Abstract
Aim
This study aimed to assess the pharmacokinetics of henagliflozin in dialysis patients with diabetes.
Methods
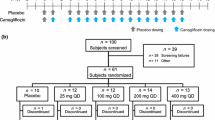
In this prospective, randomized, open-label study where 10 hemodialysis and 10 peritoneal dialysis patients with diabetes were randomized in a 1:1:1:1 ratio to oral administration of henagliflozin in doses of 5 and 10 mg/day. The pharmacokinetics of a single dose of henagliflozin on Days 1 and 2, the minimum plasma concentration (Cmin) of the steady state on Day 10, and single hemodialysis clearance of henagliflozin were measured.
Results
The mean values of Cmax were 70.2–77.0 ng/mL and 105–143 ng/mL in the 5 mg and 10 mg henagliflozin groups, respectively; the mean values of AUCinf were 777–811 h*ng/mL and 1290–1730 h*ng/mL in the 5 mg and 10 mg henagliflozin groups, respectively. The median Tmax values ranged from 1 to 3 h across the dose range. The mean values of T1/2 of henagliflozin were 14.1–14.5 and 16.2–21.0 h in the 5 mg and 10 mg groups, respectively. The Cmin values of the steady state in dialysis patients taking 5 mg and 10 mg of henagliflozin were 15.0 ± 4.4 ng/mL and 26.8 ± 16.3 ng/mL, respectively, which were 123.8% and 131.0% higher than those in diabetic patients with normal renal function, respectively. Henagliflozin concentration was decreased by 1.1% after hemodialysis treatment. No treatment-related serious adverse events or discontinuations occurred.
Conclusions
Henagliflozin at the current recommended dosage may be safe, although it is possible to result in slight accumulation in patients on dialysis.
Registration
Chinese Clinical Trial Registry number ChiCTR2200062872. The date of registration: August 22, 2022.

Similar content being viewed by others
References
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a Report of the American College of Cardiology / American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e8995–e1032.
Gilbert RE. SGLT-2 inhibition in patients with kidney disease. Diabetes Metab. 2014;40(6 suppl 1):S23–7.
De Pascalis A, Cianciolo G, Capelli I, Brunori G, La Manna G. SGLT2 inhibitors, sodium and off-target effects: an overview. J Nephrol. 2021;34(3):673–80.
Macha S, Mattheus M, Halabi A, Pinnetti S, Woerle HJ, Broedi UC. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT22) inhibitor, in subjects with renal impairment. Diabetes Obes Metab. 2014;16(3):215–22.
Devineni D, Curtin CR, Marbury TC, et al. Effect of hepatic or renal impairment on the pharmacokinetics of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Ther. 2015;37(3):610–28.
Wang L, Wu C, Shen L, et al. Evaluation of drug-drug interaction between henagliflozin, a novel sodium-glucose co-transporter 2 inhibitor, and metformin in healthy Chinese males. Xenobiotica. 2016;46(8):703–8.
Yong X, Wen A, Liu X, et al. Pharmacokinetics and pharmacodynamics of henagliflozin, a sodium glucose co-transporter 2 inhibitor, in Chinese patients with type 2 diabetes mellitus. Clin Drug investing. 2016;36:195–202.
Obokata M, Negishi K, Sunaga H, et al. Association between circulating ketone bodies and worse outcomes in hemodialysis patients. J Am Heart Assoc. 2017;6(10): e006885.
Chen Z, Li L, Zhang Y, et al. Characterization and quantitative determination of henagliflozin metabolites in humans. J Pharm Biomed Anal. 2021;192: 113632.
Davidson JA. SGLT2 inhibitors in patients with type 2 diabetes and renal disease: overview of current evidence. Postgrad Med. 2019;131(4):251–60.
Barreto J, Borges C, Rodrigues TB, et al. Pharmacokinetic properties of dapagliflozin in hemodialysis and peritoneal dialysis patients. Clin J Am Soc Nephrol. 2023. https://doi.org/10.2215/CJN.0000000000000196.
Acknowledgments
We thank the nurses who administered medicines and Miss Yini Jiang for double-checking the data. Miss Jiang received no financial support for her participation. We also appreciate the volunteers who willingly participated and were involved in this study. Leyi Gu was funded by the Program of Shanghai Academic/Technology Research Leader (22XD1431400), the Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant (20172015), and the National Nature Science Foundation Grant of China (81970610).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Science and technology Commission of Shanghai municipality and Shanghai Hengrui pharmaceutical Co. Ltd.
Conflicts of Interest
Leyi Gu worked with Shanghai Hengrui Pharmaceutical Co. Ltd. as a consultant and did not receive any remuneration or funding from the company. Li Ding, Shang Liu, Hao Yan, Zhenyuan Li, Yijun Zhou, Huihua Pang, Renhua Lu, Weiming Zhang, Miaolin Che, Lin Wang, Qin Wang, Wei Fang, Minfang Zhang, and Xiajing Che declared no conflicts of interest that might be relevant to this work.
Contribution Statement
L.G Designed the study. LD, SL, HY, ZL, YZ, and HP recruited patients and were responsible for the informed consent form signing process. LD, SL, HY, ZL, HP, RL, WZ, MC, LW, WF, QW, MZ, XC, and LG followed up with patients. LD, SL, and LG conducted the data analysis. LD and SL wrote the first draft of the manuscript, and LG edited the manuscript. LG is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability
The original contributions presented in the study are included in the Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Approval
Ethics Committee of Shanghai Jiaotong University School of Medicine, Renji Hospital (Approval number KY2022-176-B)
Consent to Participate
Written, informed consent was obtained from all participants.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, L., Liu, S., Yan, H. et al. Pharmacokinetics of Henagliflozin in Dialysis Patients with Diabetes. Clin Pharmacokinet 62, 1581–1587 (2023). https://doi.org/10.1007/s40262-023-01300-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01300-z