Abstract
Treprostinil is available in three different formulations and four different routes of administration: Remodulin® (treprostinil sodium, intravenous and subcutaneous administration), Tyvaso® (treprostinil sodium, inhaled administration), and Orenitram® (treprostinil diolamine, oral administration) for the treatment of pulmonary arterial hypertension (PAH). Pharmacokinetic studies have been performed in healthy volunteers and patients with PAH. The intent of this review is to outline pharmacokinetic considerations of the three treprostinil formulations and provide clinicians with a resource that may support clinical decisions in treating patients with PAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There are currently three formulations of treprostinil available for treatment of pulmonary arterial hypertension (PAH) in four routes of administration: intravenous (IV), subcutaneous (SC), inhaled, and oral treprostinil. Each route of administration is associated with unique pharmacokinetics, dosing considerations, and potential for route-specific adverse effects. |
Parenteral routes of administration (IV, SC) are bioequivalent at steady state, while inhaled treprostinil achieves lower systemic concentrations with localized delivery to the lungs. Oral treprostinil achieves similar systemic exposure to parenteral administration with a bioavailability of approximately 17 %. |
1 Introduction
Pulmonary arterial hypertension (PAH) is a progressive and fatal disease, characterized by increasing pulmonary vascular resistance (PVR), which may eventually lead to right ventricular failure and premature death [1]. The disease is defined by a mean pulmonary artery pressure >25 mmHg at rest, pulmonary arterial wedge pressure ≤15 mmHg, and PVR >3 Wood units. The cause of PAH is multi-factorial but may develop due to imbalances in the endothelin-1, nitric oxide, and prostacyclin pathways. These irregularities lead to increased production of vasoconstricting compounds (e.g., endothelin, thromboxane) and decreased production of vasodilators (e.g., prostacyclin), ultimately resulting in pulmonary artery vasoconstriction and endothelial cell proliferation. Currently, four classes of compounds are approved for the treatment of PAH: endothelin receptor antagonists (ERAs), phosphodiesterase type 5 (PDE-5) inhibitors, soluble guanylate cyclase stimulators, and prostacyclins.
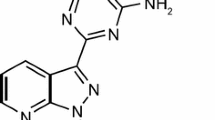
Treprostinil is a chemically stable, tricyclic analog of prostacyclin, with a molecular weight of 390.52 (C23H34NaO5). The primary mechanism of action of treprostinil is reduction in pulmonary artery pressure through direct vasodilation of the pulmonary and systemic arterial vascular beds, thereby improving systemic oxygen transport and increasing cardiac output with minimal alteration of the heart rate. Treprostinil has been shown to have high in vitro affinity for the DP1, EP2, and IP receptors (inhibition constant [K i] 4.4, 3.6, and 32 nmol/L, respectively) [2], all of which can result in dilatation of human pulmonary vasculature upon activation. For the IP receptor specifically, when endogenous prostacyclin binds, cyclic adenosine monophosphate is activated. This activation accounts for additional mechanisms of prostacyclin action, including inhibition of pulmonary artery smooth muscle cell proliferation, inhibition of platelet aggregation, and reversal of pulmonary artery remodeling [2, 3]. It has also recently been discovered that in vitro the majority of the anti-proliferating properties of treprostinil are mediated through the EP2 receptor [4].
Treprostinil is available in three separate formulations: a continuous subcutaneous (SC) and intravenous (IV) infusion (Remodulin®), a solution for inhalation (Tyvaso®), and an extended-release oral tablet (Orenitram®). Parenteral and inhaled treprostinil are formulated as the sodium salt, whereas oral treprostinil is formulated as the diolamine salt. Clinical effectiveness of these products was demonstrated by improvement in exercise capacity, as measured by change in 6-min walk distance (6MWD) [5–7]. Additionally, a placebo-controlled study examining the transition of IV epoprostenol to SC treprostinil demonstrated a delay in time to clinical worsening with treprostinil [8]. While each treprostinil formulation provides benefit to patients with PAH, different routes of administration have the potential to produce distinct adverse events. For example, 85 % of patients receiving SC treprostinil in the pivotal trial experienced infusion-site pain, while 54 and 25 % of patients experienced cough and throat irritation with inhaled treprostinil [9, 10]. With oral treprostinil, 6 % of patients experienced abdominal discomfort compared with a placebo rate of 0 % [11]. Table 1 summarizes some of the risks and benefits of each formulation. Understanding the relative pharmacokinetic differences of the available treprostinil formulations may facilitate and support clinical decision making in treating patients with PAH, especially when transitioning between treprostinil formulations. The objective of this review is to provide a summary and comparison of pharmacokinetic data from studies of treprostinil formulations performed in healthy volunteers and patients with PAH. Details of study designs and participant populations are provided in Table 2.
2 Overview of Treprostinil Formulations and Key Pharmacokinetic Data
2.1 Remodulin® (Parenteral Treprostinil Sodium) Dosing Overview
The preferred route of administering parenteral treprostinil is SC, but it can be administered by a central IV line if the SC route is not tolerated due to severe site pain or reaction [9]. The infusion rate is initiated at 1.25 ng/kg/min. If this initial dose cannot be tolerated because of systemic effects, the infusion rate should be reduced to 0.625 ng/kg/min. The infusion rate should be increased in increments of 1.25 ng/kg/min per week for the first 4 weeks of treatment. The dose should be further titrated in increments of 2.5 ng/kg/min per week, as determined by the patient’s clinical response. If tolerated, dosage adjustments may occur more frequently.
Currently, the method of parenteral treprostinil delivery involves an external delivery device. One study is ongoing in which the objective is to analyze whether an implantable intravascular delivery system for continuous drug administration is feasible. A multicenter, prospective, single-arm, non-randomized study at ten sites involving 60 implanted subjects demonstrated that use of the implantable intravascular delivery system to administer parenteral treprostinil significantly reduced the number of catheter-related complications from a pre-defined criterion of 2.5 complications per 1000 days with external delivery devices to 0.27 complications per 1000 days with the implantable delivery device (p < 0.0001) [12]. For this analysis, the mean duration of use was 367 days. The mean plasma treprostinil concentration was 10.5 ng/mL 1 week after implantation compared with a baseline level of 10.9 ng/mL, collected when the drug was being administered with the external pump [13].
2.1.1 Bioequivalence at Steady State
In a comparative pharmacokinetic crossover study, subjects received treprostinil (IV or SC) at a dose of 10 ng/kg/min for 72 h; infusions were separated by a 4-day washout period [14]. Steady-state ratios of the geometric means (IV/SC) [90 % confidence intervals (CIs)] for the area under the plasma concentration–time curve (AUC) and maximal plasma concentration (C max) were 92.9 % [89.8–96.1] and 106 % [99.4–113], respectively (Fig. 1) [14]. When considering treatment options, these findings indicate that IV and SC treprostinil are bioequivalent at steady state.
Mean plasma concentration of treprostinil following intravenous and subcutaneous infusion: a linear plot and b log-linear plot [14]. IV intravenous, SC subcutaneous
2.1.2 Long-Term Pharmacokinetic and Diurnal Variation
The steady-state pharmacokinetic and potential for diurnal variation was investigated when administered as a long-term 28-day continuous SC infusion to healthy adult volunteers [15]. The doses administered were 2.5, 5, 10, and 15 ng/kg/min, and escalations occurred every 7 days with no washout periods between escalations. Linear regression analysis of the mean steady-state treprostinil concentration versus the targeted dose yielded a fitted line with an R 2 of 0.92, demonstrating linear and dose-independent pharmacokinetics. Consistent diurnal variation cycles of two peaks and two troughs were observed over a 24-h steady-state interval for all doses, with peak concentrations approximately 20–30 % higher than trough levels. Inter-subject percentage coefficients of variation (CV%) ranged from 14 to 26 % for mean peak and trough concentrations, respectively.
2.1.3 Dose Linearity in Pulmonary Arterial Hypertension (PAH) Patients
Dose proportionality was assessed in patients receiving treprostinil by continuous IV or SC infusion at doses between 12.1 and 125 ng/kg/min [16]. Steady-state treprostinil plasma concentrations ranged from 14.9 to 18248 pg/mL. A positive correlation between the treprostinil dose and treprostinil plasma concentration following linear regression analysis was reported, with an R 2 value of 0.796. The equation describing the relationship between the treprostinil dose and steady-state plasma concentration (pg/mL) was as follows:
This study illustrated treprostinil dose–concentration linearity up to 125 ng/kg/min.
2.2 Tyvaso® (Inhaled Treprostinil Sodium) Dosing Overview
Treprostinil solution for inhalation is administered via an ultrasonic nebulizer, with the device delivering approximately 6 µg of treprostinil per breath [10]. The initial dose of Tyvaso® is 3 breaths (18 µg) four times daily, with up-titration to target maintenance dosage of 9 breaths (54 µg) four times daily as tolerated. Forty-two percent of patients in the open-label extension trial achieved a dose of at least 12 breaths (72 µg) four times daily, with qualitatively similar adverse events to the short-term placebo-controlled trial [10].
2.2.1 Absolute Bioavailability and Dose Linearity
A crossover study to determine the absolute bioavailability of inhaled treprostinil relative to IV treprostinil was performed in 18 volunteers who were randomized to receive 18 µg of inhaled treprostinil, 36 µg of inhaled treprostinil, or 15 ng/kg/min (60 min) of IV treprostinil [17]. Both C max and AUC increased proportionally following a single administration of 18 or 36 µg of inhaled treprostinil, with mean C max and AUC ± standard deviation (%CV) increasing from 0.354 ± 0.137 (38.8 %) to 0.698 ± 0.141 (20.2 %) ng/mL and from 0.2556 ± 0.0843 (33 %) to 0.6115 ± 0.1751 (28.6 %) ng·h/mL, respectively. Mean estimates of the absolute systemic bioavailability of treprostinil after inhalation were 64.4 % (18 μg, 3 breaths) to 71.6 % (36 μg, 6 breaths) relative to IV treprostinil concentrations. Treprostinil concentrations remained detectable in the plasma approximately 4 h after inhalation.
2.2.2 Maximum Tolerated Dose
The maximum tolerated dose (MTD) and dose linearity of inhaled treprostinil were evaluated in a study of volunteers randomized to receive 54, 72, 78, 84, or 90 µg of inhaled treprostinil [17]. Mean systemic exposures (AUC from time zero to infinity [AUC∞]) (%CV) after inhaled treprostinil doses of 54, 72, 78, 84, and 90 µg were 0.812 (58.1 %), 0.661 (67.3 %), 1.206 (44.3 %), 1.182 (20.2 %), and 1.579 (51.7 %) ng·h/mL, respectively. Treprostinil pharmacokinetics were dose proportional for AUC∞, AUC from time zero to time t (AUCt), and C max (mean range 790–1708 pg/mL). Adverse events of chest pain, chest discomfort, dyspnea, headache, dizziness, tremor, nausea, and vomiting in the 90 µg cohort were determined to be intolerable, and thus the MTD for a single dose of inhaled treprostinil in healthy volunteers was determined to be 84 µg.
2.2.3 Pharmacokinetics in PAH Patients
A substudy of a phase IV safety study assessed the pharmacokinetics of inhaled treprostinil following long-term administration in 17 patients with PAH receiving inhaled treprostinil for ≥30 days and on a stable dose for ≥3 days prior to pharmacokinetic collection [18]. Across all patients and doses, the time to C max (t max) ranged from 5 to 30 min. The geometric mean C max for the cohort of 11 PAH patients receiving the recommended maintenance dose of 54 µg (9 breaths) four times daily for ≥3 days was 1015.3 pg/mL (CV% = 118 %). At the same dose, the geometric AUCt was 993.6 h·pg/mL (CV% = 151 %) (Fig. 2). The observed C max appears to be consistent with those previously observed in healthy volunteers receiving inhaled treprostinil, while AUC∞ (1023.2 h·pg/mL) is 26 % higher in PAH patients than healthy volunteers [17, 18]. This effect was not limited to the inhaled formulation and was more pronounced in a systemically administered treprostinil formulation. PAH patients who were taking oral treprostinil 2 mg twice daily experienced approximately 50 % higher C max and AUC values than equivalently dosed healthy volunteers [19, 20].
Mean (±standard deviation) plasma treprostinil concentration vs. time following administration of 54 μg of inhaled treprostinil (n = 11) [18]
Additionally, studies were conducted by independent academic investigators to explore the safety and efficacy of inhaled treprostinil administered by inhalation at varying doses and durations [21, 22]. The pharmacokinetic studies demonstrated that the plasma C max of treprostinil was achieved 10–45 min after inhalation and confirmed dose-dependent plasma concentrations.
2.3 Orenitram® (Treprostinil Diolamine) Extended-Release Tablets Dosing Overview
Oral treprostinil is an extended-release tablet that utilizes osmotic release technology. Oral treprostinil has been studied as both a twice daily and three times daily regimen. Oral treprostinil is to be administered to patients at a recommended starting dose of 0.25 mg twice daily or 0.125 mg three times daily with food [11]. A three times daily dosing strategy may lower the peak to trough ratio and allow for a more rapid titration by minimizing adverse events [23]. The determination of dosing frequency is based on physician discretion. Dose titrations are recommended to occur in increments of 0.25–0.5 mg twice daily or 0.125 mg three times daily every 3–4 days, as tolerated.
The maximum dose of oral treprostinil is dependent on patient tolerability. The maximum doses studied were 12 mg twice daily in a phase III, 12-week, placebo-controlled study and as high as 27.5 mg three times daily in an open-label, long-term extension study [5, 24]. The mean dose in a controlled clinical trial at week 12 was 3.4 mg twice daily, and the open-label extension study reported mean doses of 3.1, 3.6, and 4.1 mg twice daily at 6, 12, and 24 months, respectively. Table 3 presents the AUC and C max for varying doses of oral, parenteral, and inhaled treprostinil for comparison.
2.3.1 Bioavailability and Food Effect
The bioavailability of oral treprostinil 1 mg was compared with a dose of IV treprostinil 0.2 mg over 4 h (7.6–14.7 ng/kg/min with a mean of 11.4 ng/kg/min). Based on the ratios of geometric means for AUC∞, the absolute bioavailability of oral treprostinil was 17 % (90 % CI 16–19). In this study, oral treprostinil was administered twice daily with a well-balanced 500 calorie meal based on the results of food effect studies.
Oral treprostinil administered to healthy volunteers immediately following a US Food and Drug Administration (FDA)-designated high-fat, high-calorie meal (containing approximately 800–1000 calories and approximately 50 % fat) resulted in a 49 % increase in AUC∞ and 13 % increase in C max compared with fasting conditions [11]. The t max value was delayed from 3.5 h in the fasted state to 6 h following a high-fat, high-calorie meal, with sustained plasma treprostinil concentrations observed over 12 h. Additionally, in a study of volunteers who were randomized to receive 1 mg of oral treprostinil immediately following four meal types containing varying calories and fat content, a decrease in treprostinil C max and AUC (5–15 %) was observed with decreasing caloric intake from 500 to 250 calories or increasing fat content from 30 to 50 % prior to administration [25]. Overall, these data indicate that oral treprostinil should be administered with food but with no specific caloric requirement.
2.3.2 Drug–Drug Interactions:
The majority of drug–drug interaction studies have been conducted with oral treprostinil, with the data extrapolated to apply to the IV, SC, and inhaled formulations as appropriate. Several phase I studies were conducted in healthy volunteers to evaluate the potential for drug–drug interactions with oral treprostinil [26–29]. Based on in vitro cytochrome P450 (CYP) experiments evaluating the inhibitory and induction potential of treprostinil, it is expected that treprostinil would have little potential to cause interactions with drugs metabolized by CYP isozymes. Any drugs that inhibit, induce, or are metabolized by CYP2C8, and to a lesser extent CYP2C9, may have the potential to affect the concentrations of treprostinil in the systemic circulation. Two studies were conducted with prototypical inducer (rifampin [rifampicin]) and inhibitors (gemfibrozil and fluconazole) of the CYP2C8 system to determine the effects of these drugs on the pharmacokinetics of oral treprostinil. Rifampin reduced treprostinil concentrations by 30 % and gemfibrozil increased treprostinil concentrations twofold. In the presence of gemfibrozil or other strong CYP2C8 inhibitors, the starting dose of oral treprostinil should be reduced to 0.125 mg twice daily and may be titrated by 0.125 mg twice daily as tolerated. Fluconazole, a CYP2C9 inhibitor was selected to evaluate the effect of concomitant use with treprostinil; no significant changes were noted in the presence of fluconazole.
Concomitant PAH therapies evaluated for drug–drug interaction potential include bosentan, an ERA, and sildenafil, a PDE-5 inhibitor. There were no clinically significant or evident treatment-emergent changes or adverse trends in vital signs or laboratory parameters following administration of oral treprostinil in combination with bosentan or sildenafil. Neither bosentan nor sildenafil affect the pharmacokinetics of treprostinil.
The effect of esomeprazole, a proton pump inhibitor, on treprostinil pharmacokinetics was also evaluated and no effect was found. Therefore, no dosing adjustments are recommended for concomitant use of gastric acid suppressive agents and oral treprostinil. Figure 3 displays the CIs obtained from all drug interaction studies conducted with treprostinil diolamine.
Impact of co-administered drugs on the systemic exposure of oral treprostinil 1 mg compared with oral treprostinil administered alone [28]. AUC ∞ area under the plasma concentration–time curve from time zero to infinity, bid twice daily, CI confidence interval, C max maximum concentration, PK pharmacokinetics, qid four times daily, tid three times daily
In addition, drug interaction studies were conducted with SC treprostinil sodium co-administered with warfarin (25 mg/day) in healthy volunteers and acetaminophen in healthy volunteers. There was no clinically significant effect of treprostinil on the pharmacokinetics or pharmacodynamics of warfarin. Additionally, acetaminophen did not affect the pharmacokinetics of treprostinil [30, 31].
Other important interactions to consider with all treprostinil formulations include concomitant use of antihypertensive agents, diuretics, other vasodilators, and anticoagulants. When treprostinil is used in combination with antihypertensive agents, diuretics, or vasodilators, patients may have an increased risk of symptomatic hypotension, and the antiplatelet effects of treprostinil may increase the risk of bleeding when used with anticoagulants [9–11].
2.3.3 Special Populations
Studies have been conducted in subjects with hepatic and renal impairment [32, 33]. Relative to healthy volunteers, mean oral clearance (CL/F) values in subjects with Child-Pugh class A, B, and C decreased by approximately 57, 76, and 89 %, respectively [32]. The decrease in CL/F as a function of hepatic impairment severity resulted in an increase in exposure levels of treprostinil. Relative to healthy subjects, mean AUC∞ values in subjects with mild, moderate, and severe hepatic impairment increased by factors of 2.2, 4.9, and 7.6, respectively. Mean C max values in subjects with mild, moderate, and severe hepatic impairment also increased by factors of 1.6, 4.0, and 4.8, respectively. As a result of these data, dose adjustments are suggested for patients with Child-Pugh class A hepatic dysfunction; however, oral treprostinil should be avoided in Child-Pugh class B and is contraindicated in class C patients [11]. The parenteral and inhaled treprostinil formulations bypass first-pass metabolism; therefore, the effect of hepatic impairment on pharmacokinetics is reduced.
In the renal impairment study, results demonstrated that end-stage renal disease (ESRD) did not substantially alter the pharmacokinetics of treprostinil following oral treprostinil administration. Mean plasma exposure to treprostinil in ESRD subjects post-dialysis was approximately 23 % lower than in healthy subjects with normal renal function. Hemodialysis was not found to contribute significantly to the elimination of treprostinil from the systemic circulation; treprostinil pharmacokinetics in ESRD subjects were largely comparable when oral treprostinil was administered either 4 h prior to or after dialysis [33].
2.3.4 Long-Term Dosing in PAH Patients
A study of 70 PAH patients evaluated the pharmacokinetic profile of oral treprostinil after long-term treatment for a minimum of 4 weeks at doses ranging from 0.5 to 16 mg twice daily, with 90 % of the patients receiving a dose of ≤7 mg twice daily [23]. The mean treprostinil AUC, C max, and minimum concentration (C min) increased from 5.24 to 204.09 ng·h/mL, 1.38 to 33.59 ng/mL, and 0.05 to 3.68 ng/mL between the 0.5 and 16 mg twice daily dose levels, respectively. Results of the power analysis suggest that AUC and C max increased in a dose-linear manner between 0.5 and 15 mg (slope estimates = 0.84 and 0.71, respectively). In addition, the treprostinil pharmacokinetic profile appeared to be consistent regardless of patient age, weight, sex, race, ethnicity, background therapy, or PAH etiology (including in a cohort of nine patients with connective tissue disease who classically have impaired gut motility).
2.3.5 Three Times Daily Dosing
A study evaluated the pharmacokinetic profile of oral treprostinil following administration of a dosing regimen of 0.5 mg three times daily for 7 days in healthy volunteers. On Day 7, mean steady-state plasma treprostinil concentrations were maintained above 200 pg/mL for approximately 20 h over a 24-h interval. Statistical analyses showed no significant differences in C max between Day 7 and Day 1, and no significant differences in the AUC from time zero to 6 h (AUC6) between Day 7 and Day 1, with the 90 % CI of the mean C max and AUC ratios falling within the 0.80–1.25 range, indicating equivalence. Box plots and statistical analysis showed the mean total exposure (AUC from time zero to 24 h [AUC24]) on Day 7 (after three doses) was not significantly different from three times the exposure after a single dose on Day 1 (3 × AUC∞), with the 90 % CI of the mean parameter ratios falling within the 0.80–1.25 range. This study provides support for a three times daily dosing strategy, which is currently being evaluated in all ongoing clinical trials with oral treprostinil. A cohort of 13 PAH patients were enrolled in an open-label study comparing the pharmacokinetics and tolerability of twice-daily versus three times daily dosing. Dosing oral treprostinil using a three times daily regimen resulted in higher total daily doses and AUCs while maintaining consistency in the C max and increasing the C min approximately twofold compared with twice-daily dosing. Adverse events were assessed using the Standardized Subject Impression of Change, with a net improvement observed in 12 of 13 patients with three times daily dosing. Overall, three times daily dosing resulted in a reduction in the peak to trough ratio of approximately twofold [23, 34]. These data suggest that three times daily dosing may be an alternative to twice-daily dosing to improve tolerability.
2.3.6 Oral Treprostinil as a Replacement for Parenteral Therapy
At physiological pH, treprostinil sodium (SC, IV, and inhaled formulations) and treprostinil diolamine (oral tablets) dissociate from their respective salt counterion, resulting in ionized treprostinil that can circulate freely in the plasma. Oral treprostinil dose equivalence can be estimated with the following equation:
Additionally, if there is a treatment interruption with oral therapy, SC or IV treprostinil could temporarily be initiated by calculating the parenteral dose with the following equation:
Preliminary data from a study evaluating the safety, tolerability, pharmacokinetics, and logistics of transitioning clinically stable PAH patients from parenteral (25–111 ng/kg/min) to oral treprostinil indicates that the transition is feasible in the majority of subjects. Figure 4 compares the mean concentration of parenteral treprostinil with that of oral treprostinil administered twice daily and three times daily [35].

Data from a study sponsored by United Therapeutics Corp. The PK report from this study was dated Feb 2015
Mean ± standard error of the mean treprostinil concentration vs. time plots by treatment regimen (semi-log). Tyvaso® has been omitted from this figure as its systemic effects are not comparable with parenteral Remodulin® or oral treprostinil (Data on file). Mean dose ± standard deviation of Remodulin® (n = 32): 58.2 ± 19.2 ng/kg/min; total daily mean dose ± standard deviation of oral treprostinil twice daily (n = 6): 27 ± 12.3 mg; total daily mean dose ± standard deviation of oral treprostinil three times daily (n = 26): 37.9 ± 13.8 mg. bid twice daily, tid three times daily.
3 Clinical Impact and Estimates of Pharmacokinetic Equivalents
The goal of treprostinil dosing in PAH is to establish an optimal dose at which symptoms are improved, while minimizing excessive pharmacologic effects associated with the prostacyclin class of medications (e.g., headache, nausea, diarrhea, flushing, jaw pain, vomiting). Experience with IV and SC treprostinil indicates that patients achieve a wide range of doses following long-term exposure. While many factors contribute to the dosing paradigm for each patient, pharmacokinetic data in healthy volunteers and patients with PAH indicate that the administration of IV and SC route are bioequivalent, linear up to 125 ng/kg/min, have diurnal variation of 20–30 %, and have an elimination half-life of 4 h [15].
In comparison, inhaled treprostinil is approved for a target dose of 54 µg four times daily, although data indicate some patients have tolerated doses as high as 72 µg four times daily [6]. Importantly, the effect of inhaled treprostinil may not be driven entirely by systemic plasma concentrations but rather due to the local delivery of treprostinil to the lungs [36]. Pharmacokinetic data demonstrate dose linearity up to 84 µg, absolute bioavailability of approximately 65–70 %, and a t max of 10 min.
Pharmacokinetic studies with oral treprostinil have addressed bioavailability, food effect, pharmacokinetic linearity in volunteers and PAH patients, drug interactions, and use in special populations. The major conclusions from oral treprostinil pharmacokinetic studies indicate that the bioavailability (17 %) is affected by food, is linear up to 16 mg twice daily in PAH patients, drug interactions are present with CYP2C8 inhibitors and inducers, and dose adjustments are required in patients with hepatic dysfunction but not patients with renal dysfunction, including ESRD. Most oral treprostinil studies were conducted with twice-daily dosing, but three times daily dosing has also been evaluated. Three times daily dosing demonstrated higher systemic exposure than twice-daily dosing, with a reduction in the plasma treprostinil peak to trough ratios throughout the day. Less variation in drug concentrations may reduce the occurrence or severity of adverse events and therefore improve the rate of titration [23].
In summary, each route of administration for treprostinil has unique pharmacokinetic characteristics. Importantly, regardless of route of administration, the pharmacologically active agent measured in the plasma is treprostinil and a direct comparison of systemic exposure can be obtained (Table 3). The target maintenance dose of inhaled treprostinil (54 µg four times daily) would deliver the same systemic exposure as an infused treprostinil dose of 1 ng/kg/min. When this same comparison is performed with parenteral and oral treprostinil, the following evaluation can be made: 1 mg three times daily of oral treprostinil is approximately equivalent to 5 ng/kg/min of parenteral treprostinil. Notably, this only holds true for patients who weigh approximately 70 kg and have no other confounding factors (i.e., liver dysfunction or receiving a CYP2C8 modifier). For patients who weigh less than or greater than 70 kg, estimation at an equivalent dose should be made using the equations presented in Sect. 2.3.6.
When deciding between formulations, clinicians should individualize therapy selection based on the patient’s clinical status, health literacy, quality of life, co-morbidities, and any route-specific considerations. Table 1 highlights many of the risks and benefits clinicians may consider when choosing between formulations.
4 Conclusion
In this article we have reviewed and compared pharmacokinetic data from studies performed in healthy volunteers and patients with PAH for three different formulations and four different routes of administration of treprostinil: Remodulin® (treprostinil sodium, IV and SC administration), Tyvaso® (treprostinil sodium, inhaled administration), and Orenitram® (treprostinil diolamine, oral administration). Careful consideration of these pharmacokinetic data can aid the clinician in making treatment decisions to select an appropriate route of administration, as well as to transition between formulations.
References
McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–619.
Whittle BJ, Silverstein AM, Mottola DM, et al. Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: treprostinil is a potent DP1 and EP2 agonist. Biochem Pharmacol. 2012;84(1):68–75.
Falcetti E, Hall SM, Phillips PG, et al. Smooth muscle proliferation and role of the prostacyclin (IP) receptor in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182(9):1161–70.
Patel J, Shen L, Hall S, et al. EP2 receptors play a key role in mediating the anti-proliferative activity of treprostinil in smooth muscle cells derived from the lungs of pulmonary hypertensive patients. Am J Respir Crit Care Med. 2015;191:A5954.
Jing ZC, Parihk K, Pulido T, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127(5):624–33.
Benza RL, Seegar W, McLaughlin VV, et al. Long-term effects of inhaled treprostinil in patients with pulmonary arterial hypertension: the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) study open-label extension. J Heart Lung Transpl. 2011;30(12):1327–33.
Simonneau G, Barst R, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:800–4.
Rubenfire M, McLaughlin VV, Allen RP, et al. Transition from IV epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension: a controlled trial. Chest. 2007;123(3):757–63.
Remodulin U.S. package insert. Research Triangle Park: United Therapeutics Corporation; 2014.
Tyvaso U.S. package insert. Research Triangle Park: United Therapeutics Corporation; 2014.
Orenitram U.S. package insert. Research Triangle Park: United Therapeutics Corporation; 2016.
Bourge RC, Waxman AB, Gomberg-Maitland M, et al. Treprostinil administered to treat pulmonary artery hypertension using a fully implantable programmable intravascular delivery system: results of the DelIVery for PAH trial. Chest. 2016. doi:10.1016/j.chest.2015.11.005.
Bourge R. Treprostinil administered using a fully implantable programmable intravascular delivery system: results of the Delivery for PAH trial [oral presentation]. American College of Chest Physicians Annual Meeting: Austin; 25–30 Oct 2014.
Laliberte K, Arneson C, Jeffs R, et al. Pharmacokinetics and steady-state bioequivalence of treprostinil sodium (Remodulin) administered by intravenous and subcutaneous route to normal volunteers. J Cardiovasc Pharmacol. 2004;44(2):209–14.
Wade M, Maker FJ, Roscigno R, et al. Pharmacokinetics of treprostinil sodium administered by 28-day chronic continuous subcutaneous infusion. J Clin Pharmacol. 2004;44(5):503–9.
McSwain CS, Benza R, Shapiro S, et al. Dose proportionality of treprostinil sodium administered by continuous subcutaneous and intravenous infusion. J Clin Pharmacol. 2008;48(1):19–25.
Nelsen AC, Laliberte KJ, Zaccardelli DS, et al. Pharmacokinetics of inhaled treprostinil sodium in healthy volunteers. Am J Respir Crit Care Med. 2010;181:A338.
Bourge RC, Tapson VF, Safdar Z, et al. Rapid transition from inhaled iloprost to inhaled treprostinil in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2013;31(1):38–44.
White RJ, Torres F, Allen R, et al. Pharmacokinetics of oral treprostinil sustained release tablets during chronic administration to patients with pulmonary arterial hypertension. J Cardiovasc Pharmacol. 2013;61(6):474–81.
Laliberte K, Goetz B, Phares K, et al. Sustained treprostinil plasma concentrations following administration of UT-15C (treprostinil diethanolamine) sustained release tablets in healthy volunteers [poster]. American Thoracic Society International Conference: San Francisco; 18–23 May 2007.
Channick RN, Olschewski H, Seeger W, et al. Safety and efficacy of inhaled treprostinil as add-on therapy to bosentan in pulmonary arterial hypertension. J Am Coll Cardiol. 2006;48(7):1433–7.
Voswinckel R, Enke B, Reichenberger F, et al. Favorable effects of inhaled treprostinil in severe pulmonary hypertension: results from randomized controlled pilot studies. J Am Coll Cardiol. 2006;48(8):1672–81.
White RJ, Frutiger K, Theuer A, et al. A pharmacokinetic and tolerability comparison in subjects transitioning from twice daily to three times daily dosing of oral treprostinil. Chest. 2014;146:865A.
White RJ, Jing Z, Parikh K, et al. An open-label extension trial of oral treprostinil in subjects with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187:A3270.
Lim A, Wang-Smith L, Kates J, et al. The effect of different meal compositions on the oral bioavailability of treprostinil diolamine in healthy volunteers. J Clin Pharm Ther. 2013;28(6):450–5.
Gotzkowsky SK, Dingemanse J, Lai A, et al. Lack of a pharmacokinetic drug interaction between oral treprostinil and bosentan in healthy adult volunteers. J Clin Pharmacol. 2010;50(7):829–34.
Gotzkowsky SK, Kumar P, Mottola D, et al. Lack of a pharmacokinetic interaction between treprostinil diolamine and sildenafil in healthy adult volunteers. J Cardiovasc Pharmacol. 2013;61(5):444–51.
Rollins K, Peterson L, Laliberte K, et al. Overview of the drug-drug interaction potential with treprostinil. Am J Respir Crit Care Med. 2009;197:A3367.
Rollins K, Walker S, Kates BA, et al. The effect of gastric acid suppression with esomeprazole on treprostinil diethanolamine pharmacokinetics in healthy volunteers. Am Coll Rheumatol. 2010;62(10):A597.
Wade M, Hunt TL, Lai AA. Effect of continuous subcutaneous treprostinil therapy on the pharmacodynamics and pharmacokinetics of warfarin. J Cardiovasc Pharmacol. 2003;41(6):908–15.
Rollins K, Laliberte K, Gotzkowsky SK, et al. Overview of the drug-drug interaction potential with treprostinil [abstract no. P3852]. European Respiratory Society 19th Annual Congress: Vienna; 12–15 Sep 2009.
Peterson L, Marbury T, Marier J, et al. An evaluation of the pharmacokinetics of treprostinil diolamine in subjects with hepatic impairment. J Clin Ther. 2013;38(6):518–23.
Jenkins A, Wang-Smith L, Marbury T, et al. Pharmacokinetics of treprostinil diolamine in subjects with end-stage renal disease on or off dialysis. J Cardiovasc Pharmacol. 2013;61(4):272–6.
White RJ, Frutiger K, Theuer A, et al. A pharmacokinetic and tolerability comparison in subjects transitioning from twice daily to three times daily dosing of oral treprostinil [oral presentation]. American College of Chest Physicians Annual Meeting: Austin; 25–30 Oct 2014.
White RJ, Chakinala M, Mathier M, et al. Safety and tolerability of transitioning from parenteral treprostinil to oral treprostinil in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187:A3303.
Sandifer B, Brigham K, Lawrence E, et al. Potent effects of aerosol compared with intravenous treprostinil on the pulmonary circulation. J Appl Physiol. 2005;99:2363–8.
White RJ, Chakinala M, Rischard F, et al. Safety and tolerability of transitioning from parenteral treprostinil to oral treprostinil in patients with pulmonary arterial hypertension [oral presentation]. American Thoracic Society International Conference: San Diego; 18–21 May 2014.
Barst RG, Galie N, Simonneau G, et al. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J. 2006;28(6):1195–203.
McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55(18):1915–22.
Acknowledgments
The data included in this review comes from clinical studies that were sponsored by United Therapeutics Corp (Research Triangle Park, NC, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funds were used in the preparation of this manuscript.
Conflicts of interest
Parag Kumar and Emily Thudium completed post-doctoral fellowships with United Therapeutics and Parag Kumar is currently employed by the National Institutes of Health. Kevin Laliberte, David Zaccardelli, and Andrew Nelsen are employees of United Therapeutics Corp.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kumar, P., Thudium, E., Laliberte, K. et al. A Comprehensive Review of Treprostinil Pharmacokinetics via Four Routes of Administration. Clin Pharmacokinet 55, 1495–1505 (2016). https://doi.org/10.1007/s40262-016-0409-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0409-0