Abstract
Background and Objectives
Dorzagliatin is a first-in-class small molecule glucokinase activator (GKA) that improves pancreatic insulin secretion behavior and regulates hepatic glucose conversion in a glucose concentration-dependent manner. The primary objective of this study was to develop a population pharmacokinetic model of dorzagliatin to evaluate the influence of covariates, such as demographic characteristics and liver and kidney function, on the pharmacokinetics of dorzagliatin and provide a basis for medication guidance.
Method
The pharmacokinetic data of dorzagliatin in this study came from six clinical trials. Based on the combined data, a population pharmacokinetic model of dorzagliatin was established using NONMEM software (ICON, MD, USA, version 7.4.3). The algorithm used was first-order conditional estimation with interaction (FOCEI). The dorzagliatin population pharmacokinetic modeling analysis included 1062 subjects and 7686 observable concentrations. Covariates, including age (AGE), sex (GEND), body weight (TBW), body mass index (BMI), body surface area (BSA), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (CR), creatinine clearance (CRCL), and total bilirubin (TBIL), were screened using the forward-backward method. Model evaluation was performed using goodness-of-fit plots, prediction corrected visual prediction check (pcVPC), and bootstrap.
Results
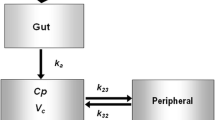
Concentration data of dorzagliatin in the dose range were best characterized by a two-compartment model with sequential zero-order then first-order absorption and first-order elimination. The final model estimated dorzagliatin data for typical male subjects (69 kg body weight, 18 U/L AST and 55 years old); the apparent total clearance (CL/F) was 10.4 L/h, apparent volume of central compartment distribution (Vc/F) was 80.6 L, inter-compartmental clearance (Q/F) was 3.02 L/h, apparent volume of peripheral compartment distribution (Vp/F) was 26.5 L, absorption rate constant (Ka) was 3.29 h-1, and duration of zero-order absorption (D1) was 0.418 h. The inter-individual variation of CL/F, Vc/F, Vp/F, and D1 was 22.5%, 14.9%, 48.8%, and 82.8%, respectively.
Conclusion
The two-compartment linear pharmacokinetic model with zero- and first-order sequential absorption adequately described the pharmacokinetic characteristics of dorzagliatin. Body weight, aspartate aminotransferase, and age had a statistically significant effect on the CL/F of dorzagliatin. Body weight and sex had a statistically significant effect on Vc/F. However, considering the clinically insignificant changes in the magnitude of steady-state exposure caused by these covariates, as well as the minimal changes in the steady-state exposure for individuals with mild and moderate impaired hepatic function and all stages of renal impairment, dose adjustments based on the tested covariates or for specific populations are deemed unnecessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Dorzagliatin pharmacokinetics are characterized by a two-compartment linear pharmacokinetic model with zero- and first-order sequential absorption. |
Body weight, age, aspartate aminotransferase, and sex were found to be significant covariates on clearance and volume; however, the impact on steady-state exposure of dorzagliatin is minimal and not expected to be clinically meaningful. |
The estimated population parameter values may assist drug labeling and clinicians in the individualization of dorzagliatin dosage regimens. |
1 Introduction
Dorzagliatin is a first-in-class small molecule glucokinase activator (GKA) that improves pancreatic insulin secretion behavior and regulates hepatic glucose conversion in a glucose concentration-dependent manner. It can also regulate glucose-controlling hormones by activating intestinal glucokinase (GK), exerting multiple synergistic hypoglycemic effects. Dorzagliatin has a unique way to activate GK, which increases the affinity of GK to its substrate (glucose) without significant alteration of the Hill coefficient. In addition, dorzagliatin increases the maximum reaction rate without significantly lowering the glucose S0.5 when glucose concentration is low. Therefore, dorzagliatin is a glucose-dependent glucokinase activator. In September 2022, dorzagliatin as monotherapy and as an add-on to metformin was approved in China for improving glycemic control in adult patients with type 2 diabetes mellitus (T2DM) [1].
The results from the first-in-human single ascending dose study of dorzagliatin showed that dorzagliatin exposure (area under the concentration–time curve [AUC0–t] and maximum concentration [Cmax]) increased dose proportionally from 5 to 50 mg [2]. Previous clinical trials in adult patients with T2DM showed that dorzagliatin had a beneficial effect on glycemic control, and all adverse events were mild in intensity and resolved without any treatment [2,3,4,5,6]. The half-life of dorzagliatin is about 4.5–8.6 h [7]. The results of phase II clinical trial demonstrated the good efficacy and safety profiles of dorzagliatin. The change in HbA1c from baseline was −1.12% (−1.39 to −0.86) in the 75 mg twice-daily group, which was better than the other dosage groups as well as the placebo group (−0.35%) [4]. The number of adverse events was similar between the treatment groups and the placebo group [4]. The results provide supporting information for dorzagliatin 75 mg twice a day as monotherapy in drug-naive patients or as an add-on therapy for patients tolerating metformin in the dosage selection of later phase III trials, which turned out to be a great success.
The aim of this study was to develop a population pharmacokinetic model to quantitatively describe the pharmacokinetic characteristics of dorzagliatin and assess the effect of relevant covariates on the pharmacokinetics of dorzagliatin based on a very large dataset obtained from the clinical development program.
2 Methods
2.1 Study Design and Population
The analysis included data from healthy volunteers and patients recruited in six clinical trials (two phase I, one phase II, and two phase III studies in T2DM patients, and one study in subjects with impaired renal function and matched healthy volunteers). Doses of dorzagliatin ranged from 25 to 400 mg/day. The details of these dosages, sample sizes, plasma sampling schedules, and study descriptions are presented in Table 1. All participants gave written informed consent before participating in the studies, and the protocols were approved by the Institutional Review Boards/Ethics Committees of the study sites.
2.2 Population Pharmacokinetic Analysis
A non-linear mixed-effects modeling approach was utilized to analyze dorzagliatin concentration versus time data [8,9,10]. NONMEM 7.4.3 software (ICON Development Solutions, Ellicott City, MD, USA); Perl Speaks NONMEM (PsN) Ver 4.2 (Uppsala University, Sweden) [11, 12], and R ver. 3.5.1 were used in the study. All models were fitted using the first-order conditional estimation (FOCE) method with interaction.
2.3 Structure Pharmacokinetic Model
A two-compartment disposition model with sequential zero- and first-order absorption processes were selected as the structural model after evaluation. The inter-individual variability (IIV) of pharmacokinetic (PK) parameters was modeled using the exponential error model:
where θi is the individual parameter estimate of the ith subject; θT is the population typical value of the PK parameter after logarithmic transformation, and ηi(ETA) is a random effect among individuals, which obeys the random inter-individual variation of a normal distribution with a mean of 0 and a variance of ω2.
Additive, proportional, and a combination of additive and proportional models were explored for residual variability. The final residual error model included additive and proportional error terms:
where C(t)is and Ĉ(t)ij are the measured and the corresponding model-predicted plasma concentration for subject i at time j. ε1ij and ε2ij are normally distributed independent residual random error terms with a mean of zero and variances of σ12 and σ22, respectively.
2.4 Covariate Analysis Development
Intrinsic covariates of age (AGE), sex (GEND), body weight (TBW), body-mass index (BMI), body surface area (BSA), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (CR), creatinine clearance (CRCL), and total bilirubin (TBIL) were assessed for their effects on dorzagliatin pharmacokinetics. For the two-compartment model, the most important parameters are clearance rate and central compartment distribution volume. If the impact of covariates on these two parameters is significant, it could potentially have a significant impact on the exposure level. Therefore, in this analysis, the focus is on significant covariates that affect these two parameters. Continuous covariates were normalized to the population median values and modeled using the following general equation:
Categorical covariates were modeled using the general equation:
where θi is the PK parameter of the ith subject, θT is the population typical value of the PK parameter after logarithmic transformation, Covi is the continuous covariate value of the ith subject, and Covpop represents the median value of the continuous variable in the population. Xi is the categorical variable index of the ith subject, where 0 value indicates the most common category of covariate and other integer values indicate other categories, kcov describes the influence of categorical covariates, and ηi is the random between-individual variable with a normal distribution of mean 0 and variance ω2.
Covariate–parameter relationships were identified based on scientific interest, mechanistic plausibility, or prior knowledge. Pairwise correlation screening was performed, and highly correlated covariates and the more clinically relevant covariates were examined further using stepwise covariate modeling approaches, including forward inclusion and backward elimination. In the forward inclusion step, covariates were added to the structure model one by one and a drop in the objective function value (OFV) of 6.63 corresponding to a p < 0.01 level for one degree of freedom was required for inclusion in the model. In the following step, each selected covariate was entered into a full model. A criterion of an increase of OFV by backward elimination from the full model of at least 10.83 corresponding to a p < 0.001 level for one degree of freedom was used to retain covariates in the model.
2.5 Model Evaluation
The model evaluation was accomplished by assessing the models for the goodness-of-fit plot, which included individual and population-predicted versus observed concentrations, conditional weighted residual versus time and population-predicted concentrations. The reliability and stability of the final population pharmacokinetic model were further assessed by a nonparametric bootstrap resampling method. One thousand bootstrap datasets were generated by randomly sampling with replacements from the original dataset to form new datasets containing the same number of patients as the original dataset. The median parameter values and nonparametric 95% confidence intervals (CI) were derived from the NONMEM fit based on the 1000 bootstraps dataset and compared with the final parameter estimates [13]. Furthermore, prediction-corrected visual predictive checks (pcVPCs) were performed to explore the predictive capability of the final PK model [14]. The final model parameter estimates and the demographics information of subjects were used for simulating 1000 new virtual clinical trials. The median and the 95% prediction interval simulated plasma concentration was plotted and compared with the observed data.
2.6 Model Simulation for Evaluating the Impact of Covariates
Sensitivity analyses were conducted to examine the impact of statistically significant covariates on the expected dorzagliatin steady-state exposure, including the peak concentration of the last administration (Cmax,ss), the trough concentration of the last dose (Cmin,ss) and the area under the plasma concentration–time curve (AUCtau,ss). AUCtau, ss was calculated using the linear trapezoidal approximation. Cmax,ss and Cmin,ss were the maximum and minimum observed post-dose concentrations in the simulated plasma concentration–time profile, respectively. The simulated exposure of patients with extreme covariate values (5th and 95th percentiles) was compared with that of a typical patient with median covariate values for each of the statistically significant covariates in the final model.
To further determine the predicted effect of covariates of interest, the steady-state exposure in each subject was simulated using the individual post-hoc parameter estimates from the final population PK (popPK) model. The purpose of this simulation was to compare exposures between BMI quartiles, age groups (age < 65 years vs. age ≥ 65 years), hepatic function groups (normal vs. mild vs. moderate), and renal function groups (normal vs. mild) [15, 16]. The differences in drug exposure up to 20% are not clinically significant. Of note, this simulation accounts for potential correlations among covariates.
3 Results
3.1 Data Summary and Demographics
A total of 7686 plasma concentrations from 1062 subjects from six clinical trials were included in the analysis. Baseline characteristics for subjects included in the analyses by study are summarized in Table 2.
3.2 Population Pharmacokinetic Model
The PopPK structural model of dorzagliatin is a two-compartment model with zero-order and first-order sequential absorption and first-order linear elimination in the central compartment. The PopPK model of dorzagliatin is composed of the following parameters: apparent total clearance (CL/F), apparent volume of central compartment distribution (Vc/F), inter-compartmental clearance (Q/F), apparent volume of peripheral compartment distribution (Vp/F), absorption rate constant (Ka), and duration of zero-order absorption (D1).
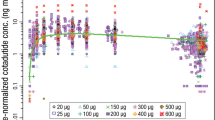
The diagnostic plots of the final PopPK model for dorzagliatin are shown in Fig. 1. The observed and predicted concentrations are in good agreement, with no obvious bias. The estimated parameters of the final model of dorzagliatin and the estimated parameters of bootstrap are compared in Table 3. For typical male subjects with a TBW of 69 kg, AST of 18 U/L, and 55 years old taking dorzagliatin in trials 201, 301, and 302, CL/F was 10.4 L/h, Vc/F was 80.6 L, Q/F was 3.02 L/h, Vp/F was 26.5 L, Ka was 3.29 h-1, and D1 was 0.418 h. The inter-individual variability of CL/F, Vc/F, Vp/F, and D1 was 22.5%, 14.9%, 48.8%, and 82.8%, respectively. The median estimated parameters of the bootstrap model and the estimated parameters of the final PopPK model are similar. The bootstrap 95% CI (presented by the 2.5th–97.5th quantile interval) is highly overlapped with the final model-predicted 95% CI, indicating that the final model is both stable and accurate. The pcVPC results presented in Fig. 2 demonstrated that the model can fully reproduce the central tendency and variability of the original data.
Diagnosis plot final dorzagliatin model. Left panel: observed dorzagliatin concentrations versus individual predicted dorzagliatin concentrations (IPRED) in logarithmic (top) and original (bottom) scale. Median panel: observed dorzagliatin concentrations versus population predicted dorzagliatin concentrations (PRED) in logarithmic (top) and original (bottom) scale. Right panel: conditional weighted residuals (CWRES) versus time after first dose (top) and population prediction (bottom). Points are individual data. Red solid lines represent the unit diagonal (left and median) or line at zero (right). Green dashed lines represent the |CWRES|=5. Blue dashed lines are smooth curves (lowess) showing the relationship between two variables.
Prediction-corrected visual predictive check (pcVPC) of dorzagliatin concentration–time profiles across all studies. The final model parameter estimates and the demographics information of subjects were used for simulating 1000 new virtual clinical trials. Points are observed concentrations, solid line represents the median observed value, and dashed lines represent 2.5th percentiles and 97.5th percentiles of the observed values. The upper and lower shaded areas represent the 95% confidence interval (CI) spread of the 2.5th–97.5th percentile predicted values, and middle shaded areas represent the 95% CI spread of medium predicted values.
Covariates were tested using the stepwise forward and backward method in NONMEM, and the final model results showed that body weight, AST, and age had an effect on CL/F of dorzagliatin, while body weight and sex had an effect on Vc/F. The correlation between covariates and post-hoc individual estimates of the corresponding parameters are illustrated in Fig. 3. Patients with higher body weight or lower age or AST tended to have higher CL; patients with higher body weight or who were male tended to have higher Vc. Other covariates, including ALT, serum creatinine, creatinine clearance, and total bilirubin had no significant effect on the pharmacokinetics of dorzagliatin.
Final model pharmacokinetic (PK) parameter–covariate relationships. Final model PK parameter–covariate relationships for baseline body weight (left panel), age (mid-top), AST (right-top), and gender (mid-bottom). The solid red lines indicate a typical (population) predicted covariate relationship. The blue squares represent the geometric mean within the group for categorical covariates. The black circles are the empirical Bayes estimates of individual PK parameters after correcting for all other covariates except for the one plotted on the x-axis. CL/F clearance, Vc/F volume distribution of central compartment
3.3 Sensitive Analysis
The final model demonstrated that body weight, AST, and age had a significant impact on the subject's CL/F, while body weight and sex had a significant impact on the subject's Vc/F. The sensitivity analysis results of the impact of these significant covariates on dorzagliatin steady-state exposure are shown in Fig. 4. The change in exposure (AUCtau,ss, Cmax,ss, and Cmin,ss) for subjects weighing 55 kg and 83 kg (10th to 90th quantiles) relative to a typical subject weighing 69 kg was 1.63% to 9.62% and −7.1% to −1.35%. Subjects with AST of 13 U/L and 28 U/L (10th ~ 90th quantile) relative to a typical subject with AST of 18 U/L had exposure changes of −5.77% to −1.73% and 2.45% to 8.24%, respectively. Subjects aged 41 years and 66 years (10th–90th quantiles) relative to a subject aged 55 years had lower (−6.82% to −2.04%) and higher (1.31% to 4.41%) exposure, respectively. The difference in AUCtau,ss, Cmax,ss, and Cmin,ss between females and males were 0.01%, 9.09%, and −11.6%, respectively. The time to starting to eat after taking the drug had a significant impact on D1; however, no difference in AUCtau,ss, and almost no difference in Cmax,ss and Cmin,ss (−1.26% and 2.71%, respectively) were observed in the comparison between started to eat ≥ 1 h after dosing and 0.5 h after dosing.
Sensitivity analysis plot comparing the effect of covariates on dorzagliatin steady-state exposure (AUCss, Cmax,ss, and Cmin,ss). Base, as represented by the black vertical line and values, refers to the predicted exposure (AUCtau,ss, Cmax,ss, and Cmin,ss) of dorzagliatin in a typical subject after 10 repeated doses of 75 mg twice a day. The black-shaded bar with value at each end shows the 5th–95th percentile exposure range across the entire population. Each blue-shaded bar represents the influence of covariates on the exposure. The label at the left end of the bar represents the covariate being evaluated. The upper and lower values for each covariate capture 80% of the plausible range in the population. The length of each bar describes the potential impact of the covariates on dorzagliatin exposure, with the percentage value in parentheses at each end representing the percent change of exposure from the base. The most influential covariates are at the top of the plot for each exposure parameter. AST aspartate aminotransferase, AUCtau,ss steady-state area under the plasma concentration–time curve, CI confidence interval, Cmax,ss steady-state maximum concentration, Cmin,ss steady-state trough concentration, Gender sex.
3.4 Impact of Covariates of Interest on Dorzagliatin Exposure
The pharmacokinetic exposure of dorzagliatin (AUCtau,ss, Cmax,ss, and Cmin,ss) and comparison is shown in Fig. 5 and Appendix Table S1–S4 (see Electronic Supplementary Material [ESM]). The range of geometric mean differences of exposure was − 10% to 5.57% across the quartiles of BMI groups compared with overall subjects. Age was a significant covariate for CL/F in the population pharmacokinetic analysis. The exposures of subjects aged ≥ 65 years was 7.08% to 13.4% higher than that for those aged < 65 years. AST was also a significant covariate for CL/F. The estimated pharmacokinetics of dorzagliatin were grouped by hepatic function and renal function according to the FDA guideline [15, 16]. The range of geometric mean differences in exposure was −20.2% to 5.67% across all hepatic function groups compared with overall subjects while it was no more than 12.4% for subjects with mild renal function compared with subjects with normal renal function.
Impact of covariates of interest on dorzagliatin steady-state exposure. Circles are the simulated dorzagliatin exposure (AUCtau,ss, Cmax,ss, and Cmin,ss) in individual subjects with 75 mg twice daily based on post-hoc pharmacokinetic parameters. The boxes represent the 25th to 75th percentiles (IQR). The solid black horizontal line in the middle of each box represents the median. The bars extend to the most extreme data point which is no more than 1.5×IQR from the box. AUCtau,ss steady-state area under the plasma concentration–time curve, BMI body mass index, Cmax,ss steady-state maximum concentration, Cmin,ss steady-state trough concentration, IQR interquartile range.
4 Discussion
This analysis included the data from six clinical studies for population pharmacokinetic analysis. The results showed that the two-compartment with zero- and first-order sequential absorption and first-order elimination pharmacokinetic model could well describe the pharmacokinetic characteristics of dorzagliatin within the dose range in this study. Body weight, AST, and age had a statistically significant effect on the CL/F of dorzagliatin. Body weight and sex have a statistically significant effect on Vc/F. Patients with higher body weight exhibited higher CL/F and Vc/F in response to dorzagliatin treatment. Additionally, patients with lower age and AST exhibited higher CL/F. Male patients had higher Vc/F than female patients. Further, patients with time to food consumption of ≥1 h after drug administration had higher D1 than those who consumed food after only 0.5 h.
During the modeling process, it was found that the data predictions from clinical studies 102, 103, and 110 had biased results, with slightly lower exposure compared with studies 201, 301, and 302. This difference in exposure may be related to the dosage formulation, as studies 301 and 302 used near commercial preparations and there may be differences in bioavailability compared with earlier formulations or for other unknown reasons. Attempts were made to find that the model was unable to correct for prediction bias by bioavailability. The three studies (201, 301, and 302) were mainly sparsely sampled, the difference in bioavailability is likely reflected in the difference in clearance rate during the modeling process. For the purpose of better parameter estimation and model application, the between-trial difference was adjusted for the clearance of subjects in trials 102, 103, and 110 using fixed effects. The estimated CL in studies 102, 103, and 110 was 22.5% higher than that in studies 201, 301, and 302.
Body weight is a statistically significant covariate on CL/F and Vc/F. For subjects with body weights of 55 kg and 83 kg (10th–90th quantiles) compared with a typical subject’s weight of 69 kg, the changes in CL/F were −5.63% to 4.83%, and the changes in Vc/F were −11.8% to 10.8%. Age is a statistically significant covariate on CL/F. For subjects aged 41 years old and 66 years old (10th–90th quantile) compared with the typical subject aged 55 years, CL/F changes were 4.06% to −2.44%. AST is a statistically significant covariate on CL/F. For subjects with AST of 13 U/L and 28 U/L (10th to 90th quantile) compared with typical subjects with AST of 18 U/L, CL/F changed by 3.41% to − 4.45%, respectively. Sex had a statistically significant impact on the Vc/F; females were 15.7% lower than male subjects. Despite statistical significance indicating a relationship between a covariate and a PK parameter, this does not necessarily imply a clinical relevance or meaningful impact on patient outcomes. It could be that the observed impact of the covariate on PK parameters is too small to have a significant impact on the overall clinical response. TBW, AGE, and AST were identified as being statistically significant baseline covariates for dorzagliatin clearance in the final PopPK model. However, the extreme values (10th–90th quantile) of a single covariate on clearance or Vc resulted in a < 12% change from a typical patient. Given the limited impact of these covariates on the parameters, it is not expected to be clinically meaningful in terms of the magnitude of the change in exposure. Other covariates including BMI, BSA, ALB, ALT, CR, CRCL, and TBIL did not have a statistically significant impact on the PK parameters.
Sensitivity analysis results further showed that body weight, AST, age, and sex had limited effect on exposure, the 10th or 90th percentile, or different sex relative to typical subjects; exposure quantitative changes are all within 12%. As expected, the time to food consumption after dosing (≥ 1 h vs. 0.5 h) had almost no influence on exposure (−1.26% to 2.71%).
The application of the model also examined the differences in exposure between different covariate groups including BMI quartiles, age groups (< 65 vs. ≥ 65 years), hepatic function (normal vs. mild vs. moderate) and renal function (normal vs. mild). Additionally, the results of a two-part renal impairment study showed limited difference between patients with end-stage renal disease (ESRD) and healthy volunteers (geometric mean ratio of ESRD to HV at 0.81 for Cmax and 1.11 for AUC), suggesting that no dose adjustment is needed for dorzagliatin in patients with T2DM at all stages of renal impairment [7]. The above differences also included the confounding effects of multiple factors. AUCtau,ss and Cmax,ss are within ±12.6% relative to the overall difference; the Cmin,ss is within ±21% of the overall difference. Of note, Cmin,ss is associated with greater variability than AUCtau,ss and Cmax,ss. Given the low IIV of dorzagliatin’s key PK parameters (CL and Vc) and the low-to-moderate effect of all statistically significant covariates on dorzagliatin exposure (AUCtau,ss, Cmaxss, and Cmin,ss), no covariate is expected to have clinically meaningful effects on dorzagliatin exposure.
5 Conclusion
The two-compartment linear pharmacokinetic model with zero- and first-order sequential absorption well described the pharmacokinetic characteristics of dorzagliatin within the dose range in this study. Body weight, age, aspartate aminotransferase, sex, mild and moderate impaired hepatic function, and all stages of renal impairment had a small effect on the exposure. The effect of these covariates on the exposure was not expected to be clinically meaningful and no dose adjustment is needed.
References
Syed YY. Dorzagliatin: first approval. Drugs. 2022;82(18):1745–50.
Xu H, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of novel glucokinase activator HMS5552: results from a first-in-human single ascending dose study. Drug Des Devel Ther. 2016;10:1619–26.
Yang W, et al. Dorzagliatin add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2022;28(5):974–81.
Zhu D, et al. Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: a dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Diabetes Endocrinol. 2018;6(8):627–36.
Zhu D, et al. Dorzagliatin in drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2022;28(5):965–73.
Zhu XX, et al. Dorzagliatin (HMS5552), a novel dual-acting glucokinase activator, improves glycaemic control and pancreatic beta-cell function in patients with type 2 diabetes: A 28-day treatment study using biomarker-guided patient selection. Diabetes Obes Metab. 2018;20(9):2113–20.
Miao J, et al. Effect of renal impairment on the pharmacokinetics and safety of dorzagliatin, a novel dual-acting glucokinase activator. Clin Transl Sci. 2022;15(2):548–57.
Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetics parameters. I. Michaelis-Menten model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm. 1980;8(6):553–71.
Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetic parameters II Biexponential model and experimental pharmacokinetic data. J Pharmacokinet Biopharm. 1981;9(5):635–51.
Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetic parameters. III. Monoexponential model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm. 1983;11(3):303–19.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)–a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75(2):85–94.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79(3):241–57.
Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol. 1997;37(6):486–95.
Bergstrand M, et al. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
FDA, Guidance for Industry Pharmacokinetics in Patients with Impaired Hepatic Function: Study Design, Data Analysis, and Impact on Dosing and Labeling. 2003. https://www.fda.gov/media/71311/download. Accessed 01 July 2023.
FDA, Guidance for Industry Pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. 2020. https://www.fda.gov/media/78573/download. Accessed 01 July 2023.
Acknowledgments
The authors acknowledge the investigators, patients, and their families who participated in these clinical trials.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by Hua Medicine.
Conflict of Interest
K.W., F.X., and Z.S. have received research funding and served in consultant roles to Hua Medicine. L.F., J.Z., Q.Z., L.C., and F.T. are employees of Hua Medicine.
Ethics Approval
Individual trial protocols were approved by the Institutional Review Boards/Ethics Committees of the study sites.
Consent to Participate
All participants gave written informed consent before participating in the respective studies.
Consent for Publication
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
K.W. wrote the manuscript. L.C. and F.T. conceptualized the research. L.F., J.Z., and Q.Z. performed the research. K.W., F.X., and Z.S. analyzed the data. All authors reviewed the key steps of the analysis, commented on intermediate versions of the manuscript, and read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, K., Feng, L., Zhang, J. et al. Population Pharmacokinetic Analysis of Dorzagliatin in Healthy Subjects and Patients with Type 2 Diabetes Mellitus. Clin Pharmacokinet 62, 1413–1425 (2023). https://doi.org/10.1007/s40262-023-01286-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01286-8