For quinolones from the BCS class III, food impact was either neutral (for ofloxacin and sitafloxacin) or negative (for levofloxacin and lomefloxacin). BCS class III drugs typically have low permeability and rapid dissolution rates, making them susceptible to changes in their dissolution process when taken with food [28]. The varying effects of food on individual quinolones in this class suggest that factors beyond physicochemical structure may play an additional role in their interactions.
4.1.2 Type of Dietary Intervention
The type of meal (e.g., high-fat, low-fat, high-protein) and the quantitative composition of the meal (e.g., caloric load, fat content) often influenced the food effect for many previously studied drugs [28, 29]. In our review, we employed the type of meal as a grouping variable in subgroup analyses when relevant. Of 19 quinolones for which food-effect data were available, only 6 were tested with more than one type of meal, namely ciprofloxacin, enoxacin, fleroxacin, levofloxacin, lomefloxacin, and ofloxacin. Interestingly, the type of meal did not explain heterogeneity in any analysed case. In general, whether the impact of food on the absorption of individual quinolones was negative, positive, or neutral, this effect was consistent across all types of tested meals (see Table S4.1 in Online Resource 4).
Regarding the impact of antacids or mineral supplements, we performed subgroup analyses with the type of intervention as a grouping factor for 3 quinolones: ciprofloxacin, gemifloxacin, and ofloxacin. Only in the case of ciprofloxacin did we reveal significant subgroup differences (see Table 3). Co-administration of ciprofloxacin with aluminium/magnesium-containing antacids and iron supplements significantly decreased the AUC (by on average 85% and 55%, respectively) and Cmax (by on average 81% and 48%). Upon co-intake with calcium supplements, the decrease of AUC was lower (by on average 35%), and the effect on Cmax varied from a significant reduction (by on average 41%) to no significant changes (depending on the meta-analysis). To address this conflicting data, we analysed studies separately. The neutral impact of calcium supplements was revealed in the study of Fleming et al, published in 1986 [30]. However, the authors described the methodology very basically (we judged the study as having a high risk of bias), and participants were patients with renal failure, which may have influenced the results. In two other studies by Lomaestro et al, calcium carbonate was given 2 h before the ciprofloxacin, either as a single dose or following three consecutive days. The authors concluded that this dosing regimen is sufficient to avoid changes in the drug bioavailability [31, 32]. In all the remaining studies, in which calcium supplements were administered with ciprofloxacin, a substantial decrease in absorption occurred, suggesting a negative interaction.
Interestingly, Frost et al noted that even with a 40% reduction in ciprofloxacin absorption when taken with calcium carbonate, urinary concentrations of ciprofloxacin remained above the MIC for susceptible uropathogens [33]. Additionally, unlike magnesium, calcium supplementation in bacterial growth medium for in vitro testing did not diminish the antibacterial activity of ciprofloxacin [33]. Therefore, calcium in the urine is unlikely to increase the MIC for susceptible bacteria. Nevertheless, the authors advised against the simultaneous intake of ciprofloxacin and calcium supplements.
For gemifloxacin and ofloxacin, although subgroup analyses by the type of supplements did not explain the heterogeneity, the time between antibiotic and supplement intake differed among the studies (see Table S4.2 in Online Resource 4). As we could not consider these factors simultaneously, we individually analysed the studies. For both gemifloxacin and ofloxacin, aluminium-magnesium and iron preparations given together with antibiotics exerted a clinically significant negative effect on bioavailability, whereas a probably clinically irrelevant effect occurred for calcium preparations. For all investigated antiacids and mineral supplements, their administration 2 hours after gemifloxacin or ofloxacin intake was sufficient to avoid negative interaction.
In cases where subgroup analyses by the type of antacid/mineral supplements were not applicable, we analysed individual study results. For enoxacin, pazufloxacin, pefloxacin, and rufloxacin, only studies with aluminium-containing antacids were available, all resulting in significantly decreased drug bioavailability (see Table S4.2 in Online Resource 4). For the remaining quinolones—fleroxacin, levofloxacin, lomefloxacin, moxifloxacin, and nemonoxacin, which were tested with different types of interventions, calcium supplements generally caused a much smaller, clinically insignificant, decrease in quinolones absorption compared to aluminium and aluminium–magnesium antacids. This difference could be due to the strength of the chemical bonds formed with these metal ions. Aluminium establishes more robust and stable chelates because its higher positive charge is concentrated in a smaller volume. In contrast, calcium ions have a larger size and lower charge density. Bonds between quinolones and calcium lengthen and weaken to avoid unfavorable bond angle distortion with the larger calcium ion [34]. We observed impaired absorption regardless of the antacid/supplement type only for norfloxacin and sitafloxacin.
Regarding the impact of beverage, we found evidence for several interventions: milk, yogurt, orange juice, grapefruit juice, and green tea (see Table S4.3 in Online Resource 4). The impact of milk or yogurt consumption was tested solely in adult volunteers, with effects varying from negative (for ciprofloxacin, norfloxacin, pazufloxacin, and prulifloxacin) to neutral (for enoxacin, fleroxacin, lomefloxacin, moxifloxacin, and ofloxacin). The impact of milk and yogurt on individual quinolone absorption was generally consistent with the effect of calcium supplements, suggesting that the most possible mechanism of negative interaction could be chelate formation with calcium ions present in dairy products.
For ciprofloxacin and levofloxacin, the effect of regular and calcium-fortified orange juice consumption was investigated, with both interventions impairing drug absorption [35,36,37]. Additionally, for levofloxacin, changes in AUC and Cmax were comparable regardless of whether the orange juice was calcium-fortified; thus, chelate formation does not seem to be the primary mechanism explaining the interaction [37]. Ciprofloxacin and levofloxacin are substrates of organic anion-transporting peptides (OATP) and possibly P-glycoprotein as well [37, 38]. Orange juice ingredients, such as heptamethoxyflavone, tangeretin, and nobiletin may act as substrates or inhibitors of these intestinal transport mechanisms, impairing the absorption of both quinolones. Additionally, the authors calculated AUC24h/MIC and Cmax/MIC ratios to investigate the clinical relevance of interaction. They revealed that for pathogens having a MIC > 0.5 mg/dL, such as, e.g., Streptococci and Staphylococci, antimicrobial activity of ciprofloxacin and levofloxacin could be suboptimal after co-intake with orange juice (especially calcium-fortified) [35,36,37].
Grapefruit juice inhibits CYP3A4 enzymes in the gut, slowing down drug metabolism and potentially impacting the bioavailability of certain medications. Recently, Cravens et al described the case of a 49-year-old healthy male who experienced Stevens-Johnson syndrome (SJS) shortly after completing a prophylactic course of ciprofloxacin following a vasectomy [39]. The patient had consumed approximately 950 mL (32 oz) of grapefruit juice daily for seven days. Although ciprofloxacin is primarily metabolized by CYP1A2 and only partially by CYP3A4, this case may represent the first reported instance of a clinically significant interaction between grapefruit juice and ciprofloxacin. However, Cravens et al highlighted that the SJS could also be caused simply by prophylactic administration of ciprofloxacin, as it is a rare but established side effect of the therapy. Based on this single case study, we cannot make recommendations against moderate grapefruit juice consumption during the treatment with ciprofloxacin.
The impact of green tea consumption was tested in only one pilot study by Matsumoto et al involving two participants [40]. Administration of pazufloxacin with green tea significantly decreased drug Cmax without changes in AUC or urinary recovery. Similar results were obtained after co-intake with milk. Matsumoto et al did not propose a possible mechanism for the observed interactions with beverages.
We found no studies investigating the interactions between quinolones and high-calorie, carbonated beverages, e.g., Coca-Cola. Although coffee appeared in several food-effect reports as the qualitative component of the tested meal (see Online Resource 4), we did not identify studies investigating the impact of coffee alone on the bioavailability of quinolones. Conversely, we found several reports of quinolone influence on the pharmacokinetics of caffeine, which is coffee’s active ingredient. Staib et al observed that simultaneous intake of ciprofloxacin or enoxacin with 220–230 mg of caffeine may inhibit caffeine elimination by decreasing its clearance and increasing AUC and half-life [41]. Healy et al confirmed these results for a 100 mg caffeine dose. Additionally, they noted that in the presence of ciprofloxacin, the rate of caffeine conversion to its metabolite—paraxanthine—was significantly delayed, which may lead to caffeine accumulation [42]. Carbo et al obtained similar results for pipemidic acid and norfloxacin [43]. In contrast, ofloxacin and lomefloxacin did not significantly alter the pharmacokinetics of caffeine [41, 44]. We excluded the above-mentioned reports from the systematic review (see Online Resource 3), as our focus was on the impact of dietary interventions on quinolones pharmacokinetics (and not the opposite). Additionally, synthetic caffeine instead of coffee was administered in these studies (so it did not meet our definition of intervention). Given the wide-spread global consumption of coffee and high-calorie carbonated beverages, good-quality studies investigating their potential impact on quinolones bioavailability would be needed.
4.1.3 Drug Formulation
Well-designed drug formulations can mitigate the impact of food on drug bioavailability [14, 28]. In quantitative synthesis, we used drug formulation as a grouping variable only for enoxacin and ciprofloxacin. For the latter antibiotic, it explained the heterogeneity of studies (see Figs. S7.12.3. and S7.14.2. in Online Resource 7).
Most food-effect studies for ciprofloxacin involved immediate-release (IR) tablet formulation, and results indicated decreased drug absorption. However, Omari et al tested two types of extended-release (ER) ciprofloxacin tablets (the marketed and experimental) and found no clinically significant changes in AUC and Cmax in fed versus fasted state [45]. Similarly, Shah et al revealed a slight, clinically irrelevant decrease in the rate of postprandial ciprofloxacin bioavailability when given as an oral suspension [46]. Lack of interaction with food makes both the ER tablet and the oral suspension of ciprofloxacin preferable formulations. Moreover, the ER formulation may provide better compliance and reduced frequency of gastrointestinal side effects compared to IR tablets [45], whereas the liquid form can be primarily used in the aging population and patients with swallowing difficulties.
Additionally, we found a significant formulation impact for delafloxacin and nalidixic acid during qualitative analysis (see Table 5).
For delafloxacin, ingesting both capsules and tablets with the high-fat meal resulted in decreased and prolonged absorption. However, the magnitude of the food effect was much higher for capsules than for tablets (Cmax lower by 50% and 21%, respectively) [47].
Ogata et al investigated the impact of food on 3 different nalidixic acid formulations: rapidly dissolving uncoated tablets, slowly dissolving uncoated tablets, and delayed-release tablets [48]. No significant changes in drug bioavailability occurred when drug release from the dosage was rapid, whereas, for the remaining formulations, the intake with a meal increased Cmax of nalidixic acid by 91–123%. Such an improvement in absorption could be due to the vigorous mixing and agitation in the gastrointestinal tract in the presence of food, resulting in enhanced drug dissolution [48].
4.1.4 Patients’ Health State
At least 95% of the reports analysed in our review involved healthy adult volunteers, which aligns with FDA recommendations. The remaining studies that recruited infected individuals were conducted in the 1980s and early 1990s, and their quality was low (high or moderate risk of bias) [30, 49,50,51,52,53].
We found only one report that considered the impact of a patient’s health state while investigating the effect of food. Pai et al examined the PK of levofloxacin without and with calcium carbonate (given 2 h after the drug) in healthy volunteers and patients with cystic fibrosis [11]. In healthy participants, the standard 2-h period between levofloxacin and calcium carbonate intake was sufficient to avoid the interaction. Meanwhile, Cmax decreased by 19% in patients with cystic fibrosis, and tmax increased by 37%. Changes in peak concentrations resulted in a lack of bioequivalence. Observed differences related to the participants’ health state can be due to the slower intestinal transit time in patients with cystic fibrosis [11]. As calcium supplements are commonly used in cystic fibrosis to prevent osteoporosis, Pai et al recommended the maximum separation time (longer than 2 h) between multivalent cations supplements and ciprofloxacin to ensure treatment efficacy [11].
4.2 Limitations of Studies Included in the Review
4.2.1 Actuality of Studies
Our systematic review encompassed food-effect studies without imposing restrictions based on publication year to ensure the broadest range of evidence. The first quinolones were initially discovered and registered in the 1960s, but their therapeutic significance substantially grew in the early 1980s with the development of fluoroquinolones [54]. Since food-effect studies are typically conducted during the introduction of the drug to the market, the oldest reports examined in our review date back to 1976 and 1979. Many of the studies we analysed were published in the 1980s and 1990s. These earlier reports often offered only rudimentary methodological details, making the risk of bias assessment more challenging.
4.2.2 Methodology of Studies
Only 60.5% of the analysed food-effect studies adhered to the study design recommended by FDA guidelines, which is an open-label, cross-over design. This might be because the initial FDA recommendations were not published until 2002, and many of the studies included were conducted before this date. When assessing the risk of bias, we encountered several concerns related to the cross-over design strategy, including the lack of separate data from each trial period, incomplete participant count disclosure within study sequences, and washout periods that we judged too short (see Online Resource 5 for detailed assessment of each study).
Approximately 86% of cross-over and 55% of all studies were randomized. However, a significant limitation was that most randomized trials did not provide comprehensive information regarding the randomization process; in most cases, the term "randomized" was the sole mention. This lack of details raises uncertainty about the authenticity of the randomness and the concealment of the allocation sequence.
Furthermore, there were eight studies in which the study design was left unspecified (see Table 1). As a result, we could not assess the quality of these trials and include them in the quantitative synthesis.
Moreover, we noted disparities in the bioanalytical techniques employed across the analysed studies. Starting in the mid-1980s, the adoption of more advanced and accurate technologies, such as high-performance liquid chromatography (HPLC) and liquid chromatography tandem mass spectrometry (LC-MS/MS), for determining antibiotic concentrations gained prominence. Most of the reports included in our review used these modern methods. However, some studies determined plasma antibiotic concentrations through microbiological assays. To ensure the comprehensiveness of the evidence pool, we did not exclude these trials, but combining the results from studies in which varied bioanalytical methods were used may contribute to increased heterogeneity.
4.2.3 Participants-related Concerns
The trials under investigation generally featured small participant numbers, with 48% of the studies enrolling fewer than 12 volunteers, which is the minimal sample size recommended by the FDA in their 2002 guidelines.
The significant limitation is the lack of data regarding the impact of food on the pediatric population, as all studies included in the review involved adult volunteers. It can partially be explained by the fact that, due to safety concerns, the use of quinolones in the pediatric population is limited to only several FDA-approved indications, such as, e.g., treatment of inhalation anthrax or complicated urinary tract infections [55].
While gender was specified in most cases (95%), there was a significant underrepresentation of females, as 58% of studies included only males. The distribution was often uneven in those studies where both genders were present, with males constituting the majority of participants.
Furthermore, only 42% of the studies provided information about the race of the participants. Among the studies that did address these factors, African Americans were notably underrepresented, appearing in only 20% of the studies. Considering these factors, translating the study results into clinical practice may pose challenges.
4.2.4 Significant Gaps in Data
We investigated the impact of dietary interventions on PK parameters and PK/PD indices of quinolones; however, only three studies included the analysis of AUC24h/MIC and Cmax/MIC ratios [35,36,37].
In our meta-analyses, of various factors regarding the impact of food, the drug formulation and type of meal did not provide sufficient explanation for the observed heterogeneity; however, we cannot definitively rule out the influence of these factors due to data gaps. While most food-effect studies (91%) did specify the drug formulation, a significant number of the examined drugs lacked assessments for all available formulations. Additionally, in 16% of the studies, the type of meal was not specified, and in 61%, the quantitative and/or qualitative composition of the meals was not mentioned. Furthermore, a recurring concern was the variation in the qualitative and quantitative components, even among identical meal types (e.g., high-fat, high-protein, low-fat, etc.).
Regarding the impact of antacids and mineral supplements, our meta-analyses indicated that the interaction outcomes for specific quinolones depended on the antacid or mineral supplement used. However, there was significant variability in the formulations and doses of identical antacid/supplement types. Additionally, 13% of the studies did not clarify which antacid/supplement formulation or dose was under investigation.
With these limitations, we categorized the studies in this review as carrying a moderate or high risk of bias (for detailed information, refer to Online Resource 5).
4.3 Limitations of the Review
The primary limitation of our review was an uneven distribution and varying quality of evidence on quinolone interactions with dietary interventions. This topic was not addressed in any studies for 2 of 22 quinolones, namely pipemidic acid and rosoxacin. Among the remaining 20 drugs, 13 were excluded from quantitative analysis for at least one intervention due to insufficiently described study design or limited data, typically confined to a single report.
We conducted meta-analyses for 12 quinolones, yet numerous studies could not be included in the quantitative synthesis. The primary reasons for exclusion were inadequate or missing data on the investigated outcomes and inappropriate or uncertain study designs. In total, 17 studies had to be excluded, potentially leading to the loss of valuable insights into the effects of dietary interventions. Additionally, 96% of all studies carried a high or moderate risk of bias, lowering the quality of the presented evidence.
We avoided combining reports with different study designs, which led to the need for multiple syntheses for each outcome, resulting in an average of 3–4 studies per meta-analysis. These separate analyses sometimes yielded different results, adding complexity to interpretation.
Cochrane guidelines recommend a minimum of 11 studies for subgroup analysis and assessing funnel plot asymmetry. In our review, no meta-analysis reached this threshold. In the review protocol, we anticipated high overall heterogeneity and thus decided to conduct subgroup analyses when potential grouping factors were present, with each subgroup containing at least 2 studies. This approach allowed us to identify the type of antacid/supplement as a possible factor explaining heterogeneity in some trials. However, caution should be taken when interpreting these results due to the mentioned limitations.
5 Summary
Optimizing quinolone use is gaining importance in light of the rise in antibiotic-resistant bacterial strains. Dietary interventions can significantly affect the bioavailability of quinolones, and the extent of this impact varies among different drugs. Several factors may influence interactions, including the physicochemical properties of drugs, the type of dietary intervention, drug formulation, and the patient’s health condition.
Our review offers a comprehensive insight into how food, antacids, mineral supplements, and beverages affect the PK parameters of individual quinolones. However, the overall quality of the available evidence is compromised due to outdated, methodologically diverse studies with high or moderate risk of bias and an uneven distribution of data among different drugs. Available data on the effect of dietary interventions on PK/PD indices are scarce; hence, research in this direction would be desirable to allow linking changes in the bioavailability of quinolones to their efficacy.
References
Yan A, Bryant E. Quinolones. StatPearls. Treasure Island: StatPearls Publishing; 2022.
Adriaenssens N, Bruyndonckx R, Versporten A, Hens N, Monnet DL, Molenberghs G, et al. Consumption of macrolides, lincosamides and streptogramins in the community, European Union/European Economic Area, 1997–2017. J Antimicrob Chemother. 2021;76:30–6.
Baggio D, Ananda-Rajah MR. Fluoroquinolone antibiotics and adverse events. Aust Prescr. 2021;44:161–4.
Hopper D, Jacoby G. Mechanisms of drug resistance: quinolone resistance. Ann N Y Acad Sci. 2015;176:139–48.
European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance in the EU/EEA—a One Health response. 2022. https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-resistance-policy-brief-2022.pdf Accessed 4 Nov 2022.
Onufrak NJ, Forrest A, Gonzalez D. Pharmacokinetic and pharmacodynamic principles of anti-infective dosing. Clin Ther. 2016;38:1930–47.
Pino-Marín DE, Madrigal-Cadavid J, Amariles P. Clinical relevance of drug interactions with antibiotics related to changes in the absorption: structured review. Ces Med. 2018;32:235–49.
Gregg CR. Drug interactions and anti-infective therapies. Am J Med. 1999;106:227–37.
Gugler R, Allgayer H. Effects of antacids on the clinical pharmacokinetics of drugs. An update. Clin Pharmacokinet. 1990;18:210–9.
Sulochana SP, Syed M, Chandrasekar DV, Mullangi R, Srinivas NR. Clinical drug–drug pharmacokinetic interaction potential of sucralfate with other drugs: review and perspectives. Eur J Drug Metab Pharmacokinet. 2016;41:469–503.
Pai MP, Allen SE, Amsden GW. Altered steady state pharmacokinetics of levofloxacin in adult cystic fibrosis patients receiving calcium carbonate. J Cyst Fibros. 2006;5:153–7.
Mallet L, Huang A. Coadministration of gatifloxacin and multivitamin preparation containing minerals: potential treatment failure in an elderly patient. Ann Pharmacother. 2005;39:150–2.
Mergenhagen KA, Wattengel BA, Skelly MK, Clark CM, Russo TA. Fact versus fiction: a review of the evidence behind alcohol and antibiotic interactions. Antimicrob Agents Chemother. 2020;64:e02167-e2219.
Wiesner A, Zagrodzki P, Paśko P. Do dietary interventions exert clinically important effects on the bioavailability of beta-lactam antibiotics? A systematic review with meta-analyses. J Antimicrob Chemother. 2024;79:722–57.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1–8.
Higgins JPT, Li T, Sterne JAC. Revised Cochrane risk of bias tool for randomized trials (RoB 20): Additional considerations for crossover trials. Cochrane Methods. 2021;1–6.
NIH. Study quality assessment tools. 2022. https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools Accessed 25 Jun 2022.
Higgins JPT, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: Combining results reported on log-transformed or raw scales. Stat Med. 2008;27:6072–92.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Deeks J, Higgins J, Altman D. Chapter 10: Analysing data and undertaking meta-analyses. In: Cochrane Handb Syst Rev Interv version 63. 2022.
Ohnishi A, Toyoki T, Yoshikawa K, Hashizume K, Tanigawa T, Tanaka T, et al. Safety, pharmacokinetics and influence on the intestinal flora of BAY 12–8039 (moxifloxacin hydrochloride) after oral administration in healthy male subjects. Jpn Pharmacol Ther. 2005;33:1029–45.
Zhang J, Zhang Y-Y, Shi Y-G, Yu J-C, Wang L. Clinical pharmacokinetic study on ciprofloxacin. Chin Pharmacol Bull. 1996;12:430–3.
FDA. Guidance for industry: food-effect bioavailability and fed bioequivalence studies. 2002. https://www.fda.gov/files/drugs/published/Food-Effect-Bioavailability-and-Fed-Bioequivalence-Studies.pdf Accessed 1 Jul 2022.
Sharma PC, Jain A, Jain S. Fluoroquinolone antibacterials: a review on chemistry, microbiology and therapeutic prospects. Acta Pol Pharm Drug Res. 2009;66:587–604.
Culley CM, Lacy MK, Klutman N, Edwards B. Moxifloxacin: clinical efficacy and safety. Am J Heal Pharm. 2001;58:379–88.
Fish DN, Chow AT. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997;32:101–19.
Blokhina SV, Sharapova AV, Ol’khovich MV, Volkova TV, Perlovich GL. Solubility, lipophilicity and membrane permeability of some fluoroquinolone antimicrobials. Eur J Pharm Sci. 2016;93:29–37.
Wiesner A, Skrońska M, Gawlik G, Marcinkowska M, Zagrodzki P, Paśko P. Interactions of antiretroviral drugs with food, beverages, dietary supplements, and alcohol—a systematic review and meta-analysis. AIDS Behav. 2023;27:1441–68.
Wiesner A, Gajewska D, Paśko P. Levothyroxine interactions with food and dietary supplements—a systematic review. Pharmaceuticals. 2021;14:206.
Fleming LW, Moreland TA, Stewart WK, Scott AC. Ciprofloxacin and antacids. Lancet. 1986;2:294.
Lomaestro BM, Bailie GR. Effect of staggered dose of calcium on the bioavailability of ciprofloxacin. Antimicrobal Agents Chemother. 1991;35:1004–7.
Lomaestro BM, Bailie GR. Effect of multiple staggered doses of calcium on the bioavailability of ciprofloxacin. Ann Pharmacother. 1993;27:1325–8.
Frost RW, Lasseter KC, Noe AJ, Shamblen EC, Lettieri JT. Effects of aluminum hydroxide and calcium carbonate antacids on the bioavailability of ciprofloxacin. Antimicrob Agents Chemother. 1992;36:830–2.
Walden DM, Khotimchenko M, Hou H, Chakravarty K, Varshney J. Effects of magnesium, calcium, and aluminum chelation on fluoroquinolone absorption rate and bioavailability: a computational study. Pharmaceutics. 2021;13:594.
Neuhofel AL, Wilton JH, Victory JM, Hejmanowsk LG, Amsden GW. Lack of bioequivalence 4f ciprofloxacin when administered with calcium-fortified orange juice: a new twist on an old interaction. J Clin Pharmacol. 2002;42:461–6.
Sultana R, Ullah A, Akbor MM, Azad MAK, Latif AHMM, Hasnat A. Effect of orange juice on bioavailability of levofloxacin. J Appl Res. 2008;8:34–42.
Wallace AW, Victory JM, Amsden GW. Lack of bioequivalence when levofloxacin and calcium-fortified orange juice are coadministered to healthy volunteers. J Clin Pharmacol. 2003;43:539–44.
Park MS, Okochi H, Benet LZ. Is ciprofloxacin a substrate of P-glycoprotein? Arch Drug Inf. 2011;4:1–9.
Cravens MG, Sherman N, Sawaya J. Ciprofloxacin-induced Stevens–Johnson syndrome with grapefruit juice consumption: a case report. Cureus. 2019;11: e3827.
Matsumoto F, Sakurai I, Imai T, Ishida Y, Takahashi T, Morita M, et al. Fundamental and clinical studies on pazufloxacin. Jpn J Chemother. 1995;43:244–56.
Staib AH, Stille W, Dietlein G, Shah PM, Harder S, Mieke S, et al. Interaction between quinolones and caffeine. Drugs. 1987;34:170–4.
Healy DP, Polk RE, Kanawati L, Rock DT, Mooney ML. Interaction between oral ciprofloxacin and caffeine in normal volunteers. Antimicrob Agents Chemother. 1989;33:474–8.
Carbo M, Segura J, De la Torre R, Badenas JM, Cami J. Effect of quinolones on caffeine disposition. Clin Pharmacol Ther. 1989;45:234–40.
Healy DP, Schoenle JR, Stotka J, Polk RE. Lack of interaction between lomefloxacin and caffeine in normal volunteers. Antimicrob Agents Chemother. 1991;35:660–4.
Omari DM, Johary D, Salem II, Najib N, Sallam AA. Bioequivalence of two oral extended release formulations of ciprofloxacin tablets in healthymale volunteers under fed and fasting conditions. J Bioequiv Availab. 2011;3:038–42.
Shah A, Liu MC, Vaughan D, Heller AH. Oral bioequivalence of three ciprofloxacin formulations following single-dose administration: 500 mg tablet compared with 500 mg/10 mL or 500 mg/5 mL suspension and the effect of food on the absorption of ciprofloxacin oral suspension. J Antimicrob Chemother. 1999;43(Suppl A):49–54.
Hoover R, Hunt T, Benedict M, Paulson SK, Lawrence L, Cammarata S, et al. Single and multiple ascending-dose studies of oral delafloxacin: effects of food, sex, and age. Clin Ther. 2016;38:39–52.
Ogata H, Aoyagi N, Kaniwa N, Ejima A. Effect of food on bioavailability of nalidixic acid from uncoated tablets having different dissolution rates. J Pharmacobiodyn. 1984;7:760–7.
Golper TA, Hartstein AI, Morthland VH, Christensen JM. Effects of antacids and dialysate dwell times on multiple-dose pharmacokinetics of oral ciprofloxacin in patients on continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1987;31:1787–90.
Pennec MPL, Kitzis MD, Terdjman M, Foubard S, Garbarz E, Hanania G. Possible interaction of ciprofloxacin with ferrous sulphate. J Antimicrob Chemother. 1990;25:184–5.
Maesen FP, Davies BI, Geraedts WH, Sumajow CA. Ofloxacin and antacids. J Antimicrob Chemother. 1987;19:848–50.
Preheim LC, Cuevas TA, Roccaforte JS, Mellencamp MA, Bittner MJ. Ciprofloxacin and antacids. Lancet. 1986;2:48.
Preheim LC, Cuevas TA, Roccaforte JS, Mellencamp MA, Bittner MJ. Oral ciprofloxacin in the treatment of elderly patients with complicated urinary tract infections due to trimethoprim/sulfamethoxazole-resistant bacteria. Am J Med. 1987;82:295–300.
Bisacchi GS. Origins of the quinolone class of antibacterials: an expanded “discovery story.” J Med Chem. 2015;58:4874–82.
Patel K, Goldman JL. Safety concerns surrounding quinolone use in children. J Clin Pharmacol. 2016;56:1060–75.
Akinleye MO, Coker HAB, Chukwuani CM, Adeoye AW. Effect of five alive fruit juice on the dissolution and absorption profiles of ciprofloxacin. Nig Q J Hosp Med. 2007;17:53–7.
Allen A, Vousden M, Porter A, Lewis A. Effect of Maalox on the bioavailability of oral gemifloxacin in healthy volunteers. Chemotherapy. 1999;45:504–11.
Allen A, Bygate E, Faessel H, Isaac L, Lewis A. The effect of ferrous sulphate and sucralfate on the bioavailability of oral gemifloxacin in healthy volunteers. Int J Antimicrob Agents. 2000;15:283–9.
Allen A, Bygate E, Clark D, Lewis A, Pay V. The effect of food on the bioavailability of oral gemifloxacin in healthy volunteers. Int J Antimicrob Agents. 2000;16:45–50.
Amsden GW, Whitaker A-M, Johnson PW. Lack of bioequivalence of levofloxacin when coadministered with a mineral-fortified breakfast of juice and cereal. J Clin Pharmacol. 2003;43:990–5.
Ball AP, Fox C, Ball ME, Brown IR, Willis JV. Pharmacokinetics of oral ciprofloxacin, 100 mg single dose, in volunteers and elderly patients. J Antimicrob Chemother. 1986;17:629–35.
Bertino JS Jr, Nafziger AN, Wong M, Stragand L, Puleo C. Effect of a fat- and calcium-rich breakfast on pharmacokinetics of fleroxacin administered in single and multiple doses. Antimicrob Agents Chemother. 1994;38:499–503.
Black HR, Israel KS, Wolen RL, Brier GL, Obermeyer BD, Ziege EA, et al. Pharmacology of cinoxacin in humans. Antimicrob Agents Chemother. 1979;15:165–70.
Borner K, Höffken G, Lode H, Koeppe P, Prinzing C, Glatzel P, et al. Pharmacokinetics of ciprofloxacin in healthy volunteers after oral and intravenous administration. Eur J Clin Microbiol. 1986;5:179–86.
Cabarga MM, Navarro AS, Gandarillas CIC, Dominguez-Gil A. Effects of two cations on gastrointestinal absorption of ofloxacin. Antimicrob Agents Chemother. 1991;35:2102–5.
Campbell NR, Kara M, Hasinoff BB, Haddara WM, McKay DW. Norfloxacin interaction with antacids and minerals. Br J Clin Pharmacol. 1992;33:115–6.
Chung DT, Tsai CY, Chen SJ, Chang LW, King CH, Hsu CH, et al. Multiple-dose safety, tolerability, and pharmacokinetics of oral nemonoxacin (TG-873870) in healthy volunteers. Antimicrob Agents Chemother. 2009;54:411–7.
Dudley MN, Marchbanks CR, Flor SC, Beals B. The effect of food or milk on the absorption kinetics of ofloxacin. Eur J Clin Pharmacol. 1991;41:569–71.
Flor S, Guay DR, Opsahl JA, Tack K, Matzke GR. Effects of magnesium-aluminum hydroxide and calcium carbonate antacids on bioavailability of ofloxacin. Antimicrob Agents Chemother. 1990;34:2436–8.
Frost RW, Carlson JD, Dietz AJJ, Heyd A, Lettieri JT. Ciprofloxacin pharmacokinetics after a standard or high-fat/high-calcium breakfast. J Clin Pharmacol. 1989;29:953–5.
Grasela TH Jr, Schentag JJ, Sedman AJ, Wilton JH, Thomas DJ, Schultz RW, et al. Inhibition of enoxacin absorption by antacids or ranitidine. Antimicrob Agents Chemother. 1989;33:615–7.
Guo B, Wu X, Zhang Y, Shi Y, Yu J, Cao G, et al. Safety and clinical pharmacokinetics of nemonoxacin, a novel non-fluorinated quinolone, in healthy Chinese volunteers following single and multiple oral doses. Clin Drug Investig. 2012;32:475–86.
Hoffken G, Borner K, Glatzel PD. Reduced enteral absorption of ciprofloxacin in the presence of antacids. Eur J Clin Microbiol. 1985;4:345.
Hoogkamer JF, Kleinbloesem CH. The effect of milk consumption on the pharmacokinetics of fleroxacin and ciprofloxacin in healthy volunteers. Drugs. 1995;49(Suppl 2):346–8.
Hooper WD, Dickinson RG, Eadie MJ. Effect of food on absorption of lomefloxacin. Antimicrob Agents Chemother. 1990;34:1797–9.
Ichihara N, Tachizawa H, Tsumura M, Une T, Sato K. Phase I study on DL-8280. Chemotherapy. 1984;32:118–49.
Jaehde U, Sörgel F, Stephan U, Schunack W. Effect of an antacid containing magnesium and aluminum on absorption, metabolism, and mechanism of renal elimination of pefloxacin in humans. Antimicrob Agents Chemother. 1994;38:1129–33.
Kalager T, Digranes A, Bergan T, Rolstad T. Ofloxacin: serum and skin blister fluid pharmacokinetics in the fasting and non-fasting state. J Antimicrob Chemother. 1986;17:795–800.
Kara M, Hasinoff B, McKay D, Campbell N. Clinical and chemical interactions between iron preparations and ciprofloxacin. Br J Clin Pharmacol. 1991;31:257–61.
Kawakami J, Matsuse T, Kotaki H, Seino T, Fukuchi Y, Orimo H, et al. The effect of food on the interaction of ofloxacin with sucralfate in healthy volunteers. Eur J Clin Pharmacol. 1994;47:67–9.
Kays MB, Overholser BR, Mueller BA, Moe SM, Sowinski KM. Effects of sevelamer hydrochloride and calcium acetate on the oral bioavailability of ciprofloxacin. Am J Kidney Dis. 2003;42:1253–9.
Kivisto KT, Ojala-Karlsson P, Neuvonen PJ. Inhibition of norfloxacin absorption by dairy products. Antimicrob Agents Chemother. 1992;36:489–91.
Lazzaroni M, Imbimbo BP, Bargiggia S, Sangaletti O, Dal Bo L, Broccali G, et al. Effects of magnesium–aluminum hydroxide antacid on absorption of rufloxacin. Antimicrob Agents Chemother. 1993;37:2212–6.
Ledergerber B, Bettex JD, Joos B, Flepp M, Lüthy R. Effect of standard breakfast on drug absorption and multiple-dose pharmacokinetics of ciprofloxacin. Antimicrob Agents Chemother. 1985;27:350–2.
Lee LJ, Hafkin B, Lee ID, Hoh J, Dix R. Effects of food and sucralfate on a single oral dose of 500 milligrams of levofloxacin in healthy subjects. Antimicrob Agents Chemother. 1997;41:2196–200.
Lehto P, Kivistö KT. Different effects of products containing metal ions on the absorption of lomefloxacin. Clin Pharmacol Ther. 1994;56:477–82.
Lehto P, Kivisto K, Neuvonen P. The effect of ferrous sulphate on the absorption of norfloxacin, ciprofloxacin and ofloxacin. Br J Clin Pharmacol. 1994;37:82–5.
Lehto P, Kivistö KT. Effects of milk and food on the absorption of enoxacin. Br J Clin Pharmacol. 1995;39:194–6.
Leroy A, Borsa F, Humbert G, Bernadet P, Fillastre JP. The pharmacokinetics of ofloxacin in healthy adult male volunteers. Eur J Clin Pharmacol. 1987;31:629–30.
Lettieri J, Vargas R, Agarwal V, Liu P. Effect of food on the pharmacokinetics of a single oral dose of moxifloxacin 400 mg in healthy male volunteers. Clin Pharmacokinet. 2001;40(Suppl 1):19–25.
Liu C, Deng K, Huang J, Yang S, Yang X, Xiang Y, et al. Bioequivalence of moxifloxacin hydrochloride tablets in healthy Chinese subjects. Chinese J Clin Pharmacol Ther. 2021;26:1393–9.
Männistö PT. Pharmacokinetics of nalidixinic acid and oxolinic acid in healthy women. Clin Pharmacol Ther. 1976;19:37–46.
Minami R, Inotsume N, Nakano M, Sudo Y, Higashi A, Matsuda I. Effect of milk on absorption of norfloxacin in healthy volunteers. J Clin Pharmacol. 1993;33:1238–40.
Mitra SK, Sundaram R. Effect of Gasex on pharmacokinetic profile of ciprofloxacin in healthy male volunteers. Indian J Exp Biol. 1997;35:799–800.
Motoya T, Shimozono T, Yamaguchi T, Shinkawa T, Nakamura K, Shimodozono Y, et al. Effects of milk and aluminum hydroxide on the absorption of norfloxacin, ciprofloxacin and tosufloxacin in healthy volunteers. J Appl Ther. 1997;1:213–7.
Nakashima M, Kanamaru M, Uematsu T, Takiguchi A, Mizuno A, Itaya T, et al. Clinical pharmacokinetics and tolerance of fleroxacin in healthy male volunteers. J Antimicrob Chemother. 1988;22(Suppl D):133–44.
Nakashima M, Uematsu T, Kanamaru M, Okazaki O, Hakusui H. Phase I study of levofloxacin, (S)-(–)-ofloxacin. Jpn J Clin Pharmacol Ther. 1992;23:515–20.
Nakashima M, Uematsu T, Kosuge K, Okuyama Y, Morino A, Ozaki M, et al. Pharmacokinetics and safety of NM441, a new quinolone, in healthy male volunteers. J Clin Pharmacol. 1994;34:930–7.
Nakashima M, Kosuge K, Uematsu T. Phase I clinical study of pazufloxacin. Japanese J Chemother. 1995;43:143–63.
Nakashima M. Pharmacokinetics of sitafloxacin (DU-6859a) in healthy volunteers. Jpn J Chemother. 2008;56:154–5.
Neuvonen PJ, Kivistö KT, Lehto P. Interference of dairy products with the absorption of ciprofloxacin. Clin Pharmacol Ther. 1991;50:498–502.
Neuvonen PJ, Kivistö KT. Milk and yoghurt do not impair the absorption of ofloxacin. Br J Clin Pharmacol. 1992;33:346–8.
Nix DE, Watson WA, Lener ME, Frost RW, Krol G, Goldstein H, et al. Effects of aluminum and magnesium antacids and ranitidine on the absorption of ciprofloxacin. Clin Pharmacol Ther. 1989;46:700–5.
Nix DE, Wilton JH, Ronald B, Distlerath L, Williams VC, Norman A. Inhibition of norfloxacin absorption by antacids. Antimicrob Agents Chemother. 1990;34:432–5.
Baxdela (Melinta Therapeutics). Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208610s000,208611s000lbl.pdf Accessed 24 Oct 2023.
Levaquin (Janssen). Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021721s020_020635s57_020634s52_lbl.pdf Accessed 24 Oct 2023.
Maxaquin (Pfizer). Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/20013s015lbl.pdf Accessed 24 Oct 2023.
Pazzucconi F, Barbi S, Baldassarre D, Colombo N, Dorigotti F, Sirtori CR. Iron-ovotransferrin preparation does not interfere with ciprofloxacin absorption. Clin Pharmacol Ther. 1996;59:418–22.
Piccolo ML, Toossi Z, Goldman M. Effect of coadministration of a nutritional supplement on ciprofloxacin absorption. Am J Hosp Pharm. 1994;51:2697–9.
Pletz MW, Petzold P, Allen A, Burkhardt O, Lode H. Effect of calcium carbonate on bioavailability of orally administered gemifloxacin. Antimicrob Agents Chemother. 2003;47:2158–60.
Polk RE, Healy DP, Sahai J, Drwal L, Racht E. Effect of ferrous sulfate and multivitamins with zinc on absorption of ciprofloxacin in normal volunteers. Antimicrob Agents Chemother. 1989;33:1841–4.
Qin F, Wang G-M, Huang J-Y, Wu J-R, Song W-J, Deng J, et al. Pharmacokinetics and bioequivalence study of two ciprofloxacin hydrochloride tablets in healthy Chinese subjects under fasting and fed conditions: a randomized, open-label, two-formulation, two-sequence, two-period, single-dose crossover study. Int J Clin Pharmacol Ther. 2020;59:804–16.
Sahai J, Healy DP, Stotka J, Polk RE. The influence of chronic administration of calcium carbonate on the bioavailability of oral ciprofloxacin. Br J Clin Pharmacol. 1993;35:302–4.
Saito A. Influence of meal on the pharmacokinetics of pazufloxacin and comparison of pazufloxacin and ofloxacin. Jpn J Chemother. 1995;43:180–9.
Saito A, Tarao F. Influence of milk on absorption of NM441, a prodrug type of quinolone antibiotic. Jpn J Chemother. 1996;44:221–8.
Segre G, Cerretani D, Moltoni L, Urso R. Pharmacokinetics of rufloxacin in healthy volunteers. Eur J Clin Pharmacol. 1992;42:101–5.
Shiba K, Saito A, Shimada J, Kaji M, Hori S, Yoshida M, et al. Effect of antacid on gastrointestinal absorption of fleroxacin in healthy humans. Chemotherapy. 1990;38:344–9.
Shiba K, Sakai O, Shimada J, Okazaki O, Aoki H, Hakusui H. Effects of antacids, ferrous sulfate, and ranitidine on absorption of DR-3355 in humans. Antimicrob Agents Chemother. 1992;36:2270–4.
Shiba K, Okazaki O, Aoki H, Shimada J, Sakai O. Influence of antacids and ranitidine on the absorption of levofloxacin in men. Drugs. 1993;46:299–300.
Shiba K, Yoshida M, Maezawa H, Sakai O. Effect of an antacid or cimetidineon gastrointestinal absorption of pazufloxacin. Jpn J Chemother. 1995;43:220–5.
Shiba K. Effect of metal-ion on sitafloxacin absorption. Jpn J Chemother. 2008;56:25–31.
Shimada J, Shiba K, Oguma T, Miwa H, Yoshimura Y, Nishikawa T, et al. Effect of antacid on absorption of the quinolone lomefloxacin. Antimicrob Agents Chemother. 1992;36:1219–24.
Somogyi AA, Bochner F, Keal JA, Rolan PE, Smith M. Effect of food on enoxacin absorption. Antimicrob Agents Chemother. 1987;31:638–9.
Stass H, Kubitza D. Effects of iron supplements on the oral bioavailability of moxifloxacin, a novel 8-methoxyfluoroquinolone, in humans. Clin Pharmacokinet. 2001;40:57–62.
Stass H, Sachse R. Effect of calcium supplements on the oral bioavailability of moxifloxacin in healthy male volunteers. Clin Pharmacokinet. 2001;40:27–32.
Stass H, Kubitza D. Effects of dairy products on the oral bioavailability of moxifloxacin, a novel 8-methoxyfluoroquinolone, in healthy volunteers. Clin Pharmacokinet. 2001;40(Suppl 1):33–8.
Stass H, Böttcher MF, Ochmann K. Evaluation of the influence of antacids and H2 antagonists on the absorption of moxifloxacin after oral administration of a 400 mg dose to healthy volunteers. Clin Pharmacokinet. 2001;40(Suppl 1):39–48.
Takazawa K, Fujita M. Effects of several drugs on absorption of fleroxacin in healthy males. Jpn J Chemother. 1995;43:830–5.
Verho M, Malerczyk V, Dagrosa E, Korn A. The effect of food on the pharmacokinetics of ofloxacin. Curr Med Res Opin. 1986;10:166–71.
Vinceneux P, Weber P, Gaudin H, Boussougant Y. Decreased absorption of pefloxacin by gastric antacids Preliminary study. Presse Med. 1986;15:1826.
Vinceneux P, Dancour A, Mahe M, Weber P, Boussougant Y. Reduced enteral absorption of pefloxacin in the presence of antacids (magnesium and aluminium hydroxydes). Med Mal Infect. 1987;17:450–3.
Wang L, Ruan Z, Zhang K, Chen J, Yang D, Shao R, et al. Pharmacokinetics and bioequivalence evaluation of ciprofloxacin tablets in healthy Chinese subjects under fasting and fed conditions. Chin J Clin Pharmacol Ther. 2021;26:1273–8.
Xia B-J, Wan X-X. Effect of food on the bioavailability of fleroxacin. Chinese J Antibiot. 2003;28:438–40 (+448).
Yamasaku F, Suzuki Y, Uno K. A comparative study of the pharmacokinetics of ofloxacin, ciprofloxacin, NY-198 and T-3262 in the same volunteers. Chemotherapy. 1988;36:195–200.
Yang D, Chen J, Wu J, Chen J, Lou H, Ruan Z, et al. Bioequivalence of norfloxacin tablets in Chinese healthy volunteers under fasting and fed condition. Chin J Clin Pharmacol Ther. 2020;25:1357–62.
Zhang Y-F, Dai X-J, Wang T, Chen X-Y, Liang L, Qiao H, et al. Effects of an Al3+- and Mg2+-containing antacid, ferrous sulfate, and calcium carbonate on the absorption of nemonoxacin (TG-873870) in healthy Chinese volunteers. Acta Pharmacol Sin. 2014;35:1586–92.
Zhao C, Lv Y, Li X, Hou F, Ma X, Wei M, et al. Effects of nemonoxacin on thorough ECG QT/QTc interval: a randomized, placebo- and positive-controlled crossover study in healthy Chinese adults. Clin Ther. 2018;40:922–83.
Acknowledgments
The main author—AW participated in the training “Statistics in medicine—a meta-analysis”, organized by the StatSoft company and financed under the Strategic Programme Excellence Initiative at Jagiellonian University (Skills Development and Engagement module).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The work was supported by a grant from the Faculty of Pharmacy under the Strategic Programme Excellence Initiative at Jagiellonian University (Research Support Module, project number: U1C/W42/NO/28.15).
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Availability of data and material
All data supporting the findings of this study are available within the paper and its Supplementary Information.
Author contributions
Conceptualization: Paweł Paśko, Agnieszka Wiesner, Paweł Zagrodzki; Systematic literature search: Agnieszka Wiesner, Paweł Paśko; Data collection and extraction: Agnieszka Wiesner, Paweł Paśko; Study risk of bias assessment: Agnieszka Wiesner, Paweł Paśko, Data analysis, and interpretation: Agnieszka Wiesner; Chemical consultation: Alicja Gawalska; Writing - original draft preparation: Agnieszka Wiesner; Writing - review and editing: Agnieszka Wiesner, Paweł Paśko, Paweł Zagrodzki, Alicja Gawalska; Supervision: Paweł Paśko. All authors read and approved the final version of the manuscript.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wiesner, A., Zagrodzki, P., Gawalska, A. et al. Together or Apart? Revealing the Impact of Dietary Interventions on Bioavailability of Quinolones: A Systematic Review with Meta-analyses. Clin Pharmacokinet 63, 773–818 (2024). https://doi.org/10.1007/s40262-024-01377-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-024-01377-0