Abstract
Introduction
Because the multimodal antidepressant vortioxetine is likely to be coadministered with other central nervous system (CNS)-active drugs, potential drug–drug interactions warrant examination.
Objective
These studies evaluated whether there are pharmacokinetic and/or pharmacodynamic interactions between vortioxetine and ethanol, diazepam, or lithium.
Methods
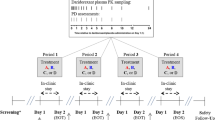
This series of phase I studies included healthy men and women (only men in the lithium study) aged 18–45 years. The ethanol study was a randomized, double-blind, two-parallel group, four-period crossover study in which subjects received a single dose of vortioxetine (20 or 40 mg) or placebo with or without ethanol, and the diazepam study was a randomized, double-blind, placebo-controlled, two-sequence, two-period crossover study in which subjects received a single dose of diazepam following multiple doses of vortioxetine 10 mg/day or placebo. These two studies evaluated the effect of coadministration on standardized psychomotor parameters and on selected pharmacokinetic parameters of each drug. The lithium study was a single-blind, single-sequence study evaluating the effect of multiple doses of vortioxetine 10 mg/day on the steady-state pharmacokinetics of lithium.
Results
Concomitant administration of vortioxetine and single doses of either ethanol or diazepam had no significant effect on the psychomotor performance of subjects compared with administration of ethanol or diazepam alone. Vortioxetine had no significant effect on the pharmacokinetics of ethanol, diazepam, or lithium, and ethanol had no significant effect on the pharmacokinetics of vortioxetine.
Conclusions
Concomitant administration of these agents with vortioxetine was generally well tolerated, with no clinically relevant drug–drug pharmacokinetic or pharmacodynamic interactions identified.
Similar content being viewed by others
No significant effect on the exposure to ethanol, diazepam, or lithium following coadministration with vortioxetine was observed. |
Coadministration of ethanol or diazepam with vortioxetine did not result in significant pharmacodynamic (i.e. psychomotor) effects. |
Coadministration of ethanol, diazepam, or lithium with vortioxetine was generally well tolerated. |
1 Introduction
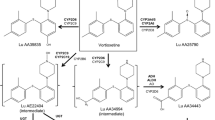
Vortioxetine is a new, multimodal antidepressant approved for the treatment of major depressive disorder (MDD) by the US FDA and the European Medicines Agency, with demonstrated efficacy in both short- and long-term trials [1–3]. The mechanism of action of vortioxetine is thought to be related to its multimodal activity: a combination of direct modulation of receptor activity and inhibition of the serotonin (5-HT) transporter. In vitro studies indicate that vortioxetine is a 5-HT3, 5-HT7, and 5-HT1D receptor antagonist, a 5-HT1B receptor partial agonist, a 5-HT1A receptor agonist, and an inhibitor of the 5-HT transporter [4, 5]. In vivo studies in rats demonstrated that vortioxetine enhances extracellular levels of serotonin, noradrenaline, dopamine, acetylcholine, histamine, and glutamate in specific brain areas [4–8]. The pharmacokinetics of vortioxetine are linear and dose proportional, with a mean terminal half-life of approximately 66 h [9, 10]. Vortioxetine is extensively metabolized primarily through oxidation via multiple cytochrome P450 (CYP) isozymes (predominantly CYP2D6) and subsequent glucuronic acid conjugation [11]. The major carboxylic acid metabolite is pharmacologically inactive [10]. Data from in vitro studies suggest that vortioxetine and its metabolites (Lu AA34443 and Lu AA39835) are unlikely to inhibit many CYP enzymes, including CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, and P-glycoprotein [10, 12]. In vitro studies also found no evidence that vortioxetine induces CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP3A4/5 [10].
Because vortioxetine is typically used chronically, coadministration with other central nervous system (CNS)-active agents, including ethanol, diazepam, and lithium, is likely. Ethanol can potentiate the pharmacologic profile of other CNS drugs; many people with alcohol use disorder are depressed and may therefore be treated with antidepressants [13]. Diazepam is a benzodiazepine with anxiolytic, sedative, muscle relaxant, anticonvulsant, and amnestic effects [14]. Metabolized primarily by CYP2C19, diazepam is commonly used as adjuvant therapy for depression and has potent CNS effects [15]. Lithium is often used in combination with other antidepressants for refractory depression and bipolar disorder treatment and has a narrow therapeutic margin [16]. Because coadministration of ethanol, diazepam, and lithium with vortioxetine is likely, the potential for drug–drug interactions (DDIs) is of clinical relevance. The objectives of these studies included evaluating potential pharmacokinetic and/or pharmacodynamic interactions between vortioxetine and ethanol, diazepam, and lithium.
2 Methods
2.1 Subjects
Subjects included healthy men and women (men only in the lithium study) aged 18–45 years inclusive (19–45 years in the diazepam study) who were capable of understanding and complying with the study protocol. Subjects were required to weigh ≥50 kg, with a body mass index between 19 and 30 kg/m2 inclusive, and to be in a good, healthy condition, as assessed by prestudy physical examination, medical history, vital signs, electrocardiograms, and results of blood biochemistry, hematology, serology tests, and urinalysis. Men with a partner of childbearing potential, as well as women of childbearing potential, were required to use adequate contraception until ≥1 month after the last dose of study medication. In the ethanol study, subjects were required to have a history of regular alcohol use, defined as 2–14 units inclusive per week, with 1 unit equivalent to 25 mL (1 oz) of spirits, 118 mL (4 oz) of wine, or 247 mL (8 oz) of beer.
2.2 Study Designs
Study designs of these phase I studies are summarized in Table 1. Detailed study descriptions are provided below. The studies were conducted according to the World Medical Association Declaration of Helsinki, the International Conference on Harmonisation (ICH) Harmonised Tripartite Guideline for Good Clinical Practice, and all applicable local regulations. Site-designated Investigational Review Boards approved the protocol and all subjects provided written informed consent.
2.2.1 Ethanol Study
This randomized, double-blind, placebo-controlled, two-parallel group, four-period crossover, single-center (UK), single-dose study investigated the DDI between vortioxetine (20 or 40 mg) and ethanol (0.6 g/kg). Subjects were screened ≤28 days prior to randomization and reported to the clinical research unit on the day prior to the study. In group 1, men and women were randomized to one of four treatment sequences that included vortioxetine 20 mg or corresponding placebo with or without ethanol. Blinding was maintained through a randomization schedule held by the dispensing pharmacist. In group 2, men were randomized to one of four treatment sequences that included vortioxetine 40 mg or corresponding placebo with or without ethanol. Ethanol 0.6 g/kg was chosen because it can be potentiated by other sedative drugs [17]. All alcohol-containing products were prohibited between 72 h prior to check-in and throughout the study, except on the dosing days where subjects received 0.6 mg/kg ethanol or matching placebo.
Subjects received a single oral dose of vortioxetine or vortioxetine–placebo, and 5 h later were administered ethanol (or ethanol–placebo) as three drinks. The administration times of vortioxetine and ethanol were selected to provide peak concentrations of both drugs at approximately the same time; therefore, the maximum pharmacodynamic interactions may be evaluated in this study. Subjects were allowed 10 min to consume each of the drinks, with total administration time not to exceed 30 min. Subjects remained in the clinic until day 6 of each treatment period, with discharge occurring after the last pharmacokinetic sample collection.
Plasma samples for vortioxetine and its metabolites were performed at predose (within 15 min) and 1, 2, 3, 4, 5.5, 6.5, 7.5, 9.5, 11.5, 13.5, 23.5, 36, 48, 72, 96, and 120 h postdose. Plasma samples for ethanol were obtained at predose and 0.5, 1.5, 2.5, 4.5, 6.5, 8.5, 18.5, and 24 h after administration of ethanol or ethanol–placebo. Standard pharmacokinetic variables for vortioxetine and its metabolites were derived, which included area under the plasma concentration–time curve (AUC) from time zero to the time of the last quantifiable concentration (AUClast), AUC from time zero to infinity (AUC∞), maximum observed plasma concentration (C max), time to C max (t max), and terminal elimination half-life (t ½). Pharmacokinetic parameters derived for ethanol plasma concentrations included AUClast, C max, and t max.
At screening, subjects completed two sessions of pharmacodynamic assessment training in which they were administered computerized cognitive functioning assessments. Cognitive functions included attention [simple reaction time (SRT), choice reaction time (CRT), digit vigilance task], working memory (numeric working memory, spatial working memory), secondary episodic memory (word recall, word recognition, picture recognition), skilled coordination (tracking), visual analog scale (VAS) mood and alertness (Bond–Lader scale), and postural stability (body sway). During the study, these pharmacodynamic assessments were performed prior to vortioxetine dosing and 1, 2, 4, 6, 8, 18, and 21 h postdose of ethanol or ethanol–placebo. The primary pharmacodynamic outcomes were the speed of detections on the digit vigilance task, body sway/postural stability, and self-rated alertness on the Bond–Lader scale.
2.2.2 Diazepam Study
This randomized, double-blind, placebo-controlled, two-sequence, two-period crossover, single-center (US) study evaluated the potential effects of multiple-dose administration of vortioxetine on the single-dose pharmacokinetics and pharmacodynamics of diazepam. Subjects aged 19–45 years were randomized to one of two sequences in which they received vortioxetine 10 mg and placebo or two capsules of placebo once daily for 21 days, with coadministration of diazepam 10 mg on day 15. Subjects then crossed over to receive the alternative therapy (i.e. placebo or vortioxetine 10 mg and placebo once daily) for the second 21-day treatment period, with diazepam 10 mg coadministered on day 15. Diazepam 10 mg was selected because it is the highest approved strength of the drug. Plasma samples for vortioxetine and its metabolites were obtained at predose on days 11–14 to assess attainment of steady-state concentrations. Beginning on day 14, samples were drawn at predose and 1, 3, 4, 6, 8, 10, 12, 16, and 24 h postdose for vortioxetine. Plasma samples for diazepam and its metabolite (N-desmethyldiazepam) were obtained beginning on day 15 at predose and 0.5, 1, 3, 4, 6, 8, 10, 12, 16, 24, 48, 72, 96, 120, 132, 144, 156, and 168 h postdose. Derived pharmacokinetic parameters for diazepam and N-desmethyldiazepam included AUClast, AUC ∞ , C max, t max, t ½, oral clearance (CL/F), and apparent volume of distribution during the terminal phase (Vz/F). For vortioxetine and its metabolites, pharmacokinetic parameters included AUC from time zero to 24 h (AUC24), C max, minimum plasma concentrations (C min), and t max.
Pharmacodynamic assessments were performed at vortioxetine steady state (day 14) and after the administration of diazepam (day 15) using a similar procedure as described for the ethanol study, except that the time points for assessment of each of the domains were different (i.e. at predose and 4.5, 6.5, 8.5, 10.5, 12.5, and 23 h postdose on days 14 and 15). Cognitive functions examined were attention (SRT, CRT, digit vigilance task), attention/psychophysiological threshold [critical flicker fusion (CFF) threshold test], working memory (numeric working memory, spatial working memory), secondary episodic memory (word recall, word recognition, picture recognition), VAS mood and alertness (Bond–Lader scale), and postural stability (body sway). Composite scores for the following major variables were derived in Statistical Analysis Software (SAS Institute, Cary, NC, USA): power of attention, continuity of attention, quality of working memory, quality of episodic secondary memory, speed of memory, and CFF threshold.
2.2.3 Lithium Study
This single-blind, single-sequence, single-center (US) study evaluated the effect of multiple doses of vortioxetine on the steady-state pharmacokinetics of lithium. The study included healthy men aged 18–45 years who received lithium 450 mg extended release (ER) twice daily, plus vortioxetine–placebo once daily on days 1–14; eligible subjects [mean serum lithium levels on days 11–13 of <1.0 mEq/L, in addition to mean serum lithium levels that varied by ≤0.2 mEq/L (20 %) between days 11 and 12 and days 12 and 13] subsequently received lithium 450 mg ER twice daily plus vortioxetine 10 mg once daily on days 15–28. Lithium 450 mg was chosen because it is a therapeutic dose expected to produce lithium concentrations of 0.6–1.2 mEq/L. Plasma samples for lithium were obtained at predose (both morning and evening) on days 11–13 and 25–27, and on days 14 and 28 at predose (morning) and 1, 2, 4, 6, 8, 10, and 12 h post-morning dose. Pharmacokinetic parameters included AUC from time 0–12 h (AUC12), C max, C min, and t max.
Urine samples were collected 0–12 h prior to predose on day 1, and 0–12 h post-morning dose on days 14 and 28 for lithium determination. Urine pharmacokinetic parameters included the total amount of lithium excreted in the urine during the sample collection interval (Ae12), renal clearance (CLR), and the fraction of drug excreted in the urine during the dosing interval (F e). Plasma samples for vortioxetine and its metabolites were obtained at predose on days 25–27, and on day 28 at predose and 1, 2, 4, 6, 8, 10, 12, and 24 h postdose.
2.3 Bioanalytical
2.3.1 Vortioxetine and Metabolites
Blood samples for the determination of plasma concentrations of vortioxetine and its metabolites Lu AA34443 and Lu AA39835 in these studies were collected in Vacutainers® containing ethylenediaminetetraacetic acid (EDTA). Plasma samples were stored at −20 °C or lower prior to the analysis at Aptuit Ltd, Edinburgh, Scotland. Plasma samples were prepared by solid-phase extraction using Varian SPEC C8 cartridges in a 96-well plate format. This was followed by separation of the analytes by high-performance liquid chromatography on an Ionosper 5C ion exchange column. The mobile phase consisted of 70 mmol/L ammonium formate (pH3) and acetonitrile (12:88). Eluting compounds were detected by tandem mass spectrometry in the positive ion mode, and the internal standards were the 13C-labeled analogs of each of these three analytes. The linear ranges for vortioxetine, Lu AA34443 and Lu AA39835 were 0.08–80, 0.2–200, and 0.04–40 ng/mL respectively. The accuracy and precision for these analytes were within 94.9–107 and 1.81–7.85 %, respectively. The lower limits of quantification were the lower end of the linear ranges for all the above assays. The bioanalytical methods used for these clinical DDI studies were adequate to characterize the plasma concentration profiles of vortioxetine and its metabolites.
2.3.2 Interacting Drugs and Metabolites
Blood samples for the determination of plasma concentrations of ethanol, diazepam, and N-desmethyldiazepam in these studies were collected in Vacutainers® containing EDTA (diazepam) or potassium oxalate/sodium fluoride (ethanol). Plasma samples were stored at −20 °C or lower prior to the analysis at PPD, Richmond, VA, USA. A liquid chromatography with tandem mass spectrometric detection method was used to analyze diazepam and N-desmethyldiazepam, and headspace gas chromatography with flame ionization detection was used to analyze ethanol, according to the validated methods from PPD (proprietary information). Blood samples for the determination of serum concentrations of lithium were collected in Vacutainers®. Serum samples were stored at −20 °C or lower prior to the analysis at Prevalere Life Sciences, LLC, Whitesboro, NY, USA. Graphite furnace atomic absorption spectrophotometry was used to analyze lithium in serum according to the validated method from Prevalere Life Sciences (proprietary information). The linear range, accuracy, and precision of these analyses were considered adequate to determine the plasma concentrations of the interacting drug and metabolite in these studies.
2.4 Safety
Safety variables for all studies included adverse events (AEs), clinical laboratory tests (e.g. hematology, serum chemistry, and urinalysis), vital signs, 12-lead electrocardiograms, and physical examination findings. AEs were obtained by observation from either the investigator or the subject.
2.5 Statistical Analysis
Statistical inference to evaluate the effect of vortioxetine on the pharmacokinetics of ethanol and diazepam was based on analysis of variance (ANOVA) models with fixed effects for sequence, period, and treatment, and a random effect for subject nested within sequence. Statistical inference to evaluate the effect of vortioxetine on the steady-state pharmacokinetics of lithium was based on an ANOVA model with a fixed effect for treatment and a random effect for subject. These ANOVA analyses were performed on the natural logarithms of the AUCs and C max of the test treatment least squares (LS) means relative to the reference treatments. The 90 % confidence intervals (CIs) were calculated by taking the antilog of the 90 % CIs for the difference between LS means on the logarithmic scale.
For pharmacodynamic test results in the ethanol and diazepam studies, an analysis of covariance with baseline in each period as covariate, fixed effects for sequence, treatment, and period, and a random effect for subject nested within sequence were performed at each time point for change from baseline.
3 Results
3.1 Subjects
In the ethanol study, 77 subjects were randomized (45 in group 1 and 32 in group 2); all received study medication and were included in the safety analysis. Twenty-eight subjects in group 1 completed the study. Reasons for discontinuation were protocol deviation (n = 8), withdrawal of consent (n = 7), and AE (n = 2). Forty-two subjects in group 1 had sufficient plasma concentration data for determination of one or more pharmacokinetic parameter and were included in the pharmacokinetic analysis, and 26 subjects were included in the pharmacodynamic analysis. In group 2, 27/32 subjects completed the study, with reasons for discontinuation including AE (n = 2) and protocol deviation, withdrawal of consent, and ‘other’ (n = 1 each). All 32 subjects were included in the pharmacokinetic and safety analyses; 22 were included in the pharmacodynamic analysis.
In the diazepam study, 54 subjects were randomized and 32 subjects completed the study. Reasons for discontinuation included protocol deviation and withdrawal of consent (n = 1 each), and ‘other’ [n = 20; including study stopped per sponsor due to false/positive methadone result on the laboratory screening test (n = 18), and tested positive for cocaine and out of creatine kinase range at period 2 check-in (n = 1 each)]. Thirty-five subjects had sufficient plasma concentrations and postbaseline pharmacodynamic assessments for inclusion in the pharmacokinetic and pharmacodynamic analyses.
The lithium study enrolled 18 men, 16 of whom had a sufficient plasma lithium concentration for inclusion in the pharmacokinetic analysis. Reasons for discontinuation included AE (n = 3), withdrawal of consent and ‘other’ (n = 1 each).
Demographics and baseline characteristics for each of the studies are summarized in Table 2.
3.2 Ethanol Study
3.2.1 Pharmacokinetics
Concomitant administration of ethanol had no significant effect on the pharmacokinetics of vortioxetine with overlapping plasma concentration curves for vortioxetine (20 and 40 mg) and its metabolites, regardless of whether vortioxetine was coadministered with ethanol or ethanol–placebo. For vortioxetine and its metabolites, the 90 % CIs for the ratio of the LS means of the test treatment (vortioxetine 20 or 40 mg plus ethanol) compared with the reference treatment (vortioxetine 20 or 40 mg plus ethanol–placebo) for AUClast and C max were within the 80–125 % no-effect boundary (Table 3). Median t max values were identical for the test and reference treatments.
Similarly, vortioxetine had no clinically meaningful effect on the pharmacokinetics of ethanol with overlapping ethanol plasma concentrations, regardless of whether the ethanol was administered with vortioxetine or vortioxetine–placebo in both groups 1 and 2. The 90 % CIs for the ratio of the LS means of the test treatment (vortioxetine 20 or 40 mg plus ethanol) compared with reference treatment (vortioxetine–placebo plus ethanol) for AUClast and C max were within the 80–125 % no-effect boundary (Table 4; Fig. 1).
Effects of multiple once-daily doses of vortioxetine 10 mg on the pharmacokinetics of diazepam and lithium, and of a single dose of vortioxetine 40 mg on the pharmacokinetics of ethanol. AUC last area under the plasma concentration–time curve from time zero to time of the last quantifiable concentration, AUC 12 AUC from time zero to 12 h, CI confidence interval, C max maximum plasma concentration, PK pharmacokinetic
3.2.2 Pharmacodynamics
For the major pharmacodynamic outcomes (i.e. speed of detections on the digit vigilance tasks, body sway/postural stability, and self-rated alertness on the Bond–Lader scale), no significant differences were observed between the combination of vortioxetine plus ethanol compared with vortioxetine–placebo plus ethanol at most time points in either group 1 or group 2, with two exceptions in group 1 post-ethanol dose: postural stability at 1 h and self-rated alertness at 2 h. Post-ethanol dose, statistically significant LS mean differences were found between vortioxetine plus ethanol and vortioxetine plus ethanol–placebo in digit vigilance speed at 1, 2, and 4 h for group 1, and at 1 and 2 h for group 2, as well as in self-rated alertness at 1 h for group 2 (Figs. 2, 3).
For other primary pharmacodynamic variables (i.e. power of attention, continuity of attention, quality of working memory, quality of episodic secondary memory, speed of memory, self-rated contentment, self-rated calmness), there were several instances of significant LS mean differences between vortioxetine plus ethanol and vortioxetine–placebo plus ethanol. In group 1, a statistically significant difference was observed in self-rated contentment (greater with active combination) at 2 h post-ethanol dose. In group 2, significant differences were noted post-ethanol dose in self-rated calmness at 1 h and speed of memory at 2 h (both less with active combination), and quality of working memory at 6 h (greater with active combination).
Between vortioxetine plus ethanol and vortioxetine plus ethanol–placebo in group 1, significant differences were found post-ethanol dose in power of attention at 1, 2, and 4 h, quality of working memory at 8 h, and quality of episodic secondary memory at 1 and 2 h (all greater with active combination), continuity of attention at 1 and 2 h (greater decrease with active combination), and speed of memory at 18 and 21 h (less decline with active combination). In group 2, significant differences were observed post-ethanol dose in power of attention at 2 h (greater with active combination) and continuity of attention at 1 h, quality of episodic secondary memory at 1 and 2 h, and speed of memory at 1 h (all greater decrease with active combination).
3.3 Diazepam Study
3.3.1 Pharmacokinetics
Vortioxetine had no clinically meaningful effect on the pharmacokinetics of diazepam. Plasma concentrations of diazepam and N-desmethyldiazepam were similar when administered alone or in combination with vortioxetine. For both diazepam and its metabolite, the 90 % CIs of the LS mean ratio of vortioxetine plus diazepam to vortioxetine–placebo plus diazepam for AUClast and C max were within the 80–125 % no-effect boundary (Table 5; Fig. 1).
3.3.2 Pharmacodynamics
Analysis of treatment differences for day 14 (prior to diazepam administration) and day 15 (after diazepam administration) mean change from baseline showed no statistically significant differences between vortioxetine plus diazepam and vortioxetine–placebo plus diazepam at any time point for any major pharmacodynamic variables (i.e. power of attention, continuity of attention, quality of working memory, quality of episodic secondary memory, speed of memory, and CFF threshold). There were several individual instances of significant differences in subtask scores on days 14 and 15, but no apparent pattern with respect to time points or treatment group differences, suggesting differences were random and likely due to the large number of subtasks and assessment times.
3.4 Lithium Study
3.4.1 Pharmacokinetics
Coadministration of vortioxetine with lithium was not associated with a significant effect on lithium pharmacokinetics. Mean serum lithium concentrations were similar when lithium 450 mg ER twice daily was administered with vortioxetine–placebo (day 14) and vortioxetine 10 mg once daily (day 28). The 90 % CI for the ratio of LS means of the test treatment (lithium plus vortioxetine 10 mg) to the reference treatment (lithium plus vortioxetine–placebo) for AUC12 and C max was within the 80–125 % no-effect boundary (Fig. 1). Overall, there was <3 % increase in AUC12 and C max values when lithium was coadministered with vortioxetine (Table 6). Urine pharmacokinetic parameters of lithium were similar with and without concomitant vortioxetine, with mean Ae12 values of 78,710 μg on day 14 and 70,500 μg on day 28. Corresponding values were 1.69 and 1.55 L/h for CLR, and 17.5 and 15.7 % for F e .
3.5 Safety
3.5.1 Ethanol Study
Thirty-six of 45 (80.0 %) subjects in group 1, and 22 of 32 (68.8 %) subjects in group 2 experienced an AE. In group 1, more subjects experienced AEs during coadministration of vortioxetine 20 mg plus ethanol (60.5 %) versus vortioxetine–placebo plus ethanol (34.3 %), as well as for vortioxetine 20 mg plus ethanol–placebo (50.0 %) compared with vortioxetine–placebo plus ethanol–placebo (34.2 %). Similarly, in group 2 more subjects experienced AEs during coadministration of vortioxetine 40 mg plus ethanol (43.3 %) compared with vortioxetine–placebo plus ethanol (31.0 %), as well as for vortioxetine 40 mg plus ethanol–placebo (36.7 %) compared with vortioxetine–placebo plus ethanol–placebo (13.3 %).
For group 1, all AEs were mild to moderate in intensity, with the most common AEs (≥10 %) with or without ethanol being headache (33.3 %), nausea (31.1 %), diarrhea (17.8 %), upper abdominal pain (15.6 %), vomiting (13.3 %), oropharyngeal pain (13.3 %), and nasal congestion (13.3 %). For group 2, all but one AEs were mild to moderate in intensity, with the most common AEs (≥10 %) being headache (25.0 %), diarrhea (25.0 %), upper abdominal pain (25.0 %), nausea (18.8 %), vomiting (15.6 %), and oropharyngeal pain (12.5 %).
3.5.2 Diazepam Study
Twenty-seven of 54 (50.0 %) subjects reported an AE, with the majority being mild in severity. The incidence was 43 % with vortioxetine plus diazepam and 37 % with vortioxetine–placebo plus diazepam. The most frequently reported (≥5 %) AEs in subjects receiving vortioxetine plus diazepam included nausea (18.2 %), headache (9.1 %), vomiting (6.8 %), and upper abdominal pain (6.8 %).
3.5.3 Lithium Study
Of the 18 subjects in the safety set, 14 (77.8 %) experienced an AE, all of which were mild in severity. The incidence of subjects experiencing AEs was 44.4 % (8/18) during days 1–14 (lithium 450 mg ER twice daily plus vortioxetine–placebo once daily), and 62.5 % (10/16) during days 15–28 (lithium 450 mg ER twice daily plus vortioxetine 10 mg once daily). Among treatment groups, the most frequently reported AEs (in two or more subjects overall) included nasal congestion (33.3 %), headache (22.2 %), increased blood pressure (11.1 %), and dizziness (11.1 %). The most frequently reported AEs that occurred in two or more of the 18 subjects receiving lithium plus vortioxetine–placebo were headache (16.7 %) and dizziness (11.1 %). For the lithium plus vortioxetine 10 mg once daily group, the only AE occurring in two or more of the 16 subjects was nasal congestion (31.3 %).
4 Discussion
Vortioxetine is an antidepressant with a multimodal mechanism of action that is approved for the treatment of MDD in regions including the US and Europe [1–3]. A previous study found that steady-state AUC and C max of vortioxetine were increased 128 and 114 %, respectively, when coadministered with bupropion [18]. In the pharmacokinetic analyses in these DDI studies, single doses of vortioxetine had no significant effect on the pharmacokinetics of ethanol, and multiple doses of vortioxetine had no significant effect on the pharmacokinetic parameters of diazepam (or N-desmethyldiazepam) or lithium. In addition, ethanol had no effect on the single-dose pharmacokinetics of vortioxetine. Multiple doses of vortioxetine were evaluated in the diazepam and lithium studies because these drugs are likely to be used together with vortioxetine chronically. The ethanol study (20 or 40 mg doses) was expected to provide exposure (C max and AUC) similar to that from multiple once-daily doses of 5 or 10 mg/day vortioxetine. A single dose design was selected as this is common in DDI studies evaluating the coadministration of CNS-active drugs with ethanol [19–21], and the results from this study are considered clinically important, considering the widespread and easy access to ethanol.
Vortioxetine is extensively metabolized, primarily through oxidation via CYP isozymes (CYP2D6, CYP3A4/5, CYP2C19, CYP2C9, CYP2A6, CYP2C8 and CYP2B6) and subsequent glucuronic acid conjugation [11]. Ethanol metabolism may lead to reduced levels of NAD (NADH), and NADH reduces the ability of the liver to produce uridine diphosphate glucuronic acid, which is necessary for glucuronidation of other drugs [25]. In addition, acute ethanol intake can inhibit CYP3A4, while chronic ethanol induces CYP2E1, CYP3A4, and CYP1A2 [26, 27]. Since ethanol may impact the oxidation and subsequent glucuronidation of vortioxetine, the potential effect of ethanol on the pharmacokinetics of vortioxetine and its metabolites was evaluated. On the other hand, lithium and diazepam are not known to be inhibitors or inducers of cytochrome enzymes or transporters, therefore the effect of lithium or diazepam on the pharmacokinetics of vortioxetine and its metabolites were not evaluated in these DDI studies.
The lack of effect of vortioxetine on diazepam pharmacokinetics was not unexpected based on in vitro microsomal data and clinical DDI studies using a cocktail approach; however, the potential DDI between vortioxetine and diazepam is of interest because both compounds are primarily converted via oxidative metabolism [11, 15]. The lack of effect of vortioxetine on the pharmacokinetics of lithium is expected because lithium is primarily eliminated via renal excretion and is not metabolized; therefore, lithium is not expected to influence the metabolism of other drugs or have its elimination affected by drugs that influence CYP isozymes. Nonetheless, lithium has a narrow therapeutic index and has been reported to cause AEs (e.g. diarrhea, confusion, tremor, dizziness, agitation) when used in combination with serotonin reuptake inhibitors [22–24].
In the pharmacodynamic evaluations in these studies, the concomitant administration of vortioxetine and single doses of either ethanol or diazepam had no significant impact on psychomotor performance compared with the administration of ethanol or diazepam alone. In the ethanol study, the concomitant use of vortioxetine had no significant overall effect on the primary pharmacodynamic outcomes, with minor exceptions at two time points with the lower dose of vortioxetine used (20 mg), which is the highest approved dose. In the diazepam study, vortioxetine had no significant effect on cognitive assessments. These results suggest that vortioxetine does not significantly enhance the CNS-depressant effects of these drugs.
The concomitant use of ethanol, diazepam, or lithium with vortioxetine was generally well tolerated. Although the rates of AEs were slightly higher when these agents were coadministered with vortioxetine compared with the administration of each agent alone, the AEs were mostly mild to moderate in severity and consistent with the safety profile of vortioxetine.
Limitations of the study include the use of healthy volunteers rather than MDD patients, and evaluation of only single doses of ethanol and diazepam. Thus, larger and longer-term trials in patients with clinical MDD are needed to confirm these findings.
5 Conclusion
The results of these trials indicate that concomitant administration of vortioxetine with ethanol, diazepam, or lithium is generally well tolerated, with no clinically relevant drug–drug pharmacokinetic or pharmacodynamic interactions identified.
References
Baldwin DS, Hansen T, Florea I. Vortioxetine (Lu AA21004) in the long-term open-label treatment of major depressive disorder. Curr Med Res Opin. 2012;28(10):1717–24.
Jacobsen PL, Harper L, Chrones L, Chan S, Mahableshwarkar AR. Safety and tolerability of vortioxetine (15 and 20 mg) in subjects with major depressive disorder: results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol. 2015;30(5):255–64.
Zhang J, Mathis MV, Sellers JW, Kordzakhia G, Jackson AJ, Dow A, et al. The US Food and Drug Administration’s perspective on the new antidepressant vortioxetine. J Clin Psychiatry. 2014;76(1):8–14.
Bang-Andersen B, Ruhland T, Jorgensen M, Smith G, Frederiksen K, Jensen KG, et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011;54(9):3206–21.
Westrich L, Pehrson A, Zhong H, Nielsen SM, Frederiksen K, Stensbol TB, et al. In vitro and in vivo effects for the multimodal antidepressant vortioxetine (Lu AA21004) at human and rat targets. Int J Psychiatry Clin Pract. 2012;16(Suppl 1):47.
Mork A, Pehrson A, Brennum LT, Moller NS, Zhong H, Lassen AB, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther. 2012;340(3):666–75.
Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015;145:43–57.
Pehrson AL, Cremers T, Betry C, van der Hart MG, Jorgensen L, Madsen M, et al. Lu AA21004, a novel multimodal antidepressant, produces regionally selective increases of multiple neurotransmitters: a rat microdialysis and electrophysiology study. Eur Neuropsychopharmacol. 2013;23(2):133–45.
Areberg J, Sogaard B, Hojer AM. The clinical pharmacokinetics of Lu AA21004 and its major metabolite in healthy young subjects. Basic Clin Pharmacol Toxicol. 2012;111:198–205.
Takeda Pharmaceuticals America, Inc. Brintellix [package insert]. Deerfield (IL): Takeda Pharmaceuticals America, Inc; 2014.
Hvenegaard MG, Bang-Andersen B, Pedersen H, Jorgensen M, Puschl A, Dalgaard L. Identification of the cytochrome P450 and other enzymes involved in the in vitro oxidative metabolism of a novel antidepressant, Lu AA21004. Drug Metab Dispos. 2012;40(7):1357–64.
Chen G, Zhang W, Serenko M. Lack of effect of multiple doses of vortioxetine on the pharmacokinetics and pharmacodynamics of aspirin and warfarin. J Clin Pharmacol. 2015;55(6):671–9.
Pettinati HM, O’Brien CP, Dundon WD. Current status of co-occurring mood and substance use disorders: a new therapeutic target. Am J Psychiatry. 2013;170(1):23–30.
Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008;118(2):69–86.
Olkkola KT, Ahonen J. Midazolam and other benzodiazepines. Handb Exp Pharmacol. 2008;182:335–60.
Nelson JC, Baumann P, Delucchi K, Joffe R, Katona C. A systematic review and meta-analysis of lithium augmentation of tricyclic and second generation antidepressants in major depression. J Affect Disord. 2014;168:269–75.
Thai D, Dyer JE, Benowitz NL, Haller CA. Gamma-hydroxybutyrate and ethanol effects and interactions in humans. J Clin Psychopharmacol. 2006;26(5):524–9.
Chen G, Lee R, Hojer AM, Buchbjerg JK, Serenko M, Zhao Z. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013;33(10):727–36.
Allen D, Lader M. Interactions of alcohol with amitriptyline, fluoxetine and placebo in normal subjects. Int Clin Psychopharmacol. 1989;4(Suppl 1):7–14.
Lemberger L, Rowe H, Bergstrom FG, Farid KZ, Enas GG. Effect of fluoxetine on psychomotor performance, physiologic response, and kinetics of ethanol. Clin Pharmacol Ther. 1985;37:658–64.
Lader M, Melhuish A, Frcka G, Fredicson O, Christensen V. The effects of citalopram in single and repeated doses and with alcohol on physiological and psychological measures in healthy subjects. Eur J Clin Pharmacol. 1986;31(2):183–90.
Adan-Manes J, Novalbos J, Lopez-Rodriguez R, Ayuso-Mateos JL, Abad-Santos F. Lithium and venlafaxine interaction: a case of serotonin syndrome. J Clin Pharm Ther. 2006;31(4):397–400.
Dalfen AK, Stewart DE. Who develops severe or fatal adverse drug reactions to selective serotonin reuptake inhibitors? Can J Psychiatry. 2001;46(3):258–63.
Hawley CJ, Loughlin PJ, Quick SJ, Gale TM, Sivakumaran T, Hayes J, et al. Efficacy, safety and tolerability of combined administration of lithium and selective serotonin reuptake inhibitors: a review of the current evidence. Int Clin Psychopharmacol. 2000;15(4):197–206.
Bodd E, Gadeholt G, Christensson PI, Mrland J. Mechanisms behind the inhibitory effect of ethanol on the conjucation of morphine in rat hepatocytes. J Pharmacol Exp Ther. 1986;239(3):887–90.
Liangpunsakul S, Kolwanker D, Pinto A, Gorski C, Hall SD, Chalansani N. Activity of CYP2E1 and CYP3A enzymes in adults with moderate alcohol consumption: a comparison with nonalcoholics. Hepatology. 2005;41(5):1144–50.
Djordejevic D, Nikolic J, Stafanovic V. Ethanol interactions with other cytochrome P450 substrates including drugs, xenobiotics, and carcinogens. Pathol Biol (Paris). 1998;46:760–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
These studies were supported by the Takeda Pharmaceutical Company Ltd, and H. Lundbeck A/S. Assistance with writing and manuscript preparation was provided by Bret Fulton, RPh, of The Medicine Group, and funded by the Takeda Pharmaceutical Company Ltd and H. Lundbeck A/S.
Conflicts of interest
Grace Chen, George G. Nomikos, John Affinito, and Zhen Zhao are employees of Takeda Development Center Americas, Inc.
Ethical approval
These studies were conducted in accordance with the World Medical Association Declaration of Helsinki, the ICH Harmonised Tripartite Guideline for Good Clinical Practice, and all applicable local regulations. Site-designated investigational review boards approved the protocols.
Informed consent
Written informed consent was obtained from all individual participants included in the studies.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chen, G., Nomikos, G.G., Affinito, J. et al. Lack of Effect of Vortioxetine on the Pharmacokinetics and Pharmacodynamics of Ethanol, Diazepam, and Lithium. Clin Pharmacokinet 55, 1115–1127 (2016). https://doi.org/10.1007/s40262-016-0389-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0389-0