Abstract
Introduction
Lasmiditan is a selective serotonin (5-HT1F) receptor agonist approved in the US for the acute treatment ofmigraine in adults. This phase I, open-label, two-cohort study assessed the pharmacokinetics (PK), safety, and tolerability of lasmiditan in patients with migraine aged 6 to < 18 years.
Methods
Cohort 1 (15 to ≤ 40 kg) and Cohort 2 (> 40 to ≤ 55 kg) received single oral doses of lasmiditan (100 mg and 200 mg, respectively).Blood samples for the assessment of PK and safety parameters were collected over a 24-h period. Follow-up was approximately 14 days after dosing.
Results
Eighteen patients received lasmiditan (11 in Cohort 1, 7 in Cohort 2) and 17 patients completed the study. One patient in Cohort 2 discontinued due to adverse events. Plasma concentrations peaked at 1.5–2 h post dose and then declined, with a terminal half-life of approximately 4 h in both cohorts. While the exposure to lasmiditan was generally similar between cohorts, PK parameters, such as apparent total body clearance and volume of distribution, were greater for the 200 mg cohort relative to the 100 mg cohort. No deaths or serious adverse events were reported. The frequency and severity of adverse events (including somnolence, dizziness, and fatigue) were generally mild and similar to those in adult studies.
Conclusion:
The PK results support weight-based dosing of lasmiditan in pediatric patients with migraine and no new safety or tolerability issues were identified. These findings support further investigation of lasmiditan as a potential treatment in pediatric patients with migraine.
Clinical Trial Registration Numbers NCT03988088 and EMEA-002166-PIP01-17M02.
Similar content being viewed by others
This study was conducted to determine the pharmacokinetic, safety, and tolerability of lasmiditan in pediatric patients (aged 6 to < 18 years) with migraine following a single oral dose of lasmiditan. |
Pharmacokinetic results support weight-based dosing of lasmiditan in pediatric patients with migraine. |
Safety and tolerability of lasmiditan were similar to that previously observed in adult studies, with no new safety findings. |
1 Introduction
Migraine is a chronic neurological disease with an estimated prevalence of approximately 7.7% in children and adolescents, and a substantial impact on the lives of affected patients and their families [1, 2]. Current acute treatment of migraine in pediatric patients relies on drugs commonly used for the adult population, such as triptans, acetaminophen, and nonsteroidal anti-inflammatory drugs [3].
Lasmiditan is a selective serotonin (5-HT1F) receptor agonist (ditan) approved in the US at doses of 50, 100, and 200 mg for the acute treatment of migraine with or without aura in adults [4, 5]. Clinical studies in adults using randomized, double-blind, placebo-controlled designs have demonstrated that lasmiditan provides effective acute treatment for migraine as determined by freedom from headache pain and most bothersome symptoms at 2 h [6, 7]. Lasmiditan was generally well tolerated in adults, and the majority of adverse events were mild to moderate [8, 9].
This is the first study to determine the pharmacokinetics (PK), safety, and tolerability of a single oral dose of lasmiditan in pediatric patients (aged 6 to < 18 years) with migraine. The data from this study will be used to facilitate global pediatric clinical development of lasmiditan.
2 Methods
2.1 Patients
Patients between 6 and < 18 years of age with a history of migraine with or without aura (as defined by the Headache Classification Committee of the International Headache Society in The International Classification of Headache Disorders, 3rd edition [10]) were eligible to participate in the study. Patients were required to have a history of migraine attack for a minimum of 6 months, reporting ≥ 2 and ≤ 15 migraine attacks per month in the 2 months before screening. Patients had to weigh between 15 and 55 kg, be able to swallow a tablet, and be migraine-free at the time of predose assessments on the day of study drug administration.
Patients were excluded if they had a history or clinical evidence of cardiovascular disease, impaired hepatic function, or a psychiatric disorder that, in the opinion of the investigator, would have interfered with adherence to study requirements or safe participation in the study. Patients were also excluded if they had used opioid- or barbiturate-containing analgesics more than three times per month in more than 2 of the past 6 months, or if they had a history of substance use disorder. Female patients of child-bearing potential were required to have a negative pregnancy test. From approximately 48 h before dosing, and up to 24 h after dosing, patients were not allowed to consume xanthine- or caffeine-containing food or drink. Patients were also asked to avoid alcoholic beverages and tobacco products during this time period.
The study protocol was reviewed and approved by an Independent Ethics Committee and/or Institutional Review Board (Western Institutional Review Board in the US, and IHL Shinagawa East One Medical Clinic Instuitional Review Board in Japan) and the study was conducted in accordance with the Declaration of Helsinki, the International Conference for Harmonisation, and Good Clinical Practice guidelines. Written informed consent was provided by all parents or legal guardians, and informed assent was obtained from each patient, if capable, before starting any study procedure. The study was conducted at five clinical sites in the US (Perseverance Research Center, Premiere Research Institute at Palm Beach Neurology, and Meridien Research) and Japan (Kurume Clinical Pharmacology Clinic and Clinical Research Hospital Tokyo).
2.2 Study Design
This phase I, open-label, single-dose, cohort study (NCT03988088/EMEA-02166-PIP01-17-M02) was designed to determine the PK, safety, and tolerability of lasmiditan in pediatric patients with migraine following a single oral dose of lasmiditan. Based on the previous PK modeling of lasmiditan in adults and the expected body weight range of patients enrolled in this study, a weight-based dosing regimen was expected to elicit exposures in pediatric patients that would be comparable with adults receiving a single 200 mg dose (the highest approved dose in the US), assuming the relationship between body weight and lasmiditan PK was similar between adult and pediatric patients. An adult dose of 200 mg was targeted because lasmiditan PK are generally dose-linear in adults. In addition, this would enable the collection of initial safety data at the highest approved adult dose in a well-controlled setting, and lasmiditan had been previously well tolerated in adults up to 400 mg in previous studies (NCT03252015, NCT00883051, NCT03465436).
Screening occurred between 3 and 28 days before lasmiditan dosing. Patients reported to the clinic and received a single oral dose of lasmiditan in tablet formation based on baseline body weight. In Cohort 1, patients weighing 15 kg to ≤ 40 kg received a single 100 mg dose of lasmiditan, and in Cohort 2, patients weighing > 40 kg to ≤ 55 kg received a single 200 mg dose of lasmiditan. Patients were allowed to leave the clinic after completing the 12-h postdose procedures, but were required to return the next day for the 24-h postdose procedures. Patients returned for the follow-up assessments approximately 14 days after the study drug administration.
Patients fasted overnight and were allowed a light breakfast 2 h before dosing. At 1 h before and after dosing, water was restricted; only up to 240 mL of water given with the dose was allowed. Patients were provided a meal approximately 2 h after dosing. Furthermore, patients were not permitted to lie supine for 2 h after dosing, unless clinically indicated or as necessary to complete study procedures.
Lasmiditan 50 and 100 mg tablets were used in this study to achieve a single total dose of either 100 or 200 mg. The investigator determined which tablet strength to administer to achieve the appropriate dose based on the patient’s ability to swallow and their preference.
The study was powered to provide at least 80% coverage probability that the 95% confidence intervals for the geometric mean of these key PK parameters will be within 70% and 140%, based on the estimated PK variability in adults [11]. Efforts were made to enroll male and female patients of varying body weights across the weight range in the two cohorts.
2.3 Assessments
2.3.1 Pharmacokinetics
For lasmiditan concentration measurements, blood samples (each a maximum of 2 mL) were collected before dosing and at 0.5, 1, 1.5, 2, 3, 4, 8, 12, and 24 h after dosing. Lasmiditan plasma concentrations were analyzed at Covance Laboratories (Madison, WI, USA) using liquid chromatography with tandem mass spectrometry detection.
The PK parameter estimates for lasmiditan were calculated by standard noncompartmental methods of analysis from plasma lasmiditan concentrations, and were listed and summarized using descriptive statistics.
2.3.2 Pharmacokinetic Modeling
The PK modeling analysis was performed using first-order conditional estimation with interaction via NONMEM version 7.4.2 (ICON, Dublin, Ireland). Previous analyses conducted using lasmiditan PK data in adult subjects across multiple clinical studies have shown that the PK of lasmiditan was best described by a two-compartment model with a series of absorption transit compartments. The PK data in pediatric patients from this study were fit to this model, with body weight included a priori on apparent clearance and volume of distribution. In addition to body weight, a covariate analysis was performed using forward addition and backward elimination to identify other potential baseline demographic factors (i.e. age, sex, race, and ethnicity) and laboratory values (i.e. albumin, bilirubin, total protein, aspartate aminotransferase, alanine aminotransferase, and estimated glomerular filtration rate) that may be used to explain interindividual variability in PK parameters. Model evaluation was performed using goodness-of-fit plots, objective function mapping, and visual predictive check (VPC).
2.3.3 Pharmacokinetic Simulations
The pediatric PK model was used to perform PK simulations to determine an appropriate body weight cut-off, resulting in a dosing regimen that provided exposures in pediatric patients comparable with those of adults at safe and efficacious doses.
The PK profiles were simulated with 1000 pediatric patients for each cohort: low-weight cohort and high-weight cohort. Based on the clinical growth charts of children and adolescents (6 to < 18 years of age), the body weight of pediatric patients participating in the lasmiditan development program was expected to span from approximately 15 to 100 kg [12]. Individual body weights were sampled using a uniform distribution from 15 to 40 kg for Cohort 1 and from > 40 to 100 kg for Cohort 2. The high-weight cohort in the simulation (Cohort 2) received a single dose of lasmiditan 50, 100, or 200 mg, whereas the low-weight cohort (Cohort 1) received a single dose of lasmiditan 25, 50, or 100 mg. As a reference, the PK profiles for adult subjects receiving a single dose of lasmiditan 50, 100, or 200 mg were also simulated.
2.3.4 Safety
Safety measurements included incidence of adverse events, clinical laboratory test results (hematology, clinical chemistry), vital sign measurements, and 12-lead electrocardiogram results. A treatment-emergent adverse event (TEAE) was defined as an adverse event that occurs postdose or that is present before dosing and becomes more severe postdose. Suicidal ideation and/or behavior was assessed using the children’s version of the Columbia-Suicide Severity Rating Scale (C-SSRS) at screening, predose, 24 h postdose, and 14-day follow-up visit. Cognition was assessed using the Cogstate Pediatric Brief Battery (measuring processing speed, attention, visual learning, and working memory) at screening, predose, 2, 8, and 24 h postdose, and 14-day follow-up visit. Electrocardiograms were assessed locally at investigator sites, and abnormal electrocardiogram findings were reported as adverse events.
2.4 Statistical Methods
Descriptive statistics [e.g. N, mean, standard deviation (SD), percentage coefficient of variation, median, minimum, and maximum] were used to summarize plasma PK. Descriptive statistics were also used to summarize adverse events, markedly abnormal clinical laboratory test results (hematology and serum chemistry), and categorical shifts in vital signs (N and percentage of patients), C-SSRS, and Cogstate Pediatric Brief Battery results (N, mean, SD, median, minimum, and maximum). The number and percentage of patients who met the criteria for significant cognitive change from baseline were summarized, where a significant cognitive change was defined as a reliable change index score less than or equal to − 1.65 for at least two of the four cognitive tasks at a given time point.
3 Results
3.1 Patient Baseline Characteristics
A total of 20 patients were screened and 18 patients were enrolled in the study and subsequently received lasmiditan. The reasons for screen failure included not meeting the entrance criteria (one patient) and withdrawal of consent (one patient). Of the enrolled patients, 11 were in Cohort 1 (15 to ≤ 40 kg) and 7 were in Cohort 2 (> 40 to ≤ 55 kg). Seventeen patients completed the study; one patient in Cohort 2 discontinued the study 4 h after receiving lasmiditan due to adverse events.
Demographic information and baseline characteristics of the study patients are listed by patient in Table 1. The patients had a mean age of 11 years (range 6–17) and experienced an average of 3.83 migraine attacks per month (range 2–8) over the 2 months before screening. The majority of patients were female (66.7%). Of the 18 patients who received lasmiditan, 8 (44.4%) were Asian (5 in Cohort 1, 3 in Cohort 2).
3.2 Pharmacokinetic Data
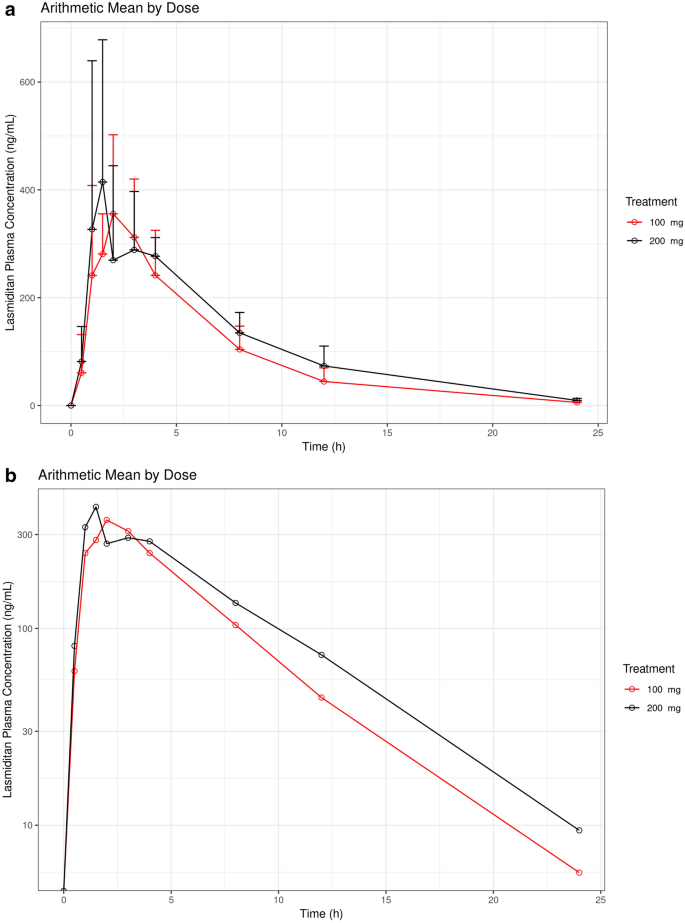
Following single-dose administration, the time course of lasmiditan concentrations was similar between pediatric patients receiving 100 mg (Cohort 1) and 200 mg (Cohort 2), with plasma concentrations peaking at a median time to reach the maximum (peak) plasma concentration following drug administration (tmax) value of 1.5–2 h postdose, and then declining, with a geometric mean terminal half-life value of approximately 4 h (Fig. 1). Exposure to lasmiditan expressed as the maximum (peak) plasma drug concentration (Cmax) and area under the plasma concentration curve (AUC) parameters were generally similar between the 2 cohorts (Table 2).
The geometric mean values for apparent total body clearance of drug from plasma after oral administration (CL/F) and apparent volume of distribution during the terminal phase after non-intravenous administration (Vz/F) were greater for the 200 mg cohort, relative to the 100 mg cohort (Table 2). This difference can be attributed to the difference in body weight between these two cohorts. Individuals with higher body weight generally exhibited higher CL/F and Vz/F values, consistent with allometric principles, which relate anatomy and physiology to body size [13].
3.3 Pharmacokinetic Modeling
Following oral administration, the lasmiditan concentration-time data were best described by a two-compartment model with first-order absorption and elimination. A series of transit compartments were used to describe temporal delays in absorption [14]. Body weight was included in this pediatric PK model on apparent clearance and volume of distribution parameters. This decision was supported by the observed trend between apparent clearance and volume of distribution versus body weight (Fig. 2). Interindividual variability was included (in exponential form) on CL/F, apparent central volume of distribution (V2/F), and mean transit time.
a Apparent clearance and b volume of distribution versus body weight in pediatric patients with migraine. Footnotes include slope (95% CI) for linear regression. CI confidence interval, CL/F apparent total body clearance of drug calculated after extravascular administration, Vz/F apparent volume of distribution during the terminal phase after extravascular administration
The parameters of the model were generally estimated with reasonable precision (≤ 40% standard error of estimate) and limited η-shrinkage (< 15%) (Table 3). The estimates for the body weight effect on apparent clearance and volume of distribution parameters were similar to the theoretical (allometric) values of 0.75 and 1, respectively. There was general concordance of the central tendency of the observed versus predicted profiles in the prediction-corrected VPC (Fig. 3). The observed concentrations fell within the 90% prediction interval computed with the PK model, with 9.55% of the data points falling outside the 5th and 95th percentiles. Thus, the VPC supports that the PK model adequately describes lasmiditan PK in pediatric patients.
None of the tested covariates significantly reduced the objective function value of the model. Consequently, no significant covariates were identified in the analysis after body weight was included in the model. This result was visually confirmed by the lack of evident trends between random effects (interindividual variability) and patient factors (electronic supplementary material 1).
3.4 Pharmacokinetic Model-Based Simulations
There was good concordance between the predicted PK profiles in pediatric and adult patients, with a high degree of overlap between the two populations, confirming that the 40 kg weight cut-off for lasmiditan dosing in pediatric patients implemented in this study was appropriate (Fig. 4). The slightly higher predicted exposure in pediatric patients, relative to that of adults, is not expected to be associated with an increase or change in the safety and tolerability of lasmiditan, based on observations in this small pediatric sample and the well-characterized safety and tolerability profile previously established in adults with doses up to 400 mg.
Predicted median and 90% prediction intervals for adult and pediatric concentrations versus time following a single dose of lasmiditan 25, 50, 100, or 200 mg. Solid red and black lines represent the medians in adult and pediatric patients, respectively; shaded region represents the 90% prediction interval. Low, mid, and high doses for adult or pediatric subjects weighing > 40 kg = 50, 100, and 200 mg for adults, respectively; low, mid, and high doses for pediatric subjects weighing ≤ 40 kg = 25, 50, and 100 mg for adults, respectively
3.5 Safety
During the study, eight patients (44.4%) experienced ten distinct types of TEAEs (Table 4). Most of the TEAEs were mild; events of fatigue (in two patients) and dizziness (in one patient) were reported as moderate in severity. There were no severe TEAEs. All TEAEs were considered possibly related to study drug by the investigator. One patient discontinued the study approximately 4 h after receiving lasmiditan due to TEAEs (i.e. lacrimation increased, ataxia, confusional state, disturbance in attention, dizziness, fatigue, irritability, and nausea). No deaths or serious adverse events were reported during the study.
The most frequently reported TEAEs (occurred in two or more patients) were dizziness, fatigue, somnolence, and ataxia. There was a higher incidence of patients with one or more TEAEs in Cohort 2 compared with Cohort 1 (Table 4).
Following lasmiditan dosing, a mean decrease in pulse of 6.4 bpm was observed at 1 h and returned to baseline between 4 and 12 h postdose. No patients met the criteria for categorical changes in blood pressure or clinical laboratory parameters based on predefined thresholds for clinically significant change. Furthermore, no patients reported suicidal ideation or behavior, and no abnormal electrocardiogram results were reported.
At 2 h postdose, seven patients (38.9%) met the criteria for significant change from baseline in cognitive performance (defined by reliable change index score less than or equal to − 1.65 for at least two tasks). Five patients were in Cohort 1, and 2 patients were in Cohort 2. One patient in Cohort 2 met the criteria at 8 h postdose but not at 2 h postdose, which is inconsistent with a drug-related effect. No patients met the criteria at 24 h postdose or at follow-up. Among the eight patients who met the criteria for significant change in cognitive performance at 2 h after dosing, the task most affected (all eight patients) was the Identification task, which measures attention. Four of the eight patients who met the criteria for significant change in cognitive performance had one or more TEAEs reported in temporal proximity to the cognitive change, which could have impacted test performance.
4 Discussion
Following single-dose administration, plasma concentrations peaked at a median tmax value of 1.5–2 h postdose and then declined, with a geometric mean terminal half-life of approximately 4 h. This is consistent with the PK previously observed in adults [15]. A relationship between PK parameters (i.e. CL/F and Vz/F) and body weight was observed whereby CL/F and Vz/F approximately doubled with a doubling of body weight, supporting a tiered dosing regimen for pediatric patients, based on body weight. This was reflected in the developed PK model describing the time course of lasmiditan concentrations in pediatric patients whereby body weight was included on apparent clearance and volume of distribution parameters of this model. No other significant covariates were identified after body weight was incorporated in the model. It is noted that the ability to identify covariates was limited, given the sample size of the study population. Subsequent model-based simulation results showed that a weight cut-off of 40 kg enables weight-based tiered dosing that yields pediatric exposures comparable with those of adults at efficacious doses.
Lasmiditan was well tolerated in pediatric migraine patients from the US and Japan when administered as a single 100 mg dose to patients 15 kg to ≤ 40 kg or as a single 200 mg dose to patients > 40 to ≤ 55 kg. No new safety findings were observed in the pediatric population receiving doses of lasmiditan comparable with the 200 mg exposure in adults. Similar to what has been observed in adult patients, TEAEs were consistent with the expected mechanism of action of lasmiditan and thus were predominantly neurologic, categorized as mild or moderately severe, and had limited duration. Some patients experienced changes in cognitive test performance at 2 h that were resolved at 8 h after dosing, consistent with cognitive data observed in driving simulation studies in healthy adults [16].
5 Conclusions
The PK results support weight-based dosing of lasmiditan in pediatric patients with migraine aged 6 to < 18 years. Safety and tolerability were similar to that observed in adult studies; no new safety issues were identified in pediatric patients with migraine. These findings support further investigation of lasmiditan as a potential treatment in pediatric patients.
References
Abu-Arafeh I, Razak S, Sivaraman B, Graham C. Prevalence of headache and migraine in children and adolescents: a systematic review of population-based studies. Dev Med Child Neurol. 2010;52:1088–97.
Kernick D, Campbell J. Measuring the impact of headache in children: a critical review of the literature. Cephalalgia. 2009;29:3–16.
Barbanti P, Grazzi L, Egeo G. Pharmacotherapy for acute migraines in children and adolescents. Expert Opin Pharmacother. 2019;20:455–63.
Nelson DL, Phebus LA, Johnson KW, Wainscott DB, Cohen ML, Calligaro DO, et al. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia. 2010;30:1159–69.
Reyvow [prescribing information]. Eli Lilly and Company; 2019. https://pi.lilly.com/us/reyvow-uspi.pdf. Accessed 4 Jun 2020.
Capi M, de Andrés F, Lionetto L, Gentile G, Cipolla F, Negro A, Borro M, Martelletti P, Curto M. Lasmiditan for the treatment of migraine. Expert Opin Invest Drugs. 2017;26(2):227–34.
Curto M, Cipolla F, Cisale GY, Capi M, Spuntarelli V, Guglielmetti M, et al. Profiling lasmiditan as a treatment option for migraine. Expert Opin Pharmacother. 2020;21(2):147–53.
Kuca B, Silberstein SD, Wietecha L, Berg PH, Dozier G, Lipton RB, COL MIG-301 Study Group. Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology. 2018;91:e2222–32.
Goadsby PJ, Wietecha LA, Dennehy EB, Kuca B, Case MG, Aurora SK, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142:1894–904.
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. https://www.ichd-3.org/wp-content/uploads/2018/01/The-International-Classification-of-Headache-Disorders-3rd-Edition-2018.pdf. Accessed 4 Jun 2020.
Wang Y, Jadhav PR, Lala M, Gobburu JV. Clarification on precision criteria to derive sample size when designing pediatric pharmacokinetic studies. J Clin Pharmacol. 2012;52(10):1601–6.
Centers for Disease Control and Prevention. Data Table of Weight-for-age Charts. https://www.cdc.gov/growthcharts/html_charts/wtage.htm#males (males) and https://www.cdc.gov/growthcharts/html_charts/wtage.htm#females (females). Accessed 4 Jun 2020.
Liu T, Ghafoori P, Gobburu JVS. Allometry is a reasonable choice in pediatric drug development. J Clin Pharmacol. 2017;57:469–75.
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34(5):711–26.
Tsai M, Case M, Ardayfio P, Hochstetler H, Willbraham D. Effects of lasmiditan on cardiovascular parameters and pharmacokinetics in healthy subjects receiving oral doses of propranolol. Clin Pharmacol Drug Dev. 2020;9:629–38.
Pearlman EM, Wilbraham D, Dennehy EB, Berg PH, Tsai M, Doty EG, Kay GG. Effects of lasmiditan on simulated driving performance: results of two randomized, blinded, crossover studies with placebo and active controls. Hum Psychopharmacol. 2020;35(5):e2732. https://doi.org/10.1002/hup.2732.
Acknowledgements
This work was sponsored by Eli Lilly and Company. The authors thank the trial investigators, trial staff, and trial patients for their contributions, and would also like to express their gratitude to Elizabeth Flate and Kent Steinriede (Syneos employees contracted by Eli Lilly and Company) and Paula Hauck (employee of Eli Lilly and Company) for medical writing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Eli Lilly and Company.
Conflict of interest
Max Tsai, Emel Serap Monkul Nery, Lisa Kerr, Rashna Khanna, Mika Komori, Ellen B. Dennehy, and Darren Wilbraham are employees and minor stock holders of Eli Lilly and Company. Paul Winner has received research grants, an honorarium, and travel reimbursements from Eli Lilly and Company.
Ethics approval
All clinical studies described herein have been approved by the appropriate Ethics Committees of all participating centers and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
In this study, a legal representative had to sign a statement of informed consent for a child to participate in the study. In addition to informed consent given by the legal representative, the child may have been required to give documented assent, if capable.
Consent for publication
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available because participants of this study did not agree for their data to be shared publicly.
Code availability
Not applicable.
Author contributions
All authors participated in the interpretation of the study results and in the drafting, critical revision, and approval of the final version of the manuscript. MT, ESMN, LK, RK, MK, EBD, and DW were involved in the study design, data collection, and data analyses. MT, EBD, RK, MK, and PW were responsible for study monitoring, and MT and LK were responsible for data analysis and plotting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tsai, M., Nery, E.S.M., Kerr, L. et al. Pharmacokinetics, Safety, and Tolerability of Lasmiditan in Pediatric Patients with Migraine. Clin Pharmacokinet 60, 819–828 (2021). https://doi.org/10.1007/s40262-020-00966-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00966-z