Abstract
Background and Objective
Daridorexant is a dual orexin receptor antagonist in clinical development for insomnia. As daridorexant is cleared mainly via cytochrome P450 (CYP) 3A4, the effect of hepatic impairment on the pharmacokinetics (PK), metabolism, and tolerability of daridorexant was evaluated. Sleep disorders are common in patients with liver cirrhosis and, therefore, sleep-promoting drugs with a better tolerability than currently available would be preferable, a premise that dual orexin receptor antagonists may fulfill.
Methods
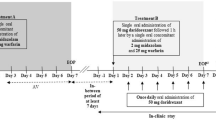
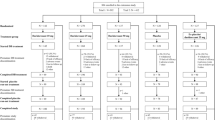
This was a single-dose, open-label, phase I study. Subjects with mild (Child–Pugh A, N = 8) or moderate (Child–Pugh B, N = 8) liver cirrhosis and matched healthy control subjects (N = 8) received 25 mg of daridorexant orally. Blood samples were collected for 72 h post-dose for PK assessments of daridorexant and three major metabolites.
Results
Compared with healthy subjects, patients showed a decrease in total daridorexant area under the plasma concentration–time curve from zero to infinity (AUC0-inf) and maximum plasma concentration with a geometric mean ratio (GMR, 90% confidence interval [CI]) of 0.51 (0.28–0.92) and 0.50 (0.35–0.72) in Child–Pugh A and 0.74 (0.39–1.41) and 0.42 (0.29–0.60) in Child–Pugh B patients, respectively. Furthermore, the median time to reach maximum plasma concentration was slightly delayed (1.0 h [90% CI 0.0–2.0] in Child–Pugh A patients and 0.5 h [90% CI 0.0–1.5] in Child–Pugh B patients), while for Child–Pugh B patients, a doubling in half-life was observed (GMR [90% CI]: 2.09 [1.32–3.30]). Considering the high plasma protein binding (> 99%) and a 1.9-fold to 2.3-fold increase in the unbound fraction in patients, the PK of unbound daridorexant was also assessed. Compared with healthy subjects, Child–Pugh B patients had a higher AUC0-inf (GMR [90% CI] 1.60 [0.93–2.73]), a lower apparent plasma clearance (GMR [90% CI] 0.63 [0.37–1.07]), and the same doubling in the half-life observed for total daridorexant, whereas maximum plasma concentration and apparent volume of distribution were not different. Unbound daridorexant PK in Child–Pugh A patients did not differ from healthy subjects. In addition, the metabolic ratios (parent to metabolite), i.e., a marker of CYP 3A4 activity, of the two most abundant daridorexant metabolites were higher in patients with liver cirrhosis compared with healthy subjects. All treatment-emergent adverse events were transient and of mild or moderate intensity and no other treatment-related effects were apparent.
Conclusions
No safety issue of concern was detected following administration of 25 mg of daridorexant in the study population. Moderate liver cirrhosis causes impaired hepatic clearance of unbound daridorexant, which prolongs the half-life. A 25-mg dose of daridorexant should, therefore, not be exceeded in Child–Pugh B patients. A dose adjustment is not required in Child–Pugh A patients, while avoidance of daridorexant in patients with Child–Pugh C cirrhosis is recommended.
Clinical Trial Registration
ClinicalTrials.gov ID: NCT03713242.
Similar content being viewed by others
References
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(5):573–85.
Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–81.
Treiber A, de Kanter R, Roch C, Gatfield J, Boss C, von Raumer M, et al. The use of physiology-based pharmacokinetic and pharmacodynamic modeling in the discovery of the dual orexin receptor antagonist ACT-541468. J Pharmacol Exp Ther. 2017;362(3):489–503.
Wang C, Wang Q, Ji B, Pan Y, Xu C, Cheng B, et al. The orexin/receptor system: molecular mechanism and therapeutic potential for neurological diseases. Front Mol Neurosci. 2018;11:220.
Muehlan C, Vaillant C, Zenklusen I, Kraehenbuehl S, Dingemanse J. Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders. Expert Opin Drug Metab Toxicol. 2020;16(11):1063–78.
Mieda M, Sakurai T. Orexin (hypocretin) receptor agonists and antagonists for treatment of sleep disorders: rationale for development and current status. CNS Drugs. 2013;27(2):83–90.
Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13(2):150–5.
Hoever P, Dorffner G, Beneš H, Penzel T, Danker-Hopfe H, Barbanoj MJ, et al. Orexin receptor antagonism, a new sleep-enabling paradigm: a proof-of-concept clinical trial. Clin Pharmacol Ther. 2012;91(6):975–85.
Dauvilliers Y, Zammit G, Fietze I, Mayleben D, Seboek Kinter D, Pain S, et al. Daridorexant, a new dual orexin receptor antagonist to treat insomnia disorder. Ann Neurol. 2020;87(3):347–56.
Muehlan C, Brooks S, Zuiker R, van Gerven J, Dingemanse J. Multiple-dose clinical pharmacology of ACT-541468, a novel dual orexin receptor antagonist, following repeated-dose morning and evening administration. Eur Neuropsychopharmacol. 2019;29(7):847–57.
Zammit G, Dauvilliers Y, Pain S, Sebök Kinter D, Mansour Y, Kunz D. Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology. 2020;94(21):e2222–32.
Boof ML, Alatrach A, Ufer M, Dingemanse J. Interaction potential of the dual orexin receptor antagonist ACT-541468 with CYP3A4 and food: results from two interaction studies. Eur J Clin Pharmacol. 2019;75(2):195–205.
Zenklusen I, Muehlan C, Ulc I, Liska J, Dingemanse J. The dual orexin receptor antagonist daridorexant does not affect the pharmacokinetics of the BCRP substrate rosuvastatin. Clin Exp Pharmacol Physiol. 2020;47(11):1843–9.
Muehlan C, Zuiker R, Peeters P, Rowles R, Dingemanse J. Pharmacokinetics and pharmacodynamics of the dual orexin receptor antagonist daridorexant in Japanese and Caucasian subjects. J Clin Psychopharmacol. 2020;40(2):157–66.
Muehlan C, Heuberger J, Juif PE, Croft M, van Gerven J, Dingemanse J. Accelerated development of the dual orexin receptor antagonist ACT-541468: integration of a microtracer in a first-in-human study. Clin Pharmacol Ther. 2018;104(5):1022–9.
Muehlan C, Boehler M, Brooks S, Zuiker R, van Gerven J, Dingemanse J. Clinical pharmacology of the dual orexin receptor antagonist ACT-541468 in elderly subjects: exploration of pharmacokinetics, pharmacodynamics and tolerability following single-dose morning and repeated-dose evening administration. J Psychopharmacol. 2020;34(3):326–35.
Muehlan C, Fischer H, Zimmer D, Aissaoui H, Grimont J, Boss C, et al. Metabolism of the dual orexin receptor antagonist ACT-541468, based on microtracer/accelerator mass spectrometry. Curr Drug Metab. 2019;20(4):254–65.
Tapper EB, Henderson JB, Parikh ND, Ioannou GN, Lok AS. Incidence of and risk factors for hepatic encephalopathy in a population-based cohort of Americans with cirrhosis. Hepatol Commun. 2019;3(11):1510–9.
Córdoba J, Cabrera J, Lataif L, Penev P, Zee P, Blei AT. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27(2):339–45.
Martin Mateos R, Garcia de la Filia Molina I, Albillos A. Pre-surgical risk assessment in patients with cirrhosis. Acta Gastroenterol Belg. 2020;83(3):449–53.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Donzelli M, Derungs A, Serratore MG, Noppen C, Nezic L, Krahenbuhl S, et al. The basel cocktail for simultaneous phenotyping of human cytochrome P450 isoforms in plasma, saliva and dried blood spots. Clin Pharmacokinet. 2014;53(3):271–82.
Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1–2):29–37.
Boss C, Gatfield J, Brotschi C, Heidmann B, Sifferlen T, von Raumer M, et al. The quest for the best dual orexin receptor antagonist (daridorexant) for the treatment of insomnia disorders. Chem Med Chem. 2020;15(23):2286–305.
Delco F, Tchambaz L, Schlienger R, Drewe J, Krahenbuhl S. Dose adjustment in patients with liver disease. Drug Saf. 2005;28(6):529–45.
Quigley EM. Gastrointestinal dysfunction in liver disease and portal hypertension: gut-liver interactions revisited. Dig Dis Sci. 1996;41(3):557–61.
Sadik R, Abrahamsson H, Björnsson E, Gunnarsdottir A, Stotzer PO. Etiology of portal hypertension may influence gastrointestinal transit. Scand J Gastroenterol. 2003;38(10):1039–44.
Thalheimer U, De Iorio F, Capra F, del Mar LM, Zuliani V, Ghidini V, et al. Altered intestinal function precedes the appearance of bacterial DNA in serum and ascites in patients with cirrhosis: a pilot study. Eur J Gastroenterol Hepatol. 2010;22(10):1228–34.
Isobe H, Sakai H, Satoh M, Sakamoto S, Nawata H. Delayed gastric emptying in patients with liver cirrhosis. Dig Dis Sci. 1994;39(5):983–7.
Pimpo MT, Frieri G, Saltarelli P, Ciccocioppo R, Aggio A, Marchetti G, et al. Effects of cisapride on abnormally prolonged endogastric alkalinity time and delayed gastric emptying in cirrhotic patients. Hepatogastroenterology. 1996;43(12):1678–84.
Usami A, Mizukami Y, Onji M. Abnormal gastric motility in liver cirrhosis: roles of secretin. Dig Dis Sci. 1998;43(11):2392–7.
Taegtmeyer AB, Haschke M, Tchambaz L, Buylaert M, Tschopl M, Beuers U, et al. A study of the relationship between serum bile acids and propranolol pharmacokinetics and pharmacodynamics in patients with liver cirrhosis and in healthy controls. PLoS ONE. 2014;9(6):e97885.
Albarmawi A, Czock D, Gauss A, Ehehalt R, Lorenzo Bermejo J, Burhenne J, et al. CYP3A activity in severe liver cirrhosis correlates with Child-Pugh and model for end-stage liver disease (MELD) scores. Br J Clin Pharmacol. 2014;77(1):160–9.
Xie F, Ding X, Zhang QY. An update on the role of intestinal cytochrome P450 enzymes in drug disposition. Acta Pharm Sin B. 2016;6(5):374–83.
Ceryak S, Bouscarel B, Fromm H. Comparative binding of bile acids to serum lipoproteins and albumin. J Lipid Res. 1993;34(10):1661–74.
Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64(12):1147–61.
Barre J, Houin G, Rosenbaum J, Zini R, Dhumeaux D, Tillement JP. Decreased alpha 1-acid glycoprotein in liver cirrhosis: consequences for drug protein binding. Br J Clin Pharmacol. 1984;18(4):652–3.
Barre J, Mallat A, Rosenbaum J, Deforges L, Houin G, Dhumeaux D, et al. Pharmacokinetics of erythromycin in patients with severe cirrhosis: respective influence of decreased serum binding and impaired liver metabolic capacity. Br J Clin Pharmacol. 1987;23(6):753–7.
Knott C, Williams CP, Reynolds F. Phenytoin kinetics during pregnancy and the puerperium. Br J Obstet Gynaecol. 1986;93(10):1030–7.
Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52(1):1–8.
Klotz U, Avant GR, Hoyumpa A, Schenker S, Wilkinson GR. The effects of age and liver disease on the disposition and elimination of diazepam in adult man. J Clin Invest. 1975;55(2):347–59.
George J, Murray M, Byth K, Farrell GC. Differential alterations of cytochrome P450 proteins in livers from patients with severe chronic liver disease. Hepatology. 1995;21(1):120–8.
Prasad B, Bhatt DK, Johnson K, Chapa R, Chu X, Salphati L, et al. Abundance of phase 1 and 2 drug-metabolizing enzymes in alcoholic and hepatitis C cirrhotic livers: a quantitative targeted proteomics study. Drug Metab Dispos. 2018;46(7):943–52.
Yang LQ, Li SJ, Cao YF, Man XB, Yu WF, Wang HY, et al. Different alterations of cytochrome P450 3A4 isoform and its gene expression in livers of patients with chronic liver diseases. World J Gastroenterol. 2003;9(2):359–63.
MacGilchrist AJ, Birnie GG, Cook A, Scobie G, Murray T, Watkinson G, et al. Pharmacokinetics and pharmacodynamics of intravenous midazolam in patients with severe alcoholic cirrhosis. Gut. 1986;27(2):190–5.
McConnell JB, Curry SH, Davis M, Williams R. Clinical effects and metabolism of diazepam in patients with chronic liver disease. Clin Sci (Lond). 1982;63(1):75–80.
Acknowledgements
The authors thank the study team of the University Hospital Basel, Basel, Switzerland (Division of Clinical Pharmacology and Toxicology) with special thanks to the study nurses Claudia Bläsi and Joyce Jesus de Santos and to Beatrice Vetter for their valuable help during the preparation and conduct of the study and the study physicians Dr. Tanja Grandinetti, Dr. Florian Pfefferkorn, and Dr. Tim Bühler, Radka Štěpánová (Aixial s.r.o., Brno, Czech Republic) for statistical analysis of the clinical data, and Susanne Globig (Department of Preclinical Pharmacokinetics and Metabolism, Idorsia Pharmaceuticals Ltd, Allschwil, Switzerland) and Mark Enzler (Swiss BioQuant AG, Reinach, Switzerland) for the bioanalytical conduct. Last but not least, the authors thank the clinical research team, i.e., Alexandre Mathis, István Kerekes, Roberta Renai, Vincent Lemoine, Priska Kaufmann, and Pascale Gasser (Department of Clinical Pharmacology, Idorsia Pharmaceuticals Ltd, Allschwil, Switzerland).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was sponsored by Idorsia Pharmaceuticals Ltd, Allschwil, Switzerland.
Conflicts of Interest
Benjamin Berger, Jasper Dingemanse, Giancarlo Sabattini, Stéphane Delahaye, and Clemens Muehlan were full-time employees of Idorsia Pharmaceuticals Ltd during the conduct of the study. Jasper Dingemanse, Stéphane Delahaye, and Clemens Muehlan own stocks (options) of Idorsia Pharmaceuticals Ltd. Urs Duthaler and Stephan Krähenbühl were employees of the University Hospital Basel (Division of Clinical Pharmacology and Toxicology) and, in addition, Urs Duthaler was also employed by the University of Basel (Department of Biomedicine) during the conduct of the study. There are no other relationships or activities that could appear to have influenced the submitted work. The University Hospital Basel (Division of Clinical Pharmacology and Toxicology) received financial compensation for the clinical conduct.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study prior to any study-mandated procedure.
Consent for publication
Not available.
Availability of data and material
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Code availability
Not applicable.
Author contributions
JD and CM designed the study. UD and SK performed the assessments and collected the data. Data were analyzed by BB, JD, GS, SD, UD, CM, and SK. BB, JD, and SK wrote the manuscript. All authors reviewed and approved the final manuscript. The authors confirm that the principal investigator for this paper is SK and that he had direct clinical responsibility for the subjects.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Berger, B., Dingemanse, J., Sabattini, G. et al. Effect of Liver Cirrhosis on the Pharmacokinetics, Metabolism, and Tolerability of Daridorexant, A Novel Dual Orexin Receptor Antagonist. Clin Pharmacokinet 60, 1349–1360 (2021). https://doi.org/10.1007/s40262-021-01028-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01028-8