Abstract
Objectives
In this in-silico study, we investigate the clinical utility of target-controlled infusion for antibiotic dosing in an intensive care unit setting using vancomycin as a model compound. We compared target-controlled infusion and adaptive target-controlled infusion, which combines target-controlled infusion with data from therapeutic drug monitoring, with conventional (therapeutic drug monitoring-based) vancomycin dosing strategies.
Methods
A clinical trial simulation was conducted. This simulation was based on a comprehensive database of clinical records of intensive care unit patients and a systematic review of currently available population-pharmacokinetic models for vancomycin in intensive care unit patients. Dosing strategies were compared in terms of the probability of achieving efficacious concentrations as well as the potential for inducing toxicity.
Results
Adaptive target-controlled infusion outperforms rule-based dosing guidelines for vancomycin. In the first 48 h of treatment, the probability of target attainment is significantly higher for adaptive target-controlled infusion than for the second-best method (Cristallini). Probability of target attainments of 54 and 72% and 47 and 59% for both methods after 24 and 48 h, respectively. Compared to the Cristallini method, which is characterized by a probability of attaining concentrations above 30 mg.L−1 > 65% in the first few hours of treatment, adaptive target-controlled infusion shows negligible time at risk and a probability of attaining concentrations above 30 mg.L−1 not exceeding 25%. Finally, in contrast to the other methods, the performance of target-controlled infusion is consistent across subgroups within the population.
Conclusions
Our study shows that adaptive target-controlled infusion has the potential to become a practical tool for patient-tailored antibiotic dosing in the intensive care unit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this proof-of-principle simulation study using vancomycin as a model antibiotic for intensive care unit patients, we showed for the first time that adaptive target-controlled infusion could achieve a higher degree of target attainment, achieve the target faster, and minimize potential toxic overshoots compared with conventional vancomycin dosing guidelines. |
Clinical pharmacologists involved in personalized antibiotic dosing should know that the (adaptive) target-controlled infusion technology has a substantial track record and user base in anesthesia and could be very useful for antibiotic dosing. |
1 Introduction
Target-controlled infusion (TCI) is a technique of infusing intravenous drugs to achieve a user-defined predicted (‘target’) drug concentration in a specific body compartment or tissue of interest [1]. Target-controlled infusion systems are based on population-pharmacokinetic (PopPK) [pharmacodynamics] models. For each individual patient, the optimal infusion rate is calculated based on the patient’s covariates (e.g. age, weight, estimated creatinine clearance) and the user-defined target (plasma) concentration. For the open-loop TCI systems, the infusion scheme is static, whereas for the closed-loop TCI systems, on-line feedback from measured variables (e.g., processed electroencephalography variables [2], blood pressure [3], intermittently or continuously measured blood concentrations [4], exhaled drug concentrations [5]) is used to adjust the infusion rates continuously [6]. Target-controlled infusion systems have been used in the field of anesthesia for research and clinical purposes for over two decades with an estimated 2.6 million patients in Europe receiving one or more drugs by TCI per annum [7].
Compared to manually controlled infusions, theoretically TCI systems have the advantage of achieving the target (plasma) concentration faster without a significant overshoot of the target. This feature would be particularly useful in the setting of antibiotic dosing where early and appropriate therapy are likely associated with improved patient survival [8,9,10]. Although PopPK models are available for antibiotics, to our knowledge, no attempts have been made to evaluate TCI systems in the field of antimicrobial therapy.
Optimized dosing of antibiotics is usually based on nomograms which define an initial dosing regimen based on patient covariates. However, in the critically ill, these fixed dosing regimens frequently result in a significant proportion of patients not achieving the therapeutic target [11]. For vancomycin, several studies demonstrated insufficient target attainment in the first 3 days of therapy [12,13,14,15].
Therefore, in vulnerable patient populations these nomograms are sometimes combined with therapeutic drug monitoring (TDM). This TDM-guided treatment individualization has been shown to increase clinical efficacy for vancomycin [16] and reduce mortality rates during aminoglycoside therapy [17].
Target-controlled infusion systems could replace these nomogram-based dosing regimens, as it is known from anesthesiology that TCI is generally more accurate compared with manually controlled infusions [7]. At the same time, closed-loop control of the TCI systems based on TDM data could be accomplished through the use of Bayesian statistics.
At present, Bayesian forecasting is used by different TDM software such as DoseMe® (DoseMe Pty Ltd., Brisbane, QLD, Australia), InsightRx® (Insight Rx Inc., San Francisco, CA, USA), and MwPharm® (Mediware a.s., Prague, Czech Republic). Some of these platforms require trained personnel to handle the software and/or interpret the output, thereby potentially hindering their widespread use. Moreover, for the dosage adjustments, these systems depend on humans, whose knowledge, judgment, and skill set may not entirely align with the model-informed precision dosing paradigm [18, 19]. In contrast, TCI systems provide direct communication with the infusion pump, allowing fast and optimal individualization of the patient’s infusion regimen.
In this simulation study, we investigated whether TCI and/or a TDM-based closed-loop TCI system [named ‘adaptive TCI (aTCI)’ hereafter] would be useful for dosing antibiotics in intensive care unit patients. We compare the performance of this system to currently used (rule-based) dosing guidelines. Vancomycin was chosen as a model drug owing to (1) the availability in the literature of different PopPK models, (2) the plethora of existing dosing guidelines, (3) the frequent use of continuous infusion for administering vancomycin [20], and (4) the widespread use of TDM to monitor and adjust vancomycin dosing regimens.
2 Materials and Methods
2.1 Sampling of Virtual Intensive Care Unit Patients and Generation of Vancomycin Pharmacokinetic Parameters
Patient records were randomly sampled from the MIMIC-III database [21, 22]. This database comprises comprehensive clinical data of > 10,000 patients from different hospitals/intensive care units (ICUs). Based on the covariates within these records (age, weight, height, sex, serum creatinine), vancomycin pharmacokinetic (PK) parameters were generated, and different PopPK models were used.
These models were identified though a PubMed search (until 27 September, 2017) using search terms: “vancomycin AND population AND pharmacokinetics [Title/Abstract]” OR “vancomycin AND pharmacokinetics AND critically ill [Title/Abstract]”. We restricted the search to vancomycin models for adult patients only, thereby excluding patients undergoing Continuous renal replacement therapy, hemofiltration, and Extracorporeal membrane oxygenation. In addition, models were retained only if they were built on a dataset with a sample size > 100 subjects and if the model’s performance was externally validated (i.e., prospective validation or validation using a different cohort from the same trial).
The 10,000 sampled subjects were randomly allocated to one of the PopPK models to generate vancomycin PK parameters. The weight attributed to the PopPK models during the random allocation was proportional to the square root of the size of the model building datasets. Vancomycin PK parameters were generated taking into account the published covariate models as well as the unknown (exponential) inter-individual variability around the typical population parameters. Parameter uncertainty was not taken into account.
2.2 Vancomycin Dosing Guidelines
A PubMed search (until 27 September, 2017) was conducted using the search term: “vancomycin AND infusion AND critically ill [Title/Abstract]”. References of retrieved articles were screened for additional references. Only dosing guidelines developed for continuous infusion were considered. We specifically looked for publications that included recommendations for a loading dose and defined rules to handle TDM data throughout therapy.
2.3 Target Controlled Infusion and Bayesian Feedback
We choose the Thomson model [23] for implementation in our (virtual) TCI system. This choice was based on the Thomson model [23] being based on the biggest dataset to date, the extensive validation of the model, and the broad range of patient characteristics that were included. Prior to the availability of TDM data, the TCI system uses the covariate relationships contained in the Thomson model [23] to calculate the optimal infusion regimen to approach the target as fast as possible (these static infusion regimens are hereafter referred to as ‘TCI’). The calculations are based on the numeric approximation of the two-compartmental model as described by Shafer et al. [24]. To avoid safety issues such as the “red man syndrome” [25] owing to high infusion rates that are too high and in line with the summary of product characteristics [26] for vancomycin, the maximum infusion rate was set to 0.6 g.h−1.
After TDM data become available, the empirical Bayes estimates (EBEs) for the PK parameters are found by minimizing the objective function (Eq. 1). For our simulation study, the EBEs were derived though NONMEM (Version 7.3; Icon Development Solutions, Hanover, MD, USA). However, in a TCI system, this is generally accomplished through minimization of the objective function with respect to the EBEs using Powell’s conjugate direction method [27].
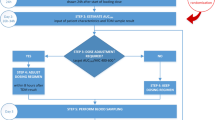
In this equation, the sum of squared distances between the measured vancomycin concentrations (Cobs) and the predictions (Cpred) and the squared distances between the typical PK parameters (TVV1, TVV2, TVCL, and TVQ2) and the EBEs are weighted by their respective variance terms (RUV, ωV1, ωV2, ωCL, and ωQ2). Both the typical PK parameters and the associated variance terms were calculated from the Thomson model. By minimizing this function, the individual’s PK parameters (EBEs) that best describe the observations are found. Subsequently, these EBEs are used to re-calculate the optimal infusion regimen (or the amount of time to stop the infusion) to attain the user-defined target as fast as possible (this approach is hereafter referred to as ‘aTCI’). A graphical representation of this process is shown in Fig. S1 of the ESM.
2.4 Clinical Trial Simulation
An overview of the entire simulation workflow is shown in Fig. S2 of the ESM. Virtual subjects were included in the trial randomly between 00:00 and 23:59 on day 1 of the study. Therapeutic drug monitoring samples were simulated at 08:00 at days 2, 3, up to day 7. Therapeutic drug monitoring data were used to individualize the vancomycin dosing regimen only if the subjects had been taking vancomycin for over 8 h. This results in the first TDM sample being taken between 8 and 32 h after initiation of the treatment.
The entire population of virtual subjects was used to evaluate the different dosing methods. All conditions, time of entry into the trial, and time of first TDM sample were identical across the different dosing methods. For each subject, the initial vancomycin dosing regimen was calculated and the concentration–time profile generated up to the time of the first TDM sample. The simulated TDM sample was based on the PopPK model used to generate the subject’s vancomycin PK parameters, taking into account the model’s associated residual unexplained variability (i.e., a ‘noisy’ TDM measurement was simulated instead of the ‘true’ underlying concentration). Based on the TDM sample, the dosing regimen was adjusted. Afterwards the concentration–time profile up to the next TDM sample was simulated, again using the PopPK model used to generate the subject’s vancomycin PK parameters. This process was repeated up to 168 h after treatment initiation.
As we focused on the comparison between dosing regimens to steer the ‘true’ individual concentrations towards the target exposure, the subject’s ‘true’ individual concentration–time profile (i.e., without residual unexplained variability) was used to calculate the performance metrics. Based on these concentration–time profiles, time on target (TOT) was calculated for each subject. It was expressed as the percentage of the treatment period for which the simulated concentrations were within the target exposure. The attainment of excessive vancomycin concentration was similarly expressed as the proportion of the treatment period for which the simulated vancomycin concentrations were above 30 mg.L−1. For the remainder of the paper, we refer to the latter as ‘time at risk’ (TAR).
The probabilities on the population level of attaining target vancomycin concentrations (PTA) or attaining concentrations above 30 mg.L−1 (PTOX) were also derived. For this, for each simulated time point, the proportion of individuals with concentrations within target exposures or above 30 mg.L−1 were calculated. Finally, we calculated the time to steady-state target attainment (TTT) for all simulated scenarios. This was defined as the time after which simulated concentrations remained within the target exposure (i.e., settling time for the dosing procedure).
All simulations were conducted in R® (R Foundation for Statistical Computing, Vienna, Austria) using packages deSolve [28] and devtools [29].
3 Results
3.1 Virtual Subjects and Clinical Trial Simulation
Our PubMed search identified seven PopPK models [23, 29,30,31,32,33,34] and four dosing guidelines [13, 35,36,37] that fulfilled all our criteria. An overview of the PopPK models and the dosing guidelines are given in Tables 1 and 2, respectively. As a benchmark for the effectiveness of TDM-based dosing vs. naïve dosing, we included one frequently referenced dosing guideline that does not include rules for handling TDM data [38].
An overview of the characteristics of the virtual subjects is shown in Table S1 of the ESM. The median time for the first TDM sample was approximately 20 h. Vancomycin PK parameters varied considerably between the different groups.
Figure 1 shows the time course of the ‘true’ individual vancomycin concentrations as well as the simulated TDM samples and ensuing dosage adjustments for a representative subject of the Thomson group. Figures S3–8 of the ESM show the simulations for a subject from the other groups.
Simulated ‘true’ individual concentrations and therapeutic drug monitoring samples for a representative individual from the Thomson group. The horizontal red lines show the boundaries for the targeted vancomycin exposure according to the different dosing methods. aTCI adaptive target-controlled infusion, TCI target-controlled infusion
3.2 Target Attainment, Time to Steady-State Target Attainment, and Time at Risk
Figure 2 shows the distribution of TOT and TAR for the different dosing methods. A naïve application of TCI that does not use TDM-based Bayesian feedback (TCI) was also evaluated. Time on target is highest for aTCI with an inter-quartile range from 53 to 91%. In comparison, TOT for the other methods is lower. The methods that do not use TDM to individualize treatment show a bimodal distribution with a significant proportion of the population having very low TOT (e.g., 50% of subjects in the Pea and TCI group have a TOT below 25 and 22%, respectively). The median TAR is markedly higher for the Ampe (23%) and Cristallini (5.5%) method compared with aTCI (0.0%). The method of Pea has the lowest TAR owing to the fact that a lower Css is targeted, i.e., 20 mg.L−1 instead of 25 mg.L−1.
Violin plot showing the distribution of time on target and time at risk for the different simulated dosing guidelines. For clarity, individual estimates (circles) are shown for only 2000 virtual subjects out of 10,000. The 25th, 50th, and 75th percentiles of the empirical cumulative distribution function are shown with vertical lines in the violin plots. These were estimated on all the data. The shape of the violin shows the empirical cumulative density curve. Time on target and time at risk are calculated as the proportion of the treatment when vancomycin concentrations are within the target exposure or above 30 mg.L−1. Target vancomycin concentrations are between 20 and 30 mg.L−1 for all methods except Pea where concentrations between 15 and 25 mg.L−1 are targeted. aTCI adaptive target-controlled infusion, TCI target-controlled infusion
The methods are compared in more detail in Table 3. Based on all data, aTCI has the highest median TOT (77.4%). The Cristallini method, which ranks second, performs less well (71.6%). At the same time, the median TAR and median TTT is lowest in the aTCI group. The proportion of virtual subjects not attaining the target after 7 days of treatment ranges between > 60% for the non-individualized methods and 16% for the Cristallini method. At 25%, aTCI performs similar to the methods of Saugel (22%), Ampe (25%), and Ocampos-Martinez (24%).
As our aTCI system is based on the Thomson model and this model was also used to generate vancomycin PK parameters for 20% of the virtual subjects, we wanted to evaluate whether this confounded our results. Therefore, a subgroup analysis was performed across the different PopPK models. As seen from Table S2 of the ESM, aTCI has the highest median TOT, the lowest median TAR, and the lowest TTT in respectively five, six, and five groups out of seven.
Finally, we wanted to evaluate the performance of the methods across different subgroups within the simulated population. The results were stratified with respect to body weight or estimated renal function. In general, the performance of aTCI is similar across subgroups. Moreover, for each of the subgroups, aTCI has the highest median TOT. For the other methods, TOT decreased and TTT increased significantly in subject with low body weight (< 58 kg) and subjects with low renal function (< 60 mL.min−1). Subjects with high renal function (> 120 mL.min−1) and high body weight (> 110 kg) appear to be at risk for excessive vancomycin concentrations when dosed using the Cristallini method (9.7%) and the Ampe method (24%).
3.3 Probability of Target Attainment and Probability of Attainment of Excessive Vancomycin Concentrations within the Population
Figures 3 and 4 show the PTA and the probability of attainment of excessive vancomycin concentrations (PTOX) within the population as a function of time. In the first few hours, TCI, aTCI, and the method of Pea attain PTAs above 50%. However, for both TCI and the method of Pea, PTAs worsen and beyond 48 h their PTAs are lowest. The PTAs for the other methods first decrease (slightly) before starting to increase owing to the effect of TDM-based treatment individualization. The increase in PTA is fastest in the aTCI group with the maximum PTA of 77% being reached by 51 h. The method of Cristallini achieves a maximum PTA of approximately 84%, but requires 109 h to do so. The methods of Ocampos-Martinez, Saugel, and Ampe approach maximum PTAs of 78, 78, and 78% by 123, 125, and 124 h, respectively.
Looking at the possibility of attaining excessive vancomycin concentrations (i.e., Cp > 30 mg.L−1), the method of Cristallini stands out with a PTOX > 65% in the first few hours. During this period, the median predicted vancomycin concentration is 66 mg.mL−1. This PTOX prevails up to approximately 20 h. The second highest ranking method was Ampe with a PTOX and associated median vancomycin concentration of 38% and 33 mg.L−1, respectively. After 24 h, PTOX is similar across methods ranging between 15 and 28%. The only exception is the method of Pea (6%), which targets a lower Css compared with the other methods.
4 Discussion
Through a clinical trial simulation, we showed that aTCI outperforms rule-based dosing guidelines for vancomycin. With a PTA of 70% reached within 42 h, aTCI is much faster than the second best method (Cristallini), which requires 70 h to achieve a PTA of 70%. Consequently, in the first 48 h of treatment, PTAs are significantly higher for aTCI than for the Cristallini method (PTAs of 54 and 72% and 47 and 59% for both methods after 24 and 48 h, respectively). Compared with the Cristallini method, which is characterized by a PTOX > 65% in the first few hours of treatment, aTCI shows negligible time at risk and a PTOX not exceeding 25%. Finally, in contrast to the other methods, the performance of aTCI is consistent across subgroups within the population.
Our approach of combining physiological data from an ICU database with different published PopPK models resulted in the generation of a very heterogeneous virtual population. However, looking at the publications describing the dosing guidelines, it appears that our simulation reproduces some features of those clinical trials. Ocampos-Martinez et al. [13] showed that the PTA is approximately 40, 50, and 70% 1, 2, and 3 days after treatment initiation. Our simulations showed a PTA of 41, 55, and 64% after 1, 2, and 3 days of treatment. Furthermore, Cristallini et al. [36] showed that 86, 28, and 17% of patients attained excessive vancomycin concentrations after 4, 12, and 24 h, respectively. This is also in good agreement with the simulated dynamics in our simulation where 68, 32, and 20% of patients attained excessive vancomycin concentrations 4, 12, and 24 h after treatment initiation. Overall, these agreements between the simulated and reported performance characteristics inspire confidence in the conclusions drawn from our simulation study.
There are limitations to our proposed methodology. First, in our simulations, we assumed that intra-individual variability is negligible. From the literature, it seems that indeed for vancomycin intra-individual variability has little clinical relevance [39]. Nevertheless, for other antibiotics it might be necessary to consider intra-individual variability as it is not entirely clear whether this would equally impact the performance of the different dosing methods. Second, the performance metrics used to compare the methods were based on the interpretation of the authors of the dosing guidelines [13, 36, 37] and align with recent work by Zasowski et al. [40] However, these might be judged too high or too low by others (see Filippone et al. [41] for a detailed discussion of vancomycin-induced nephrotoxicity).
The lower steady-state PTA for aTCI is indicative of a mismatch between the clearance model in the TCI system and the clearance models that were used for the trial simulation. By combining seven PopPK models for our simulations we are over-emphasizing this mismatch. This led to a very conservative yet robust estimate of the performance characteristics of the aTCI system. In our opinion, this inspires confidence with respect to the generalizability of our conclusions. Furthermore, in clinical practice, it is likely that when a patient’s TDM samples are consequently off-target the plasma target is changed to overcome the imprecision of the TCI system. As such, we feel that in clinical practice, steady-state PTA will likely be higher than what is seen from our simulations.
At present, there is no consensus on the ‘best’ vancomycin model and associated covariates. A pooled analysis of the data could alleviate some of this uncertainty. In line with what was done for propofol by Eleveld et al. [42] this pooled analysis could form the basis for a “general purpose vancomycin PK model”. Such a model would dramatically increase the performance of an aTCI system. Indeed, as seen from Table 3, when aTCI is based on a model that adequately describes the PKs within the population (as does our aTCI system for the Thomson group), TOT becomes very high with minimal time to steady-state target attainment and a minimal proportion of subjects not attaining the target.
Adapted TCI uses Bayesian forecasting to individualize dosing regimens based on TDM data. Approaches using more traditional PK modeling software have been described by others in the field of antibiotic [43, 44], antifungal [45, 46] and antiviral therapy [47]. Although aTCI and more traditional TDM software use a similar theoretical framework for dosage individualisation, aTCI has some advantages. First, as shown in this study, target attainment is fast with minimum overshoot of the target concentration. Second, in contrast to the other methods, dosage adjustments with aTCI are continuous and are not limited to discrete changes in infusion rates. We hypothesize that this means dosage individualization would be more precise with aTCI as compared to other methods. Evidently, this remains to be confirmed in future prospective evaluations. Finally, aTCI has the practicable advantage of flexibility, i.e., it facilitates the use of a patient-tailored target.
It is noteworthy to appreciate that TCI has been around for decades and is currently used in operating rooms and ICUs across the world (except for USA). In our opinion, this poses the opportunity to introduce model-informed personalized dosing of antibiotics via a dosing device that is already somewhat familiar to most practitioners. We believe that this familiarity could facilitate the widespread implementation of model-informed precision dosing.
5 Conclusions
Through our clinical trial simulation, we showed that the performance of aTCI is superior compared to currently used dosing guidelines. In an era where model-informed precision dosing is gaining popularity and treatment individualization is increasingly used in conjunction with TDM, we feel that aTCI would be a useful addition to the already available set of tools for patient-tailored antibiotic dosing.
References
Struys MM, De Smet T, Glen JI, Vereecke HE, Absalom AR, Schnider TW. The history of target-controlled infusion. Anesth Analg. 2016;122(1):56–69.
De Smet T, Struys MM, Neckebroek MM, Van den Hauwe K, Bonte S, Mortier EP. The accuracy and clinical feasibility of a new Bayesian-based closed-loop control system for propofol administration using the bispectral index as a controlled variable. Anesth Analg. 2008;107(4):1200–10.
Luginbuhl M, Bieniok C, Leibundgut D, Wymann R, Gentilini A, Schnider TW. Closed-loop control of mean arterial blood pressure during surgery with alfentanil: clinical evaluation of a novel model-based predictive controller. Anesthesiology. 2006;105(3):462–70.
van den Berg JP, Eleveld DJ, De Smet T, van den Heerik AVM, van Amsterdam K, Lichtenbelt BJ, et al. Influence of Bayesian optimization on the performance of propofol target-controlled infusion. Br J Anaesth. 2017;119(5):918–27.
Colin P, Eleveld DJ, van den Berg JP, Vereecke HE, Struys MM, Schelling G, et al. Propofol breath monitoring as a potential tool to improve the prediction of intraoperative plasma concentrations. Clin Pharmacokinet. 2016;55(7):849–59.
Absalom A, Struys M. An overview of TCI and TIVA. Belgium: Academia Press; 2007.
Absalom AR, Glen JI, Zwart GJ, Schnider TW, Struys MM. Target-controlled infusion: a mature technology. Anesth Analg. 2016;122(1):70–8.
Roberts JA, Kumar A, Lipman J. Right dose, right now: customized drug dosing in the critically ill. Crit Care Med. 2017;45(2):331–6.
Zelenitsky S, Rubinstein E, Ariano R, Iacovides H, Dodek P, Mirzanejad Y, et al. Vancomycin pharmacodynamics and survival in patients with methicillin-resistant Staphylococcus aureus-associated septic shock. Int J Antimicrob Agents. 2013;41(3):255–60.
Moore RD, Smith CR, Lietman PS. The association of aminoglycoside plasma levels with mortality in patients with Gram-negative bacteremia. J Infect Dis. 1984;149(3):443–8.
Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: Defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–83.
Baptista JP, Sousa E, Martins PJ, Pimentel JM. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39(5):420–3.
Ocampos-Martinez E, Penaccini L, Scolletta S, Abdelhadii A, Devigili A, Cianferoni S, et al. Determinants of early inadequate vancomycin concentrations during continuous infusion in septic patients. Int J Antimicrob Agents. 2012;39(4):332–7.
Li J, Udy AA, Kirkpatrick CM, Lipman J, Roberts JA. Improving vancomycin prescription in critical illness through a drug use evaluation process: a weight-based dosing intervention study. Int J Antimicrob Agents. 2012;39(1):69–72.
Nunn MO, Corallo CE, Aubron C, Poole S, Dooley MJ, Cheng AC. Vancomycin dosing: assessment of time to therapeutic concentration and predictive accuracy of pharmacokinetic modeling software. Ann Pharmacother. 2011;45(6):757–63.
Ye Z-K, Tang H-L, Zhai S-D. Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis. PLoS One. 2013;8(10):e77169.
Whipple JK, Ausman RK, Franson T, Quebbeman EJ. Effect of individualized pharmacokinetic dosing on patient outcome. Crit Care Med. 1991;19(12):1480–5.
Tucker GT. Personalized drug dosage: closing the loop. Pharm Res. 2017;34(8):1539–43.
Darwich AS, Ogungbenro K, Vinks AA, Powell JR, Reny JL, Marsousi N, et al. Why has model-informed precision dosing not yet become common clinical reality? Lessons from the past and a roadmap for the future. Clin Pharmacol Ther. 2017;101(5):646–56.
Blot S, Koulenti D, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. Does contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI study. Crit Care. 2014;18(3):R99.
Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035.
Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–20.
Thomson AH, Staatz CE, Tobin CM, Gall M, Lovering AM. Development and evaluation of vancomycin dosage guidelines designed to achieve new target concentrations. J Antimicrob Chemother. 2009;63(5):1050–7.
Shafer SL, Siegel LC, Cooke JE, Scott JC. Testing computer-controlled infusion pumps by simulation. Anesthesiology. 1988;68(2):261–6.
Sivagnanam S, Deleu D. Red man syndrome. Crit Care. 2002;7(2):119.
Vancomycin: summary of product characteristics. Available from: http://bijsluiters.fagg-afmps.be/. [Accessed 28 Feb 2018].
Powell MJD. An efficient method for finding the minimum of a function of several variables without calculating derivatives. Computer J. 1964;7(2):155–62.
Soetaert K, Petzoldt T, Setzer RW. Solving differential equations in R: Package deSolve. J. Stat. Softw. 2010;33(9):25.
Buelga DS, de Gatta MD, Herrera EV, Dominguez-Gil A, Garcia MJ. Population pharmacokinetic analysis of vancomycin in patients with hematological malignancies. Antimicrob Agents Chemother. 2005;49(12):4934–41.
Sanchez JL, Dominguez AR, Lane JR, Anderson PO, Capparelli EV, Cornejo-Bravo JM. Population pharmacokinetics of vancomycin in adult and geriatric patients: comparison of eleven approaches. Int J Clin Pharmacol Ther. 2010;48(8):525–33.
Revilla N, Martin-Suarez A, Perez MP, Gonzalez FM, de Gatta Fernandez, Mdel M. Vancomycin dosing assessment in intensive care unit patients based on a population pharmacokinetic/pharmacodynamic simulation. Br J Clin Pharmacol. 2010;70(2):201–12.
Lin WW, Wu W, Jiao Z, Lin RF, Jiang CZ, Huang PF, et al. Population pharmacokinetics of vancomycin in adult Chinese patients with post-craniotomy meningitis and its application in individualised dosage regimens. Eur J Clin Pharmacol. 2016;72(1):29–37.
Purwonugroho TA, Chulavatnatol S, Preechagoon Y, Chindavijak B, Malathum K, Bunuparadah P. Population pharmacokinetics of vancomycin in Thai patients. ScientificWorldJournal. 2012;2012:762649.
Medellin-Garibay SE, Ortiz-Martin B, Rueda-Naharro A, Garcia B, Romano-Moreno S, Barcia E. Pharmacokinetics of vancomycin and dosing recommendations for trauma patients. J Antimicrob Chemother. 2016;71(2):471–9.
Ampe E, Delaere B, Hecq JD, Tulkens PM, Glupczynski Y. Implementation of a protocol for administration of vancomycin by continuous infusion: pharmacokinetic, pharmacodynamic and toxicological aspects. Int J Antimicrob Agents. 2013;41(5):439–46.
Cristallini S, Hites M, Kabtouri H, Roberts JA, Beumier M, Cotton F, et al. New regimen for continuous infusion of vancomycin in critically ill patients. Antimicrob Agents Chemother. 2016;60(8):4750–6.
Saugel B, Gramm C, Wagner JY, Messer M, Lahmer T, Meidert AS, et al. Evaluation of a dosing regimen for continuous vancomycin infusion in critically ill patients: an observational study in intensive care unit patients. J Crit Care. 2014;29(3):351–5.
Pea F, Furlanut M, Negri C, Pavan F, Crapis M, Cristini F, et al. Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrob Agents Chemother. 2009;53(5):1863–7.
Staatz CE, Byrne C, Thomson AH. Population pharmacokinetic modelling of gentamicin and vancomycin in patients with unstable renal function following cardiothoracic surgery. Br J Clin Pharmacol. 2006;61(2):164–76.
Zasowski EJ, Murray KP, Trinh TD, Finch NA, Pogue JM, Mynatt RP, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2017;62(1):e01684. https://doi.org/10.1128/aac.01684-17.
Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther. 2017;102(3):459–69.
Eleveld DJ, Proost JH, Cortinez LI, Absalom AR, Struys MM. A general purpose pharmacokinetic model for propofol. Anesth Analg. 2014;118(6):1221–37.
van Lent-Evers NA, Mathot RA, Geus WP, van Hout BA, Vinks AA. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit. 1999;21(1):63–73.
van Rijn SP, Zuur MA, van Altena R, Akkerman OW, Proost JH, de Lange WC, et al. Pharmacokinetic modeling and limited sampling strategies based on healthy volunteers for monitoring of ertapenem in patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2017;61(4):e01783. https://doi.org/10.1128/aac.01783-16.
Neely M, Margol A, Fu X, van Guilder M, Bayard D, Schumitzky A, et al. Achieving target voriconazole concentrations more accurately in children and adolescents. Antimicrob Agents Chemother. 2015;59(6):3090–7.
van Wanrooy MJ, Proost JH, Rodgers MG, Zijlstra JG, Uges DR, Kosterink JG, et al. Limited-sampling strategies for anidulafungin in critically ill patients. Antimicrob Agents Chemother. 2015;59(2):1177–81.
Neely M, Jelliffe R. Practical therapeutic drug management in HIV-infected patients: use of population pharmacokinetic models supplemented by individualized Bayesian dose optimization. J Clin Pharmacol. 2008;48(9):1081–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by departmental funding.
Conflict of interest
Pieter Colin and Stijn Jonckheere have no conflicts of interest directly relevant to the content of this study. Michel Struys via his research group/department has received grants and funding from The Medicines Company (Parsippany, NJ, USA), Masimo (Irvine, CA, USA), Fresenius (Bad Homburg, Germany), Acacia Design (Maastricht, the Netherlands), and Medtronic (Dublin, Ireland) and honoraria from The Medicines Company (Parsippany, NJ, USA), Masimo (Irvine, CA, USA), Fresenius (Bad Homburg, Germany), Baxter (Deerfield, IL, USA), Medtronic (Dublin, Ireland), and Demed Medical (Temse, Belgium). He is an editorial board member to the British Journal of Anaesthesia and a senior editor to Anesthesia & Analgesia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Colin, P.J., Jonckheere, S. & Struys, M.M.R.F. Target-Controlled Continuous Infusion for Antibiotic Dosing: Proof-of-Principle in an In-silico Vancomycin Trial in Intensive Care Unit Patients. Clin Pharmacokinet 57, 1435–1447 (2018). https://doi.org/10.1007/s40262-018-0643-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-018-0643-8