Abstract
Background
Prescribing information instructs taking oral semaglutide (a glucagon-like peptide-1 analogue) in the fasting state, followed by a post-dose fasting period of ≥ 30 min. This trial compared the recommended dosing schedule with alternative schedules.
Methods
This was a randomised, single-centre, multiple-dose, open-label, five-armed, parallel-group trial in healthy subjects who received once-daily oral semaglutide (3 mg for 5 days followed by 7 mg for 5 days). Subjects (n = 156) were randomised to five dosing schedules: 2-, 4-, or 6-h pre-dose fast followed by a 30-min post-dose fast (treatment arms: 2 h–30 min, 4–30 min, 6 h–30 min); 2-h pre-dose fast followed by an overnight post-dose fast (treatment arm: 2 h–night); or overnight pre-dose fast followed by a 30-min post-dose fast (reference arm: night–30 min). Semaglutide plasma concentration was measured regularly until 24 h after the 10th dose. Endpoints included area under the semaglutide plasma concentration–time curve during a 24-h interval after the 10th dose (AUC0–24h) (primary endpoint) and maximum observed semaglutide plasma concentration after the 10th dose (Cmax) (secondary endpoint).
Results
Compared with an overnight pre-dose fast (reference arm: night–30 min), shorter pre-dose fasting times in the 2 h–night, 2 h–30 min, 4 h–30 min, and 6 h–30 min treatment arms resulted in significantly lower semaglutide AUC0–24h and Cmax after the 10th dose (estimated treatment ratio ranges: 0.12–0.43 and 0.11–0.44, respectively; p < 0.0001 for all comparisons). Semaglutide AUC0–24h and Cmax after the 10th dose were similar for the 2 h–30 min and 2 h–night treatment arms.
Conclusion
This trial supports dosing oral semaglutide in accordance with prescribing information, which requires dosing in the fasting state.
Trial Registration
ClinicalTrials.gov (NCT04513704); registered August 14, 2020.
Plain Language Summary
Oral semaglutide is a human glucagon-like peptide-1 analogue that has been approved for the treatment of type 2 diabetes. It has been established that taking oral semaglutide with food or large volumes of water decreases absorption of the drug in the body. Current prescribing information instructs taking oral semaglutide on an empty stomach (known as the fasting state), with 120 mL/4 oz of water, then waiting for at least 30 min before consuming any food, water, or taking other oral medications. This study investigates whether different dosing schedules for oral semaglutide could potentially offer more flexibility to patients in the timing of their oral semaglutide dosing. The trial, conducted in healthy volunteers, compares the dosing schedule described in the prescribing information with different fasting times before (pre-dose) and after (post-dose) taking oral semaglutide during the day or evening, to see if there were any effects on the concentration of drug in the body. Compared to the recommended overnight fasting period, shorter pre-dose fasting periods of 2–6 h with a 30-min post-dose fast considerably reduced semaglutide exposure in the body. Similarly, semaglutide exposure was also reduced with a 2-h pre-dose fast combined with post-dose overnight fasting. These findings further support the current prescribing information, which states that patients should take their oral semaglutide dose after an overnight fast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Oral semaglutide is a human glucagon-like peptide-1 analogue that has been formulated for oral administration. Since taking oral semaglutide with food reduces semaglutide exposure, the approved label instructs to dose oral semaglutide on an empty stomach (fasting state) followed by post-dose fasting of at least 30 min. |
The trial included 156 healthy subjects who received oral semaglutide according to various alternative dosing schedules to explore if different pre- and post-dose fasting times could provide similar semaglutide exposure to the reference dosing schedule. |
Compared with an overnight pre-dose fast, shorter pre-dose fasting times of 2–6 h resulted in significantly lower exposure to semaglutide. This trial supports dosing of oral semaglutide on an empty stomach in accordance with the prescribing information. |
1 Introduction
Oral semaglutide (Rybelsus®) is a novel tablet formulation of the glucagon-like peptide-1 analogue semaglutide, which is indicated as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes [1]. Oral semaglutide is co-formulated with the absorption enhancer sodium N-(8-[2-hydroxybenzoyl] amino) caprylate (SNAC), a small fatty acid derivative, which facilitates gastric absorption of semaglutide, allowing clinically relevant exposure after oral dosing [2]. SNAC promotes absorption of semaglutide across the mucosa of the stomach via effects on transcellular pathways. It also has a localised buffering effect on pH that protects semaglutide from proteolytic degradation by gastric enzymes and improves the solubility of semaglutide [2].
Exposure to semaglutide is limited following oral administration in the fed state [3], due to detrimental dilution effects on SNAC and semaglutide, that lead to greatly reduced semaglutide absorption [2]. Therefore, current dosing recommendations instruct patients to take oral semaglutide in the fasting state at least 30 min before the first intake of food, drink, or other oral medications [1, 3]. Waiting less than 30 min to eat or drink after semaglutide dosing may decrease absorption and waiting longer than 30 min may increase absorption [1, 3]. It is also recommended that oral semaglutide is administered with no more than 120 mL/4 oz of water, based on the dosing conditions used in the oral semaglutide Phase III PIONEER programme [1, 3]. While the current standard dosing regimen of oral semaglutide is well established, there remains interest in studying the dosing schedule due to the direct effect of pre- and post-dose fasting time on the absorption of oral semaglutide. Investigating the impact of different dosing schedules on the bioavailability of oral semaglutide could potentially offer further dosing options to patients, which may ultimately improve their medication adherence.
The objective of the present trial was to explore whether alternative dosing schedules of oral semaglutide during the day or evening, with different pre- and post-dose fasting times, could provide similar exposure to dosing on an empty stomach, as per alignment with the instructions in the semaglutide prescribing information [1].
2 Methods
2.1 Trial Design
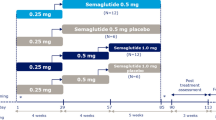
This was a randomised, single-centre, multiple-dose, open-label, five-armed, parallel-group trial in healthy subjects (Fig. 1a). The trial was conducted in the UK between August 2020 and May 2021 and was registered at ClinicalTrials.gov (NCT04513704). All subjects were treated with oral semaglutide co-formulated with SNAC. Four of the treatment arms tested alternative dosing schedules for oral semaglutide with dosing in the evening:
-
Pre-dose fasting times of 2, 4, or 6 h followed by a 30-min post-dose fast (treatment arms 2 h–30 min, 4 h–30 min, 6 h–30 min).
-
Pre-dose fasting time of 2 h followed by a post-dose overnight fast (treatment arm 2 h–night).
Trial design (a) and overview of fasting and mealtime requirements (b). aOvernight fast of at least 6 h; due to the timing of the evening snack at the clinical site, the average overnight fast for treatment arm night–30 min was approximately 11 h. bOral semaglutide was to be dosed at 8 am (treatment arm night–30 min) or 8 pm (all other treatment arms) ± 2 h. Water intake was allowed in the specified fasting periods except from 2 h prior to dosing (all treatment arms) and until 30 min (treatment arms: 2 h–30 min, 4 h–30 min, 6 h–30 min, and night–30 min) or 2 h (treatment arm: 2 h–night) post-dosing. cSubjects were expected to be asleep during this time period. The post-dose meal was the first meal served for the 2 h–night treatment arm
The fifth treatment arm was included as a reference and followed dosing recommendations in the oral semaglutide prescribing information [1], consisting of an overnight pre-dose fast of at least 6 h, treatment administration in the morning, and then a 30-min post-dose fast (night–30 min treatment arm) followed by a standardised breakfast meal. Due to the timing of the evening snack at the clinical site, the duration of the overnight pre-dose fast for this arm was approximately 11 h.
2.2 Participants
Eligible subjects were men and women aged ≥ 18 to ≤ 64 years with a body mass index of ≥ 20 to ≤ 29.9 kg/m2. Subjects had to be considered generally healthy, as judged by the investigator, based on their medical history, physical examination, vital signs, electrocardiogram, and clinical laboratory tests performed during the screening visit.
Subjects were excluded if they had glycated haemoglobin ≥ 6.5% (48 mmol/mol) at screening; presence or symptoms of clinically significant gastrointestinal disorders; personal or first-degree relative history of multiple endocrine neoplasia type 2 or medullary thyroid cancer, or of major surgical procedures involving the stomach; or presence or history of pancreatitis. Subjects who smoked > 5 cigarettes per day or who were not able or willing to refrain from smoking whilst at the clinical site were excluded. Use of prescription medicinal products or non-prescription drugs or herbal products (including St John’s wort), except for highly effective contraceptive methods, hormone replacement therapy, routine vitamins, topical medication not reaching the systemic circulation, and occasional use of paracetamol, was not permitted from within 14 days prior to the day of screening to 24 h after the last dosing. Subjects avoided any permitted oral medications from 2 h prior to, and until 30 min (treatment arms: 2 h–30 min, 4 h–30 min, 6 h–30 min, and night–30 min) or 2 h (treatment arm: 2 h–night) after each administration of oral semaglutide.
2.3 Procedures
Following an initial screening visit, subjects were randomised to the four alternative dosing schedule arms and the reference arm (1:1:1:1:2, respectively). Subjects were assigned a unique randomisation number in ascending numerical order for males and descending numerical order for females, to ensure that sex was balanced across the treatment arms. The randomisation number encoded the subject’s assignment to one of the treatment arms according to the randomisation list generated before the trial, using block randomisation (randomisation lists were provided by PAREXEL International, Nottingham, UK). The first day of dosing was the day after randomisation. Subjects stayed in-house on the site from randomisation until the end of sampling on Day 11 (24 h after 10th dose) and were dosed at the clinical site once daily for 10 days according to their randomised treatment schedule (Fig. 1a); a 3-mg oral semaglutide tablet was administered for the first 5 days, followed by a 7-mg oral semaglutide tablet on the last 5 days. Each dose was taken with no more than 120 mL/4 oz of water. A follow-up visit was scheduled 5 weeks after last dosing.
During the on-site period, subjects were served standardised meals at pre- and post-dosing according to the dosing schedule of their randomised treatment arm and were instructed to consume the complete meal within 30 min of serving. No food intake was allowed for at least 4 h prior to the standardised pre-dose meal (treatment arms: 2 h–30 min, 4 h–30 min, 6 h–30 min, and 2 h–night) (Fig. 1b). Standardised meals consisted of a pre-dose meal of approximately 760 kcal and a post-dose meal of approximately 380 kcal. The macronutrient composition of all meals was approximately 36% of energy from fat, 17% from protein, and 47% from carbohydrates. All standardised meals were weighed before serving. While participants were instructed to consume the whole meal, in cases where it was partially consumed, the weight of the meal remaining was recorded.
Blood samples for measuring semaglutide concentration in plasma were taken pre-dose on Days 1 to 9 (0–30 min prior to dosing) and at predefined time points before and after the 10th dose on Days 10 and 11 (pre-dose: 0–5 min prior to dosing; post-dose: 10, 20, 30, 40, and 50 min and 1, 1.5, 2, 2.5, 3, 4, 6, 12, and 24 h post-dose).
2.4 Assessments
Plasma semaglutide concentration was measured at a specialist laboratory using a validated liquid chromatography with tandem mass spectrometry assay, as described previously [4].
Safety assessments included adverse events, concomitant illness, medical history, clinical laboratory tests, vital signs, physical examinations, and technical complaints relating to medicine defects.
2.5 Objectives and Endpoints
The primary objective was to compare the pharmacokinetic profiles of semaglutide in healthy subjects for four different combinations of pre- and post-dose fasting times with a reference dosing schedule. The secondary objective was to compare the pharmacokinetic profiles of semaglutide in healthy subjects dosed according to the same pre-dose fasting time but two different post-dose fasting times. The primary and secondary objectives had the same primary and supportive secondary endpoints. The primary endpoint was the area under the semaglutide plasma concentration–time curve during a 24-h interval after the 10th dose of oral semaglutide (AUC0–24h,sema,Day10). Supportive secondary endpoints included the maximum observed semaglutide plasma concentration after the 10th dose of oral semaglutide (Cmax,sema,Day10) and the time to Cmax after the 10th dose (tmax,sema,Day10).
2.6 Statistical analysis
Sample size was calculated based on the precision of the ratio of AUC0–24h,sema,Day10 between treatment groups using a two-sided 95% confidence interval (CI). The primary endpoint was assumed to have a log-normal distribution and the standard deviation (SD) of the log-transformed AUC0–24h,sema,Day10 was assumed to be the same for the different dosing schedules tested; no adjustment for multiplicity was made. Based on data from previous pharmacokinetic trials of oral semaglutide using a similar 10-day design and trial population [3,4,5,6], the SD of the log-transformed AUC0–24h,sema,Day10 was estimated to be 0.55. Based on this, it was assumed that 24 evaluable subjects would be required for each of the investigated dosing schedules and 48 evaluable subjects would be required for the reference dosing schedule in order to provide an 80% probability that the 95% CI for the true ratio between groups for AUC0–24h,sema,Day10 would fall between 0.75*\(\widehat{R}\) and 1.34*\(\widehat{R}\), where \(\widehat{R}\) was the unknown estimate of the ratio.
Analyses of pharmacokinetic endpoints were conducted in the full analysis set (FAS; all subjects who were randomised and exposed to at least one dose of trial product), while safety analyses were conducted in the safety analysis set (SAS; all subjects who were exposed to at least one dose of trial product). The AUC0–24h,sema,Day10 (primary endpoint) was calculated using the linear trapezoidal method based on observed semaglutide concentrations and actual measurement times. The Cmax,sema,Day10 was calculated as the maximum of all observed valid semaglutide concentrations from nominal time 0–24 h after the 10th dose, whilst tmax,sema,Day10 was calculated as the actual time after the 10th dose to the Cmax.
Statistical analysis of the primary endpoint was performed using a linear normal model for censored data with log-transformed AUC0–24h,sema,Day10 as a dependent variable, dosing schedule group (five levels) and sex as fixed effects, and a residual variance parameter for each dosing group. All statistical analyses were performed in SAS® software (version 9.4). From this model, the estimated mean difference in log-transformed AUC0–24h,sema,Day10 (described as AUC0–24h Day 10 from here on) between pairs of treatment groups was estimated and back-transformed to the original scale and presented as a ratio of geometric means together with the corresponding two-sided 95% CI and p value. Cmax,sema,Day10 (described as Cmax Day 10 from here on) was analysed as for the primary endpoint except the model only allowed for left-censoring. The tmax,sema,Day10 (described as Tmax Day 10 from here on) was summarised descriptively.
3 Results
3.1 Subject Disposition and Baseline Characteristics
In total, 367 subjects were screened and 156 were enrolled, randomised, and exposed to trial products: 25 subjects in treatment arms 2 h–30 min and 6 h–30 min, 27 subjects in treatment arms 4 h–30 min and 2 h–night, and 52 subjects in the reference arm (night–30 min) (Fig. S1 of Online Resource 1). Overall, 154 subjects completed the trial and two subjects were prematurely withdrawn before trial completion: one subject was withdrawn from reference arm night–30 min by the investigator due to an adverse event suggestive of COVID-19 infection and one subject withdrew themself from treatment arm 2 h–night.
All 156 subjects who received trial treatment were included in the analyses. Three subjects were excluded from all AUC0–24h and Cmax Day 10 analyses: two withdrew from the trial before the 10th dose (one subject in each of the 2 h–night and night–30 min treatment arms as described above) and one subject had an incomplete Day 10 pharmacokinetic profile (6 h–30 min treatment arm).
Subject demographic and baseline characteristics are shown in Table 1 and were consistent across the five treatment arms.
3.2 Semaglutide Pharmacokinetics
Estimated geometric mean semaglutide concentration–time profiles following the 10th dose of semaglutide are shown in Fig. 2 for all five dosing schedules, and semaglutide AUC0–24h and Cmax on Day 10 (from 0–24 h after the last dose) by dosing schedule are displayed in Fig. 3. Exposure to semaglutide appeared to be greater in the treatment arms that employed longer pre-dose fasting periods (Figs. 2 and 3). Compared with the overnight pre-dose fasting period employed in the reference arm (night–30 min), shorter pre-dose fasting periods of 2, 4, and 6 h in the 2 h–30 min, 4 h–30 min, and 6 h–30 min treatment arms resulted in significantly lower semaglutide AUC0–24h and Cmax on Day 10 (estimated treatment ratio ranges: 0.12–0.43 and 0.12–0.44, respectively; p < 0.0001 for all comparisons; Fig. 4).
Semaglutide dosing interval profiles after the 10th dosing. Geometric means are plotted and were estimated by dosing schedule arm using a linear log-normal model accounting for censoring. Treatment arm name denotes pre-dose and post-dose fasting period; for example, 2 h–30 min indicates a pre-dose fast of 2 h and a post-dose fast of 30 min
Pharmacokinetic parameters for semaglutide after the 10th dosing: AUC0–24h,sema,Day10 and Cmax,sema,Day10. Boxes correspond to the 25th, 50th (median), and 75th percentiles, and solid symbols correspond to the estimated geometric means. Open circle symbols represent outliers: subjects with values for AUC0–24h,sema,Day10 or Cmax,sema,Day10 that are above the 95th percentile or below the 5th percentile. Whiskers correspond to the 5th and 95th percentiles. The geometric means were estimated using (a) a linear log-normal model accounting for censoring and (b) a linear normal model accounting for left-censoring. Percentiles are computed on imputed values. Treatment arm name denotes pre-dose and post-dose fasting period; for example, 2 h–30 min indicates a pre-dose fast of 2 h and a post-dose fast of 30 min. Three subjects (one each from the 6 h–30 min, 2 h–night, and night–30 min arms) were excluded from the statistical analysis: two subjects withdrew from the trial before the 10th dose and one subject had an incomplete Day 10 profile. AUC0–24h,sema,Day10 area under the semaglutide plasma concentration–time curve during a 24-h interval after the 10th dose of oral semaglutide, Cmax,sema,Day10 maximum observed semaglutide plasma concentration after the 10th dose of oral semaglutide
Estimated treatment ratios for primary and secondary objectives. The endpoint was log-transformed and analysed in a linear normal model for censored data with dosing schedule group (five levels) and sex as fixed effects, and a residual variance parameter for each dosing group. Three subjects (one each from the 6 h–30 min, 2 h–night, and night–30 min arms) were excluded from the statistical analysis: two subjects withdrew from the trial before the 10th dose and one subject had an incomplete Day 10 profile. Treatment arm name denotes pre-dose and post-dose fasting period; for example, 2 h–30 min indicates a pre-dose fast of 2 h and a post-dose fast of 30 min. AUC0–24h,sema,Day10 area under the semaglutide plasma concentration–time curve during a 24-h interval after the 10th dose of oral semaglutide, CI confidence interval, Cmax,sema,Day10 maximum observed semaglutide plasma concentration after the 10th dose of oral semaglutide, ETR estimated treatment ratio
A 2-h pre-dose fast followed by an overnight post-dose fast, as employed in treatment arm 2 h–night, also resulted in significantly lower semaglutide AUC0–24h and Cmax on Day 10 compared with the reference arm (night–30 min) (both p < 0.0001; Fig. 4).
The semaglutide exposure levels in the 2 h–30 min and 2 h–night treatment arms were similar and low, and the longer post-dose fasting in treatment arm 2 h–night did not appear to affect the pharmacokinetics of semaglutide (Figs. 2, 3, and 4).
There appeared to be greater variability in semaglutide AUC0–24h and Cmax on Day 10 when a 2-h pre-dose fasting period was used compared with longer pre-dose fasting periods (Fig. 3). Five subjects in treatment arm 2 h–30 min and eight subjects in treatment arm 2 h–night had no quantifiable exposure to semaglutide.
The median Tmax on Day 10 ranged from 0.8–1.0 h and appeared to be similar across the treatment arms (Table 2).
3.3 Safety
Overall, 290 treatment-emergent adverse events were reported by 93 (59.6%) subjects (Table 3). Gastrointestinal disorders were the most frequently reported adverse events, largely driven by nausea and abdominal distension. The majority of adverse events were mild in nature and none were serious or fatal. One subject in the reference arm (night–30 min) discontinued treatment due to an adverse event indicative of COVID-19 infection. No clinically significant laboratory abnormalities related to treatment were observed.
4 Discussion
Studies have previously established that food [2, 3] or other oral medications [7] may impact the absorption of oral semaglutide. Consequently, the current prescribing information for oral semaglutide recommends taking the tablet on an empty stomach with no more than 120 mL/4 oz of water at least 30 min before the first food, drink, or other oral medications of the day [1]. There remains a need to explore alternative dosing schedules that could increase the convenience for patients while achieving comparable treatment targets to standard dosing recommendations. The objective of this trial was to investigate whether alternative dosing schedules could be used to offer greater flexibility in the timing of oral semaglutide dosing.
First, the trial investigated whether the fasting time prior to dosing could be reduced from an overnight fast. Compared with the reference dosing schedule for oral semaglutide (overnight pre-dose fast followed by a 30-min post-dose fast), shorter daytime pre-dose fasting periods of 2–6 h with a 30-min post-dose fast resulted in substantial and statistically significant decreases in semaglutide exposure. As the duration of the pre-dose fasting period decreased, there was a corresponding decrease in semaglutide exposure.
The trial also investigated whether an overnight fast after dosing, where no post-dose food intake would disturb semaglutide absorption, could outweigh the negative impact of a pre-dose meal on absorption. However, the alternative dosing schedule used in the 2 h–night treatment arm resulted in a substantial and statistically significant decrease in exposure compared with the reference treatment arm (night–30 min). In addition, for subjects with a 2-h pre-dose fast, increasing the length of the post-dose fast did not seem to affect the absorption of oral semaglutide, with similar exposure levels observed between the 2 h–30 min and 2 h–night treatment arms, indicating that the disruption of semaglutide absorption caused by food components in the stomach at dosing cannot be compensated by longer post-dose fasting. A trial in healthy male subjects has previously shown that longer post-dose fasting leads to a significant increase in semaglutide exposure [3]; however, the subjects had fasted overnight prior to dosing, which may explain the different findings compared with the current trial. The present findings are supported by a previous food-effect trial in healthy subjects with oral semaglutide, which demonstrated that eating a high-fat meal 30 min prior to dosing leads to limited or no measurable semaglutide exposure [3]. Together, these studies indicate that semaglutide exposure is significantly decreased with pre-dose fasts up to 6 h compared with the reference dosing schedule (overnight fast), supporting the recommendation for administration on an empty stomach.
Exposure to semaglutide for all alternative treatment arms was reduced by more than 50% compared with the exposure observed following an overnight fast in the reference arm (night–30 min), even when subjects fasted for 6 h prior to dosing (6 h–30 min treatment arm) (Fig. 2). Due to the practical timing of serving the evening snack at the clinical site (with no other food available to subjects besides scheduled meals), the average overnight fast for the reference treatment arm (night–30 min) was approximately 11 h. Therefore, the greater exposure observed in this arm compared with the 6 h–30 min arm may be exaggerated by the longer pre-dose fasting period implemented in the present trial.
It should also be noted that subjects in the alternative treatment arms (2 h–30 min, 4 h–30 min, 6 h–30 min, and 2 h–night), with lower semaglutide exposure, were dosed in the evening with pre-dose fasting during the day, whereas subjects in the reference arm night–30 min were dosed in the morning with pre-dose fasting overnight. As circadian rhythms are prevalent within physiological processes within the gut [8], there is the potential for some time-of-day variability in the absorption of orally administered drugs and in the speed of gastric emptying following a meal [8, 9]. However, this dosing time-dependence is more common amongst drugs with shorter half-lives, such as less than 15 h, whereas oral semaglutide has a long half-life of approximately 1 week [1, 10].
The safety profile of oral semaglutide in this trial was consistent with the known safety profile for the drug [11]. No pharmacodynamic markers were assessed, as the objective was to evaluate the pharmacokinetics of oral semaglutide in healthy volunteers. There was greater variability in exposure to semaglutide in the 2-h pre-dose fasting arms than with longer pre-dose fasting periods. This variability could be partially explained by the lower semaglutide exposure in the 2-h pre-dose fasting arms, with five subjects in treatment arm 2 h–30 min and eight subjects in treatment arm 2 h–night having no quantifiable exposure. This study further supports the current prescribing information, which instructs taking oral semaglutide in a fasting state followed by a 30-min post-dose fast, and that any deviation from the current guidelines may influence the absorption of oral semaglutide. As per the exploratory nature of the trial, no confirmatory hypotheses were tested, and no multiplicity control strategy was considered.
5 Conclusion
Pre-dose fasting periods of 2, 4, and 6 h, and 2 h pre-dose fasting combined with post-dose overnight fasting, significantly reduced semaglutide exposure compared with pre-dose overnight fasting, in accordance with the current prescribing information. The results of the present trial support that the optimal dosing schedule for oral semaglutide is that recommended by the current prescribing information [1], which requires dosing on an empty stomach followed by a post-dose fast of at least 30 min.
Change history
28 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40262-023-01276-w
References
US Food and Drug Administration. Rybelsus® (semaglutide) tablets: prescribing information; 2023. https://www.accessdata.fda.gov/spl/data/fa74bbc6-5386-425e-8d4a-6151795ba57b/fa74bbc6-5386-425e-8d4a-6151795ba57b.xml. Accessed March 31, 2022.
Buckley ST, Bækdal TA, Vegge A, Maarbjerg SJ, Pyke C, Ahnfelt-Ronne J, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(467):eaar7047. https://doi.org/10.1126/scitranslmed.aar7047.
Bækdal TA, Breitschaft A, Donsmark M, Maarbjerg SJ, Sondergaard FL, Borregaard J. Effect of various dosing conditions on the pharmacokinetics of oral semaglutide, a human glucagon-like peptide-1 analogue in a tablet formulation. Diabetes Ther. 2021;12(7):1915–27. https://doi.org/10.1007/s13300-021-01078-y.
Bækdal TA, Thomsen M, Kupcova V, Hansen CW, Anderson TW. Pharmacokinetics, safety, and tolerability of oral semaglutide in subjects with hepatic impairment. J Clin Pharmacol. 2018;58(10):1314–23. https://doi.org/10.1002/jcph.1131.
Bækdal TA, Breitschaft A, Navarria A, Hansen CW. A randomised study investigating the effect of omeprazole on the pharmacokinetics of oral semaglutide. Expert Opin Drug Metab Toxicol. 2018;14(8):869–77. https://doi.org/10.1080/17425255.2018.1488965.
Granhall C, Sondergaard FL, Thomsen M, Anderson TW. Pharmacokinetics, safety and tolerability of oral semaglutide in subjects with renal impairment. Clin Pharmacokinet. 2018;57(12):1571–80. https://doi.org/10.1007/s40262-018-0649-2.
Hauge C, Breitschaft A, Hartoft-Nielsen ML, Jensen S, Bækdal TA. Effect of oral semaglutide on the pharmacokinetics of thyroxine after dosing of levothyroxine and the influence of co-administered tablets on the pharmacokinetics of oral semaglutide in healthy subjects: an open-label, one-sequence crossover, single-center, multiple-dose, two-part trial. Expert Opin Drug Metab Toxicol. 2021;17(9):1139–48. https://doi.org/10.1080/17425255.2021.1955856.
Ayyar VS, Sukumaran S. Circadian rhythms: influence on physiology, pharmacology, and therapeutic interventions. J Pharmacokinet Pharmacodyn. 2021;48(3):321–38. https://doi.org/10.1007/s10928-021-09751-2.
Goo RH, Moore JG, Greenberg E, Alazraki NP. Circadian variation in gastric emptying of meals in humans. Gastroenterology. 1987;93(3):515–8. https://doi.org/10.1016/0016-5085(87)90913-9.
Ruben MD, Smith DF, FitzGerald GA, Hogenesch JB. Dosing time matters. Science. 2019;365(6453):547–9. https://doi.org/10.1126/science.aax7621.
Thethi TK, Pratley R, Meier JJ. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab. 2020;22(8):1263–77. https://doi.org/10.1111/dom.14054.
Acknowledgements
Medical writing and editorial support were provided by Nicole Cash, PhD, and Abbey Pearson, MSci, of Axis, a division of Spirit Medical Communications Group Limited (and were funded by Novo Nordisk Inc.) in accordance with Good Publication Practice 3 (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This trial and medical writing support for the publication were funded by Novo Nordisk A/S, Søborg, Denmark.
Conflict of interest
MvH, TBJ, CB, and TAB are employees, and MvH, TBJ, and TAB are shareholders, of Novo Nordisk A/S, the sponsors of this trial. PF is an employee of PAREXEL International.
Availability of data and material
Data are available upon reasonable request. Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at novonordisk-trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in data sets in a de-identified/anonymised format.
Ethics approval
The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by an independent ethics committee (South Central—Berkshire B Research Ethics Committee).
Consent for publication
Not applicable.
Code availability
Not applicable.
Consent
All participants provided written informed consent.
Author contributions
MvH, TAB, CB, and TBJ designed the trial; PF, MvH, TAB, and TBJ conducted the trial/performed data collection; CB, TAB, TBJ, and MvH analysed the data; all authors drafted and revised the manuscript and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
van Hout, M., Forte, P., Jensen, T.B. et al. Effect of Various Dosing Schedules on the Pharmacokinetics of Oral Semaglutide: A Randomised Trial in Healthy Subjects. Clin Pharmacokinet 62, 635–644 (2023). https://doi.org/10.1007/s40262-023-01223-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01223-9