Abstract
It is well established that variations in genes can alter the pharmacokinetic and pharmacodynamic profile of a drug and immunological responses to it. Early advances in pharmacogenetics were made with traditional genetic techniques such as functional cloning of genes using knowledge gained from purified proteins, and candidate gene analysis. Over the past decade, techniques for analysing the human genome have accelerated greatly as knowledge and technological capabilities have grown. These techniques were initially focussed on understanding genetic factors of disease, but increasingly they are helping to clarify the genetic basis of variable drug responses and adverse drug reactions (ADRs). We examine genetic methods that have been applied to the understanding of ADRs, review the current state of knowledge of genetic factors that influence ADR development, and discuss how the application of genome-wide association studies and next-generation sequencing approaches is supporting and extending existing knowledge of pharmacogenetic processes leading to ADRs. Such approaches have identified single genes that are major contributing genetic risk factors for an ADR, (such as flucloxacillin and drug-induced liver disease), making pre-treatment testing a possibility. They have contributed to the identification of multiple genetic determinants of a single ADR, some involving both pharmacologic and immunological processes (such as phenytoin and severe cutaneous adverse reactions). They have indicated that rare genetic variants, often not previously reported, are likely to have more influence on the phenotype than common variants that have been traditionally tested for. The problem of genotype/phenotype discordance affecting the interpretation of pharmacogenetic screening and the future of genome-based testing applied to ADRs are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adverse drug reactions can often result from underlying genetic factors. |
Human genomes harbour many rare genetic variants that may contribute to unusual drug responses or adverse drug reactions. |
The application of modern genomic methods such as genome-wide association studies and next-generation sequencing is helping to clarify these genetic risk factors. |
As generation of genomic data becomes more routine in the clinical setting, knowledge of genetic variation that contributes to adverse drug reactions could be of predictive value, even for adverse drug reactions that are rare. |
1 Introduction
The variability between individuals in their response to drugs has been recognised for several decades. Historically, pharmacogenetic effects were noted as early as 510 B.C. when Pythagoras noted that ingestion of fava beans resulted in the acute sickness and death of some individuals [1]. Twenty centuries later, it was discovered that a defect in the glucose-6-phosphate dehydrogenase enzyme was associated with haemolytic anaemia after exposure to fresh fava beans or drugs such as primaquine, aspirin or phenacetin [1]. This discovery was followed by the characterisation of genetic variation in the pseudocholinesterase enzyme underlying the prolonged response to choline esters during anaesthetic induction [2, 3], and later the genetic variation in acetylator enzymes resulting in variable response to the drug isoniazid [4]. The discovery of polymorphic cytochrome P450 enzyme (CYP)2D6, was not until the late 1970s [5, 6] to late 1980s when mutations associated with debrisoquine metabolism were characterised [7, 8]. These genetic variants caused changes in the pharmacokinetic or pharmacodynamic profile of a drug, therefore impacting efficacy and often resulting in drug-induced toxicity [7].
Our understanding of genetic factors that underpin adverse drug reactions (ADRs) has grown through the last few decades as genetic technologies have become increasingly sophisticated. Although the primary focus of these technologies has been the mapping, identification and analysis of genes that contribute to disease, these tools have also been applied to explore the variability in human drug responses. Pharmacogenetics, like human genetics in general, began with the analysis of traits encoded by a single gene, simply because such traits were more amenable to study. These traits, referred to as being monogenic or Mendelian in nature, arise from mutation of a single causative gene, and they generally display clear familial inheritance patterns. One example of such a Mendelian pharmacogenetic trait is the ryanodine receptor mutations that cause malignant hyperthermia after administration of general anaesthetics, in an autosomal dominant fashion (meaning only one copy of the gene, or allele, need be mutated) [9, 10]. However, we now recognise that relatively few traits are truly monogenic, and most result from the interaction of many genetic and environmental factors. Most common diseases and other phenotypes such as height and weight fall into this category, and we refer to these as complex traits. There is increasing evidence that many drug responses are also complex traits. Although we have yet to completely describe the genetic architecture of any complex human trait, it is clear that in general many genes, each of small effect size, contribute to such phenotypes (Fig. 1).
Monogenic and complex traits. Monogenic traits arise from mutation of a single gene, and usually display clear familial patterns of inheritance, reflecting whether the trait occurs when one allele (dominant) or both alleles (recessive) are disrupted. Complex traits arise from the input of polymorphic variation in several to many genes, each of which contributes a small effect to the trait. Complex traits have some degree of familiality, but do not display the classical patterns of inheritance seen in monogenic traits. Modified from [165]
2 Genetic Technologies and ADRs
2.1 Linkage Mapping
One of the most productive early methods for exploring monogenic traits was linkage analysis, which involved tracking the pattern of inheritance of DNA markers within families displaying the trait or disease, to map the location of the underlying causative gene [11]. Although a very productive approach in studies of human genetic disease [12], linkage mapping has not been an avenue widely available for pharmacogenetic studies simply because it is rare for pharmacogenetic phenotypes to be defined in all members of large families. Even those ADRs that may result from the effect of a single major gene will often not be recognised as such, unless multiple members of a family have been exposed to the same or similar drugs.
2.1.1 Early Genetic Studies on ADRs
Despite the inability to widely apply linkage methods to the analysis of pharmacogenetics, some important early advances were made using functional candidate gene analysis, where variation in genes functionally linked to the relevant phenotype were studied in groups of subjects. For example, the debrisoquine/sparteine metabolism phenotype, which essentially behaves as a monogenic trait, was first observed in individuals [5, 6], then further characterised using a gene cloning and identification method that depended on an understanding of the function of the gene of interest, leading to the description of CYP2D6 and the main poor metaboliser variants [7, 8].
Similarly, the thiopurine methyltransferase (TPMT) gene was isolated after purification and amino-acid sequencing of the protein, which provided information that led to molecular cloning of the gene [13, 14]. Neither of these classic pharmacogenes, which can contribute to ADRs, was identified in linkage studies, although the phenotypes they caused were recognised to track within families. Rather, detailed prior pharmacological investigation was required to pinpoint the relevant protein, which then led to isolation of the genes.
Such candidate gene studies, where “educated guesses” of genes likely to underpin a phenotype, were the predominant approach in genetics and pharmacogenetics [15, 16] until the advent of genome-wide association studies (GWAS) [17–19].
2.1.2 Genome-Wide Association Studies
GWAS have enabled the precise and effective discovery of genes underpinning complex diseases and traits, including drug treatment responses [20, 21]. These studies require the high-throughput analysis of single nucleotide polymorphisms (SNPs), i.e., variations in single base pairs, throughout the genome. SNPs can impact the way a protein is coded in a particular gene, the way it is spliced, expressed, or regulated. When these changes occur in genes coding for enzymes, transporters, cell membrane receptors, intracellular receptors, or components of ion channels, they may change the pharmacokinetic or pharmacodynamic profile of a drug, affecting its efficacy and its likelihood of causing ADRs [22, 23]. These SNPs are catalogued by unique identifiers (called Reference SNP cluster ID, or “rs” numbers, as listed in Table 1) [24].
GWAS methodology was made possible by the convergence of several lines of investigation. First, the efforts directed at cataloguing human genetic variation, particularly SNPs, allowed development of very rich maps illustrating the correlations (linkage disequilibrium) between alleles of SNPs in the human genome [25, 26]. Second, commercial interests led to the development of several platforms for massively parallel analysis (genotyping) of SNPs on “gene chips”. Third, the mathematical and computational tools necessary for processing the very large datasets generated by genotyping many thousands of SNPs in hundreds or thousands of subjects were developed [27]. One further factor was essential for the success of GWAS. It became increasingly clear that for truly complex traits, large cohorts of cases and controls would be needed to identify the many genes of small effect underlying each trait; this realisation drove extensive international collaborations on human complex disease studies, in a way not previously seen in biomedical science [28].
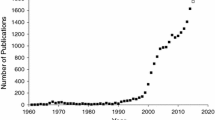
Since the first application of GWAS technology [29], over 2000 genes contributing to complex traits have been identified using this method [20, 21]. Although the primary application of GWAS has been to the understanding of human diseases and other complex traits, the method has been increasingly employed to study the genetics of drug responses and adverse drug reactions [22, 30, 31]. A major challenge for the application of GWAS to ADRs is the problem of collecting sufficient samples, given the rarity of these phenotypes. This requires concerted international collection and aggregation of samples, such as is being mediated by the International Serious Adverse Events Consortium [32], EUDRAGENE [33], and other national and international consortia [34, 35]. One of the surprises resulting from the application of GWAS to ADRs has been that even with small numbers of subjects relative to those needed for studies of complex disease, single genes have been clearly identified as contributing risk factors for some ADRs. Good examples are the association of variants in SLC01B1, the gene for the organic anion-transporting polypeptide OATP1B1, with statin-induced myopathy [36], and the association of human leukocyte antigen (HLA)-B*5701 variants with drug-induced liver injury (DILI) from flucloxacillin [37].
As well as discovering genetic variants underpinning several ADRs, GWAS have also extended existing knowledge of pharmacogenetic processes. For example, in warfarin dose response, variants of VKORC1 and CYP2C9 had long been recognised as major factors. GWAS initially confirmed the role of these two genes [38] and then revealed an additional gene, CYP4F2, with a relatively minor role [39]. The range of GWAS studies now published in pharmacogenetics makes it clear that technology is no longer the main limiting factor for understanding genetic factors influencing ADRs [22, 30], but rather it is often the timely identification, consenting and collection of subjects to add into such studies that limits progress.
2.1.3 Next-Generation Sequencing Methods
Over the past decade, methods for DNA sequencing have undergone dramatic improvements. These methods, appropriately named next-generation sequencing (NGS) technologies, have vastly increased the scale of DNA sequencing while also reducing the unit cost [40, 41]. Although the first human genome project took over a decade and cost some USD 3 billion, NGS advances now mean that a human genome can be sequenced in a few hours for less than USD $2000.
Although whole genome sequencing (WGS) is now possible [42, 43], the datasets that result and the processing power required to effectively analyse them, means that they have not yet been widely employed. Instead, analysis of only a subset of the genome, known as the exome, has been the preferred method for many initial studies [44] (Fig. 2). The exome spans all exons, or protein coding regions, of an individual’s DNA, and the process of whole exome sequencing (WES) allows physical “capture” and then sequencing of most exons from an individual in one NGS work flow. Application of WES means that variations in protein-coding regions of any gene can be identified, rather than focusing on one or a few genes as in traditional candidate gene studies [44, 45]. Although it has been a very informative technology, WES has some significant limitations, the most important of which is its inability to identify variants located outside of exons, in regulatory regions (introns), or in regions not known to be associated with any genes [46, 47]. In addition, exome data are not well suited to the identification of major structural variations seen in the genome, known as copy number variations. These limitations, combined with the recent availability of newer massive-throughput DNA sequencers, mean that WGS is gaining in popularity and this, rather than WES, may soon become the dominant approach for genome analysis [48].
The evolution in genomic technologies. Pharmacogenetic analysis has evolved from analysing one gene (in a few patients) and a few single nucleotide polymorphisms (SNPs) in a candidate gene study, to genome-wide association studies (GWAS), which look through a library of up to a million SNPs in groups of patients by using high-throughput genotyping systems (referred to as a SNP chips or SNP array). Next-generation sequencing has now taken a further step by enabling researchers to sequence the protein coding part of the genome (approximately 1 %)—whole exome sequencing (WES), or even the entire genome—whole genome sequencing (WGS)
Below we summarise current knowledge of genetic loci that have been identified as significant pharmacogenetic markers for pharmacokinetic or pharmacodynamic drug profiles or individual immunologic responses to drugs leading to ADRs. Where available, we will identify advances in pharmacogenomics that have arisen through application of the newer genetic technologies, including GWAS and NGS.
3 Drug-Metabolising Enzymes
The initial focus of pharmacogenetics was the polymorphisms affecting drug-metabolising enzymes (DMEs). Up to 60 % of all drug-induced toxicity is associated with polymorphic CYPs, also referred to as ‘phase one enzymes’ [49]. The CYP gene superfamily account for the majority (86 %) of all DMEs. Polymorphisms in CYP genes result in four major phenotypes with respect to drug metabolism, poor metaboliser, intermediate metaboliser, extensive metaboliser or ultra-rapid metaboliser. A particular polymorphism may therefore result in therapeutic failure or toxicity, with a reverse effect if the drug is a pro-drug [50].
Although to date there are relatively few publications that use NGS methods to investigate drug-induced ADRs and associations with variants in CYP enzymes, a study by Gordon et al. applied targeted NGS of multiple genes from over 14,000 subjects, to illustrate the extent of potentially deleterious variation in 12 CYP genes. The authors focussed on a set of 12 CYP genes that they described as being responsible for 75 % of all drug metabolism through oxidation reactions [51]. A total of 219 likely functional variants across 12 CYP genes were discovered, and variants were found to be abundant, occurring at an average of one per 17 bases sequenced [51].
Below we summarise clinically important polymorphisms affecting widely used drugs metabolised by CYP2D6, CYP3A4, CYP2C9 and CYP2C19.
3.1 CYP2D6
The gene coding the CYP2D6 enzyme is one of the best studied pharmacogenes. Approximately 25 % of all drugs, including a number of antidepressants, anti-arrhythmics, beta-blockers, opioid analgesics and anti-cancer agents, are metabolised through CYP2D6, which also happens to be one of the most polymorphic enzymes with over 100 known allelic variants. A 20-fold inter-individual variation in steady-state plasma concentrations of nortriptyline, a substrate for CYP2D6, following a standard daily dose over 2 weeks, was first reported in 1967 [52]. It is now known that polymorphisms in the CYP2D6 gene can result in a range of effects from complete loss of enzyme activity through deletion of the gene (poor metaboliser status), or extensive activity, through duplication of the gene (ultra-rapid metaboliser status) [50]. Common CYP2D6 variants are shown in Table 1.
Commonly prescribed analgesics such as codeine, dihydrocodeine, tramadol and morphine, are largely metabolised through hepatic CYP2D6. The Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine recommend using alternative analgesics in patients who have been genotyped as ultra-rapid metabolisers (an individual carrying more than two functional alleles) or poor metabolisers (an individual carrying no functional CYP2D6 alleles) [53]. Ultra-rapid metabolisers are likely to excessively metabolise the parent compound, in the case of codeine to morphine resulting in ADRs ranging from mild nausea, vomiting and drowsiness to more severe, but rare circulatory depression, shock or cardiac arrest [53–55]. In contrast, poor metabolisers have low codeine-morphine conversion and complain of poor or no analgesia [56, 57]. The CPIC has also recommended that poor and ultra-rapid metabolisers use morphine and non-opioid analgesics instead of tramadol, oxycodone and hydrocodone, as their metabolism is also affected by CYP2D6 polymorphisms [58].
A large proportion of antidepressant and antipsychotic drugs are metabolised predominantly through the activity of CYP2D6. CPIC dosing guidelines are currently available for several such drugs, mostly tricyclic antidepressants. These guidelines recommend a 25–50 % reduction in dose for intermediate-poor metabolisers, and an alternative drug for ultra-rapid metabolisers [59]. Studies conducted in patients taking amitriptyline showed that CYP2D6 poor metaboliser status resulted in impaired drug metabolism, hence elevated amitriptyline plasma concentrations and an increased risk of ADRs, discontinuation of therapy or switching to another drug [60, 61]. According to the catalogue of published GWAS [62], a study in 435 patients with major depressive disorder found single nucleotide polymorphisms in the CYP2D6 and CYP2C19 genes to be significantly associated with measured plasma concentrations of citalopram, escitalopram and their metabolites [63].
A difference in the CYP2D6*4 allele frequency was noted between 75 patients with atorvastatin-related myopathy and 188 atorvastatin-tolerant controls and between 61 patients with simvastatin-related myopathy and 188 controls although the difference was only significant for atorvastatin. Other studies have not confirmed this association but the possible effect on a particular statin may have been masked by several statins being studied as a group [59].
3.2 CYP3A4
Compared with CYP2D6, CYP3A4 is responsible for the metabolism of a greater proportion of drugs, up to 50–60 %, but is “strongly conserved”, meaning that the CYP3A4 gene is not as polymorphic as the CYP2D6 gene. While several polymorphisms within the CYP3A4 gene have been identified, current consensus is that SNPs affecting this gene generally have minimal clinical significance. While SNPs within the CYP3A4 gene may contribute to inter-individual differences, they occur at low population allele frequencies and are not reported to affect the pharmacokinetics and/or pharmacodynamics of CYP3A4 substrates in a major way [64]. However, while being extremely rare (<0.06 % in Caucasians) [65], the CYP3A4*20 variant was recently identified in a cohort of eight patients who had experienced severe paclitaxel-induced neuropathy, which is known to be dose dependent [66]. Using WES, two patients were found to have the rare CYP3A4*20 allele, a premature stop codon that leads to an abnormally shortened protein, and one patient had a CYP3A4*25 variant, a missense mutation causing the substitution of a different amino acid in the resulting protein. Both variants confer significantly reduced CYP3A4 expression. Analysis of DNA from an independent cohort of 228 patients treated with paclitaxel indicated a 1.3- to 2.0-fold increased risk of paclitaxel-induced neuropathy in patients carrying CYP3A4 variants that reduced enzyme expression compared with wild-type CYP3A4 [66].
A related enzyme, CYP3A5, is considered to have similar substrate specificity to CYP3A4. However, it is subject to more polymorphisms. Only the CYP3A5*1 (WT) allele is recognised as functional, the remaining CYP3A5 variants (*2–*11) are non-functional. With respect to clinical significance, differences in tacrolimus [67] concentrations were reported in subjects with the CYP3A5*3 variant compared with those with CYP3A5*1 and it was concluded that a higher dose of the drug may be required to maintain optimal blood concentrations in expressors of the functional variant [67].
Atorvastatin is a substrate for CYP3A5. Evidence that CYP3A5 polymorphisms are clinically important in the metabolism of this drug is conflicting but in an exploratory analysis of patients with atorvastatin-related myopathy, the CYP3A5*3 allele was associated with the degree of serum creatine kinase (CK) elevation [68].
3.3 CYP2C9
The enzyme CYP2C9 metabolises approximately 15 % of all clinical drugs including some oral hypoglycaemics, nonsteroidal anti-inflammatory drugs, diuretics, antiepileptic drugs, angiotensin converting enzyme inhibitors and, in particular, several drugs with a narrow therapeutic index such as warfarin (S-warfarin) and phenytoin [64]. Two commonly occurring missense mutations in the CYP2C9 gene,*2 and *3, decrease enzyme function by 30 and 90 %, respectively. Two large, recent randomised controlled studies applied rapid turnaround CYP2C9 genotyping tests to assess the benefits of genotype-guided dosing of warfarin. Pirmohamed et al. concluded that genotyping (CYP2C9*2, CYP2C9*3) prior to initiation of warfarin therapy resulted in a significantly (P < 0.001) greater number of patients remaining within the target therapeutic range and significantly (P < 0.001) fewer incidences of over-anticoagulation, defined as an international normalized ratio (INR) >4 [69]. However, a similarly designed trial, the Clarification of Optimal Anticoagulation through Genetics (COAG) trial did not show any benefit of genotype-guided dosing [70]. Similarly, various meta-analyses have reported mixed results. For example, a meta-analysis of nine trials conducted in 2001–2013 concluded that there was no significant clinical benefit of genotype-guided warfarin dosing on either time in the recommended therapeutic range or the risk of an INR >4. The authors noted that the nine trials used different genotyping methods as well as different genotype-based warfarin dosing algorithms, which may have resulted in skewed outcome definitions [71]. However, a further meta-analysis of 10 studies concluded that genotype-based dosing of warfarin increased the percentage of time in the therapeutic range and reduced the risk of haemorrhagic complications [72]. CPIC guidelines on genotype-guided warfarin dosing were last published in 2011 [73], and are currently under review based on the findings discussed above. The guidelines published in 2011 recommended the use of genetic-guided algorithms available on http://www.warfarindosing.org. Dosing algorithms taking genetics into account outperform non-genetic algorithms [73].
Polymorphisms in CYP2C9 have also been associated with phenytoin ADRs. Chung et al. used a GWAS to investigate genetic variants associated with phenytoin-related severe cutaneous adverse reactions. They discovered a cluster of 16 SNPs within the CYP2C gene locus [74] associated with phenytoin-related ADRs. Further sequencing of alleles in this region identified a significant association between the missense variant rs1057910 (CYP2C9*3) and the severe forms of phenytoin-related cutaneous adverse reactions often referred to as SCARs This SNP-ADR association was then validated through analysis of further samples from 210 patients with phenytoin-related SCARs and 3655 controls, and an odds ratio of 11.0 (95 % confidence interval 6.2–18.0, P < 0.00001) was reported [74]. Delayed plasma clearance of phenytoin was detected in patients with SCARs, especially CYP2C9*3 carriers.
3.4 CYP2C19
The enzyme CYP2C19 is known to metabolise 10 % of all commonly used medicines including proton pump inhibitors, tricyclic antidepressants, SSRIs, SNRIs, barbiturates, and the antiplatelet drugs clopidogrel, ticlopidine, and prasugrel. The CYP2C19 gene has at least 24 known variants with CYP2C19*2 and CYP2C19*3 the major polymorphisms resulting in poor metaboliser status and CYP2C19*17, a polymorphism resulting in increased CYP2C19 expression and activity [64]. There is substantial literature on the association between CYP2C19 poor metaboliser status and diminished response to clopidogrel, and therefore an increased risk of further cardiovascular events [75]. As clopidogrel is a prodrug it requires biotransformation to its active form in the liver by CYP2C19, CYP1A2 and CYP2B6. A GWAS (Pharmacogenomics of Antiplatelet Intervention) reported an association with 13 SNPs in the genomic region where the CYP2C18-CYP2C19-CYP2C9-CYPC8 genes are located [76]. Further analysis showed that the variant rs12777823 was strongly correlated with the CYP2C19*2 variant, and was associated with a greater number of cardiovascular events or death within 1 year of follow-up (20.9 %) compared with controls (10 %) [76].
Meta-analyses of randomised clinical trials evaluating CYP2C19 genotype status and the increased risk of secondary cardiovascular events have reported mixed results. Mega et al. conducted a meta-analysis of nine studies incorporating 9685 patients who had undergone percutaneous intervention and/or had acute coronary syndrome [77]. In the population studied, 71.5 % were non-carriers (i.e., CYP2C19 WT), 26.3 % had one reduced function allele (*2 or *3), and 2.2 % had two reduced functional alleles. The authors reported a significantly increased risk of major cardiovascular events, particularly stent thrombosis in patients with one (P < 0.0001) or two (P = 0.001) CYP2C19 reduced function alleles [77]. Similarly, Hulot et al. reported that CYP2C19*2 allele was associated with a 30 % increased risk of a major cardiovascular event and increased mortality in patients on clopidogrel therapy. Like the previous study, subjects with either heterozygote or homozygote CYP2C19*2 alleles were adversely affected [78]. However, two further meta-analyses have reported that the genetic association between CYP2C19 genotype and clinical efficacy of clopidogrel is not consistent or substantial enough to recommend genotyping prior to therapy [79, 80]. The CPIC guidelines for clopidogrel indicate that clinicians should consider alternative anti-platelet agents (prasugrel or ticagrelor) in patients genotyped to be CYP2C19 intermediate (*1/*2, *1/*3, *2/*17) or poor (*2/*2, *3/*3 or *2/*3) metabolisers [81].
4 Phase II Enzymes
Polymorphisms in genes for phase II DMEs are a further source of variation in drug response. Polymorphic phase two DMEs include N-acetyl transferase type 2 (NAT2) associated with isoniazid toxicity; thiopurine methyltransferases (TPMT) associated with thiopurine toxicity; dihydropyrimidine dehydrogenase (DPD) associated with 5-fluorouracil toxicity, and uridine diphosphate-glucuronosyl transferases (UGT) associated with irinotecan toxicity [82, 83]. TPMT and NAT2 are discussed below and DPD and UGT are summarised in Table 2.
4.1 TPMT
Depending on ethnicity, it is estimated that one in 150–300 individuals carry two deficient TPMT alleles resulting in lack of TPMT activity [84]. As TPMT catalyses the s-methylation of thiopurine drugs, which are highly toxic and have a narrow therapeutic index, accumulation of parent drug and/or metabolites can lead to thiopurine toxicity in haematopoietic tissues [14, 85]. Expression of non-functional and/or reduced function alleles (Table 3) has been associated with a range of adverse events ranging from cessation of therapy in up to 25 % of patients to severe and life-threatening myelosupression [86–88].
CPIC have published guidelines on the prescribing of three drugs (azathioprine, mercaptopurine and thioguanine) known to be influenced by TPMT polymorphisms [89]. The CPIC guidelines indicate that pre-emptive TPMT genetic testing can provide customised dosing to reduce the likelihood of serious and fatal ADRs such as myelosupression [88, 90, 91]. A recent retrospective study conducted in a French university hospital concluded that pre-emptive TPMT genotyping improved non-compliance and allowed the identification of patients at high risk of toxicity [92].
4.2 NAT
N-Acetyltransferase enzymes, NAT1 and NAT2 metabolise and detoxify therapeutic drugs through acetylation. Notably, variability in response to the antituberculosis drug isoniazid is associated with polymorphisms in the NAT2 gene [93]. Phenotypically, NAT2 polymorphisms confer either slow or fast acetylator status. Slow acetylators, often expressing two reduced function and/or inactive alleles, are reported to be at a greater risk of isoniazid toxicity, particularly DILI and peripheral neuropathy. In contrast, fast acetylators are likely to show reduced efficacy [93, 94]. Several retrospective studies have sought to determine whether genotyping of NAT2 status may have prevented isoniazid induced toxicity. One such study conducted by Ng et al. genotyped 26 patients with a history of liver injury as a result of a drug regimen containing isoniazid. Patients and ethnically matched controls were genotyped for three major NAT alleles (NAT2*5, NAT2*6 and NAT2*7), and it was observed that NAT2 genotypes predictive of slow acetylator phenotype were associated with an increased risk of isoniazid-induced DILI [95]. Azuma et al. also showed that NAT2 genotype-based dosing of isoniazid compared with standard treatment was beneficial. The clinical trial showed that with genotype-guided dosing there were no cases of isoniazid-induced DILI amongst slow acetylators, compared with 78 % of slow acetylators in the standard treatment group. With respect to fast-acetylators and treatment failure, genotyping resulted in a lower incidence of treatment failure (15 %) when compared with the standard treatment group (38 %) [96].
4.3 Drug Transporters
Polymorphisms also affect transporters and therefore drug distribution. For example, polymorphisms in the organic anion transporting polypeptides, also referred to as the solute carrier organic anion transporters (SLCOs), are one of the most discussed polymorphisms known to affect the transport (influx) of statins, and hence impaired efficacy and/or toxicity [97–99]. Two SNPs in the SLCO1B1 gene, rs2306283 and rs4149056 are associated with statin-associated myopathy [100]. These variants were initially identified through a GWAS conducted on the SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) cohort of 80 confirmed cases of myopathy and 90 controls [36]. Further GWAS of similar cohorts such as GoDARTs (Genetics of Diabetes Audit and Research in Tayside) and STRENGTH (Statin Response Examined by Genetic Haplotype Markers) have replicated the results. The composite endpoint was any adverse effect that led to discontinuation or myalgia or serum creatine kinase level more than three times the upper limit of normal. For this endpoint there was a gene–dose effect relationship with 19, 27 and 50 % of patients affected with no, one or two SLCO1B1*5 alleles respectively [101, 102]. Interestingly, the association of rs4149056 with statin-induced myopathy has only been clearly established for simvastatin [100].
Further studies have identified polymorphisms in other drug transporters such as the ATP-binding cassette family (ABC), specifically ABCB1 and ABCG2, which are efflux transporters that modulate intestinal drug absorption and tissue penetration [103] and have been associated with statin-induced muscle myopathy as shown through elevations in plasma creatine kinase measurements. As expected, adverse reactions to a range of drugs (Table 4) have been linked with transporter polymorphisms, mainly by way of candidate gene association studies.
Zolk and Fromm also identified polymorphisms in four genes, SLCO1B1, SLC22A2, ABCB11 and ABCB1, which are associated with increased susceptibility to ADRs in general [104].
5 Pharmacodynamic Responses
5.1 Drug-Induced Long QT Syndrome
Long QT syndrome (LQTS) is a condition with symptoms of syncope, seizures and often fatal ventricular arrythmias of the torsade de pointes type. Initially, by studying patients with congenital LQTS, genes that code for sodium and potassium ion channels within cardiomyocytes were implicated in the disorder [105]. The first mutations in the potassium channel genes KCNQ1 and KCNH2 (HERG) and the SCN5A cardiac sodium channel gene (originally named LQT1, LQT2 and LQT3 respectively), were first discovered in the 1990s [106]. Individuals with drug-induced (also referred to as acquired) LQTS present with the same symptoms and are often carriers of KCNH2 or SCN5A mutations [105, 106]. Various drug classes (antibiotics, antipsychotics, chemotherapeutics, antiemetics, opioid analgesics and anti-arrhythmics) have been associated with drug-induced LQTS [107]. The association of KCNH2 and SCN5A mutations with LQTS has been confirmed through four major GWAS published in the last decade [108–111]. Recently, Weeke et al. identified, through whole exome sequencing, rare amino acid coding variants that further increase the risk of drug-induced LQTS. There were more unique or rare amino acid coding variants (37 %) in a cohort of 65 patients with previously confirmed drug-induced LQTS compared with 148 (21 %) drug-exposed controls [112]. Similarly, Ramirez et al. have used NGS methodology to assess the presence of rare variants in a cohort of patients with drug-induced LQTS. It was reported that 11 of the 31 patients carried a novel missense mutation that matched a known congenital LQTS mutation [113].
5.2 Warfarin and Vitamin K Epoxide Reductase Complex
Warfarin exerts its anticoagulant effect by inhibiting the vitamin K epoxide reductase complex, subunit 1 (VKORC1), part of an enzyme that had long been sought as a target of warfarin but for which the gene was not identified until 2004 [114]. The identification of this enzyme, as well as linkage studies carried out in warfarin-resistant rat strains, rapidly led to identification of variants in the VKORC1 gene, which impacted on warfarin response [115], and in some patients, warfarin resistance [116].
The missense mutations CYP2C9 *2 and *3 and the VKORC1 variants identified are evidence of a combined effect of pharmacogenes influencing both the pharmacokinetics and pharmacodynamics of a medicine. GWAS initially confirmed the role of these two genes [38] and revealed an additional gene, CYP4F2, with a minor role [39].
5.3 HLA Locus
The HLA, also known as the human major histocompatibility complex (MHC), is a family of over 200 genes that are located close together on chromosome 6. The MHC genes are categorised into three classes, of which class I (HLA-A, HLA-B and HLA-C genes) and class II (HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DRA and HLA-DRB1) are relevant to this review (Fig. 3).
Location and structure of the human leukocyte antigen (HLA) locus. The large cluster of genes that comprise the major histocompatibility complex (MHC) is located on the short arm of chromosome 6. This region includes some 240 genes and spans some 3.6 million base pairs of DNA. The class I and class II genes are most relevant for adverse drug reactions. There are three main class I genes, called HLA-A, -B and -C, and the class II region includes the genes for the α and β chains of the antigen-presenting MHC class II molecules HLA-DR, -DP, and -DQ
It is estimated that up to a third of drug-induced ADRs are unpredictable hypersensitivity reactions, i.e. Type B ADRs [117, 118], and a large proportion of these are mediated through the interaction of the drug and/or metabolite with HLA proteins. Importantly, this interaction only occurs when specific HLA alleles are present [118, 119]. The HLA-drug (hapten) complex can go on to elicit an immune response. One mechanism proposed is presentation of the hapten to a naïve lymphocyte via its T-cell receptor, which may initiate an immunological response dependent on the HLA molecule, antigen-presenting cell and cytokine environment [118].
One HLA–ADR association with considerable clinical utility is that of the haplotype (group of alleles) called HLA-B*5701 and hypersensitivity to the antiretroviral drug abacavir [120]. This was originally described by two groups who essentially used a candidate gene approach, by careful HLA typing of subjects who experienced the hypersensitivity reaction and recognition that a specific haplotype of HLA-B was over represented in this group [120, 121]. DNA tests for HLA-B*5701 alleles are now widely employed before prescription of abacavir [122, 123].
More recently, application of GWAS methodology has revealed other associations of drug hypersensitivity with a range of HLA alleles. The strongest pharmacogenetic associations have been reported for flucloxacillin-associated hepatotoxicity (also referred to as DILI) with HLA-B*5701, carbamazepine-induced Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) with HLA-B*1502 in Han Chinese, allopurinol-induced severe cutaneous ADRs with HLA-B*5801 in Han Chinese and abacavir-induced hypersensitivity syndrome with HLA-B*5701 [118, 124].
In the case of carbamazepine-induced SJS, Chung et al. showed complete penetrance of the HLA-B*1502 allele, i.e. all individuals with the mutation exhibited SJS, giving a positive predictive value of 93.6 %. In this particular study of Han Chinese, 100 % of the cohort of 44 patients expressed the HLA-B*1502 allele in comparison to 3 % in the carbamazepine-tolerant population (3/101) and 8.6 % in the general Han Chinese population (8/93) [125]. For flucloxacillin-induced DILI, a GWAS (the DILIGEN study) conducted in 51 cases and 282 controls showed that the rs2395029 SNP was significantly associated with flucloxacillin-induced DILI. The SNP was confirmed to be in linkage disequilibrium with the HLA-B*5701 allele, and carriers of this polymorphism were at 80-fold greater risk of developing flucloxacillin-induced DILI. Unlike the previously discussed HLA allele (HLA-B*1502), which was common in the Han-Chinese population, HLA-B*5701 has a high allelic frequency in northern European populations when compared with African or Asian populations [37]. Other ADRs associated with the HLA locus derived from GWAS are listed in Table 5.
Other genetic associations of HLA alleles with ADRs were recognised as early as the 1990s. For example, clozapine-induced agranulocytosis has been associated with haplotypes of HLA-B38, HLA-DR4 and HLA-DQ3 [126]. Recently, WES and GWAS methods have identified an association between clozapine-induced agranulocytosis/granulocytopenia and the HLA-DQB1 and HLA-B alleles. This latest study by Goldstein and colleagues confirms previous studies [127–129] and provides further evidence to attribute the association to two amino acids, a glutamine at position 126 of HLA-DQB1, and a threonine at position 158 of HLA-B [130]. Furthermore, the authors used molecular docking to show that clozapine binds with high affinity to the HLA-B*39 antigen-presenting peptide. Docking studies showed that clozapine had low affinity for HLA-A proteins. However, the authors noted that the set of variants identified may not be robust enough to identify a “safe-clozapine” group, as the sensitivity and specificity was low (0.36 and 0.89, respectively) [130].
With respect to thiopurine drugs (azathioprine or mercaptopurine), pancreatitis is an unpredictable ADR reported to occur in up to 4 % of patients. A recent study identified an association between azathioprine and mercaptopurine-induced pancreatitis with two HLA alleles (HLA-DQA1*02:01–HLA-DRB1*07:01 haplotype) in the class II region. It was reported that patients heterozygous for a specific SNP (rs2647087) had a 9 % risk of developing pancreatitis, whereas homozygotes had a 17 % risk [131].
HLA genotyping prior to the commencement of carbamazepine or allopurinol prescription is becoming an indispensable tool to prevent ADRs in patients of south-east Asian descent. In fact, several drugs now have updated safety labels, or boxed warnings recommending HLA genotyping prior to drug prescription [132–134]. CPIC guidelines are currently available for four drug-induced ADRs with HLA allele associations, allopurinol-HLA-B*5801, carbamazepine HLA-B*1502, abacavir-HLA-B*5701 and phenytoin-HLA-B*1502 [59].
6 ADRs Determined by Multiple Pharmacogenes
By grouping genotypes according to their kinetic, dynamic or immunological influences on the development of ADRs there is the danger of not seeing the complexity of the genetic influences that may lead to the development of one ADR. Recent studies have revealed some hitherto unexpected associations which identify more than one pharmacogene associated with a single ADR. As previously discussed, algorithms generated from warfarin dosing studies and GWAS have identified variants of CYP2C9 and VKORC that demonstrate clinical utility in warfarin dosing [39].
Similarly, the association between CYP2C9*3 and phenytoin-related severe cutaneous adverse reactions discovered by a GWAS is unlikely to be a complete explanation because these reactions have immunological characteristics. In a study by Chung et al., an association between HLA-B*1502 and phenytoin-related SJS/TEN was also shown. Chung et al. proposed that interplay between delayed clearance and the accumulation of reactive phenytoin metabolites due to genetic variants of DMEs together with individual immunogenicity might facilitate the development of phenytoin-related cutaneous adverse reactions [64]. The CPIC guidelines recommend at minimum, a 25 % reduction in the starting dose of phenytoin in CYP2C9 intermediate metabolisers, and at minimum, a 50 % reduction in phenytoin dose in poor metabolisers. Additionally, regardless of CYP2C9 status, the CPIC guidelines recommend using an anticonvulsant other than carbamazepine or phenytoin if the patient is a carrier of HLA-B*1502 unless the benefits of treatment outweigh the risks of developing SJS/TEN [135].
Statins are another group of drugs known to have a number of pharmacogenes associated with altered drug disposition [136]. Statins are associated with myopathy, ranging in severity from asymptomatic increases in creatine kinase to myalgia or muscle weakness, to fatal rhabdomyolysis. Even the less serious forms can lead to non-adherence. However, members of this therapeutic group vary in their degree of lipophilicity and metabolic pathways. Recently, concerns have been raised about a disproportionate increase in the risk of myopathy with high-dose simvastatin. As discussed, for statins, SLCO1B1 variants affecting SLCO influx transporter activity appear to be the most important genetic determinants for the development of myopathies, although the strongest evidence is for simvastatin [36, 101, 137]. There is also evidence of a contribution from polymorphisms in the ABCB1 and ABCG2 efflux transporter genes. Surprisingly for the statins, which are substrates for CYP3A4 and 5 enzymes, the evidence that variants of CYP3A4 and 5 may contribute to myopathy is not conclusive and may vary between statins. An interesting development comes from a study which reported potential protection against statin-related myopathy through a variant (rs1719247) of the gene for the glycine amidinotransferase (GATM) mitochondrial enzyme that catalyses the rate-limiting step in the biosynthesis of creatine. This hypothesis was tested in two groups of patients with a resulting meta-analysis odds ratio of 0.6 (95 % confidence interval 0.45–0.81) for the association of this GATM SNP with myopathy [138].
The finding that the strongest relationship between SLCO1B1*5 and statin-related myopathy is for simvastatin is interesting and has practical implications as a study of clinical trial data and reported ADRs concluded that high-dose simvastatin (80 mg daily) carries a greater risk of fatal myopathy than 80 mg atorvastatin and lower doses of rosuvastatin. Because the SEARCH study did not demonstrate a difference in the development of cardiovascular events between low- and high-dose simvastatin, the US Food and Drug Administration advised that simvastatin 80 mg daily should not be prescribed for patients who had not already tolerated it for a year, and that alternative agents should be used if lipid targets could not be reached with lower doses of simvastatin [139].
Recently, there have been reports of patients expressing autoantibodies to the HMG-CoA reductase enzyme which results in immune-mediated myositis and necrotising myopathy. In a recent study of patients with idiopathic inflammatory myopathy, the presence of anti-HMGCR antibodies was significantly (P < 0.0001) associated with statin exposure and HLA-DRB1*11 [140].
Last, it is important to note that patients may sometimes carry novel variants that affect drug disposition. For example, a recent study described the whole-gene sequencing of CYP2D6 and CYP2C19 in a patient with severe adverse effects to venlafaxine or combined therapy with nortriptyline and fluoxetine. Chua et al. identified one novel mutation in the CYP2D6 gene and three novel mutations in the CYP2C19 gene, meaning the function of both genes was compromised hence providing an explanation for their reported adverse effects to anti-depressants [141]. This case reinforces the notion that rare genetic variants, often not previously reported, are likely to have more substantial phenotypic effects than common variants. Had traditional genetic testing solely of “known” common variants [142] been conducted in this case, the patient may have been incorrectly classified as having intermediate CYP2D6 metabolic status, and normal CYP2C19 function. Although this study was conducted with Sanger sequencing, NGS methods make the wider analysis of all relevant variation in pharmacogenes, beyond solely the common variants, a much more accessible prospect.
7 Limitations of Using Genome Sequencing in Clinical Decision Making
As pharmacogenetic testing makes its way into the clinical setting, it is not likely to entirely displace standard therapeutic drug monitoring or measurement of other phenotypic variables for narrow-therapeutic index drugs or drugs intended for the treatment of life-threatening diseases, such as azathioprine, phenytoin and warfarin. The concordance between genotype and phenotype is not absolute as the phenotype can be influenced by other factors such as drug–drug interactions, age, sex and co-morbid conditions [143]. For example, azathioprine-treated patients are at risk of dose-dependent myelosuppression. Neither pre-emptive TPMT genotyping nor phenotyping by enzyme activity in red blood cells can be regarded as sufficiently predictive methods. However, they can be complementary [144]. Identifying patients with null TPMT activity through genotyping (TPMT*3 and TPMT*2) can identify up to 95 % of such patients but this concordance rate drops to 86 % when classifying patients with intermediate enzyme activity. The results of genotyping can be used to make recommendations about azathioprine avoidance or dose reductions. However, it is important to note that the TPMT genotype is not the sole reason for increased risk of myelosuppression in patients taking azathioprine and determining TPMT enzyme activity and/or monitoring of 6-thioguanine nucleotide concentrations is still recommended [145, 146].
Phenoconversion, the transient conversion of genotypic extensive metabolisers to phenotypic poor metabolisers, is a phenomenon that needs to be considered in the context of genotype/phenotype concordance. Phenotypic changes may occur during the course of treatment because of co-prescription of other interacting drugs [147]. Phenoconversion has also been clinically associated with elevated cytokines present during inflammatory disease states. Shah and Smith have summarised probe studies showing rates of phenoconversion of genotypic CYP2D6 EMs to phenotypic PMs for various CYP2D6 inhibitors. They also discuss co-morbidities and present evidence for conversion of genotypic EMs to phenotypic PMs because of reduced CYP2D6, NAT2 and CYP3A4 activity in some human immunodeficiency virus-infected patients, and reduced CYP2C19 activity in studies of patients with liver disease or advanced cancer [147, 148].
8 Conclusion: Personal Genomes and the Future of Pharmacogenetic Testing
It is clear that application of new genetic technologies is enhancing our understanding of pharmacogenetics, and clarifying the genetic underpinnings of various ADRs. Although many ADRs are likely to be complex phenotypes, resulting from interactive effects of numerous genetic susceptibility alleles and environmental factors, a surprising range of ADRs appear to have a less complex genetic basis, and in many demonstrated cases, only one or a few genes appear to largely determine susceptibility to the specific ADR.
Clarification of genetic susceptibility factors for ADRs is clearly of fundamental importance, and such knowledge extends our understanding of pharmacology, genetics and immunology. Beyond such intrinsic value, however, will it ever be possible to routinely apply such knowledge to predict and prevent occurrence of ADRs? Given the rarity of many relevant gene variants, the relatively slow turnaround times and costs of conventional tests (if they are even accessible), and often limited evidence base to support the clinical utility of predictive testing, it is unlikely that many of the genetic variants described in this review could currently be used to predict likelihood of an ADR (within the conventional testing paradigm). However, genomic medicine is moving apace, and the application of NGS methods, including WES and WGS, is being explored in many areas [45, 149–151] with a number of centres evaluating the prospective application of high-throughput pharmacogenetic analysis, with decision support, in the hospital setting [152, 153]. It is foreseeable that a single NGS test spanning all clinically actionable genotypes, including those relevant to drug responses, could be established [154, 155]. Such a test would need to be carried out only once, and it could include variants that are rare and therefore uneconomic to test for in a traditional diagnostic pathology setting. Establishment of such a genome-based test will require resolution of many problems, particularly relating to Mendelian disease, such as the management of incidental findings, the problem of assigning function to novel variants, and storage and management of the data. However, it is conceivable that such a test could provide information on all variants likely to impact on pharmacokinetics and ADRs, in an affordable format. An important interim step on the path to such a goal, therefore, is to build an extensive and robust evidence base for all genetic factors that may contribute to good or bad drug responses.
References
Alving AS, Carson PE, Flanagan CL, Ickes CE. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science. 1956;124(3220):484–5.
Hodgkin W, Giblett ER, Levine H, Bauer W, Motulsky AG. Complete pseudocholinesterase deficiency: genetic and immunologic characterization. J Clin Investig. 1965;44:486–93. doi:10.1172/JCI105162.
Kalow W, Genest K. A method for the detection of atypical forms of human serum cholinesterase; determination of dibucaine numbers. Can J Biochem Physiol. 1957;35(6):339–46.
Evans DA, Manley KA, Mc KV. Genetic control of isoniazid metabolism in man. Br Med J. 1960;2(5197):485–91.
Eichelbaum M, Spannbrucker N, Steincke B, Dengler HJ. Defective N-oxidation of sparteine in man: a new pharmacogenetic defect. Eur J Clin Pharmacol. 1979;16(3):183–7.
Mahgoub A, Idle JR, Dring LG, Lancaster R, Smith RL. Polymorphic hydroxylation of debrisoquine in man. Lancet. 1977;2(8038):584–6.
Gonzalez FJ, Skoda RC, Kimura S, Umeno M, Zanger UM, Nebert DW, et al. Characterization of the common genetic defect in humans deficient in debrisoquine metabolism. Nature. 1988;331(6155):442–6. doi:10.1038/331442a0.
Skoda RC, Gonzalez FJ, Demierre A, Meyer UA. Two mutant alleles of the human cytochrome P-450db1 gene (P450C2D1) associated with genetically deficient metabolism of debrisoquine and other drugs. Proc Natl Acad Sci. 1988;85(14):5240–3.
MacLennan DH, Duff C, Zorzato F, Fujii J, Phillips M, Korneluk RG, et al. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990;343(6258):559–61. doi:10.1038/343559a0.
McCarthy TV, Healy JM, Heffron JJ, Lehane M, Deufel T, Lehmann-Horn F, et al. Localization of the malignant hyperthermia susceptibility locus to human chromosome 19q12-13.2. Nature. 1990;343(6258):562–4. doi:10.1038/343562a0.
Botstein D, White RL, Skolnick M, Davis RW. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980;32(3):314–31.
Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The human gene mutation database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133(1):1–9. doi:10.1007/s00439-013-1358-4.
Honchel R, Aksoy IA, Szumlanski C, Wood TC, Otterness DM, Wieben ED, et al. Human thiopurine methyltransferase: molecular cloning and expression of T84 colon carcinoma cell cDNA. Mol Pharmacol. 1993;43(6):878–87.
Weinshilboum R. Thiopurine pharmacogenetics: clinical and molecular studies of thiopurine methyltransferase. Drug Metab Dispos. 2001;29(4 Pt 2):601–5.
Ingelman-Sundberg M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci. 2004;25(4):193–200. doi:10.1016/j.tips.2004.02.007.
Goldstein DB, Ahmadi KR, Weale ME, Wood NW. Genome scans and candidate gene approaches in the study of common diseases and variable drug responses. Trends Genet. 2003;19(11):615–22. doi:10.1016/j.tig.2003.09.006.
Grant SF, Hakonarson H. Recent development in pharmacogenomics: from candidate genes to genome-wide association studies. Expert Rev Mol Diagn. 2007;7(4):371–93. doi:10.1586/14737159.7.4.371.
Daly AK. Genome-wide association studies in pharmacogenomics. Nat Rev Genet. 2010;11(4):241–6. doi:10.1038/nrg2751.
Nelson MR, Bacanu SA, Mosteller M, Li L, Bowman CE, Roses AD, et al. Genome-wide approaches to identify pharmacogenetic contributions to adverse drug reactions. Pharmacogenomics J. 2009;9(1):23–33. doi:10.1038/tpj.2008.4.
Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90(1):7–24. doi:10.1016/j.ajhg.2011.11.029.
Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42(Database issue):D1001–6. doi:10.1093/nar/gkt1229.
Daly AK. Using genome-wide association studies to identify genes important in serious adverse drug reactions. Annu Rev Pharmacol Toxicol. 2012;52:21–35. doi:10.1146/annurev-pharmtox-010611-134743.
Nelson MR, Wegmann D, Ehm MG, Kessner D, St Jean P, Verzilli C, et al. An abundance of rare functional variants in 202 drug target genes sequenced in 14,002 people. Science. 2012;337(6090):100–4. doi:10.1126/science.1217876.
Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29(1):308–11.
Sachidanandam R, Weissman D, Schmidt SC, Kakol JM, Stein LD, Marth G, et al. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 2001;409(6822):928–33.
The International HapMap Consortium. The international HapMap project. Nature. 2003;426(6968):789–96.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi:10.1086/519795.
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–78.
Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–9. doi:10.1126/science.1109557.
Motsinger-Reif AA, Jorgenson E, Relling MV, Kroetz DL, Weinshilboum R, Cox NJ, et al. Genome-wide association studies in pharmacogenomics: successes and lessons. Pharmacogenet Genomics. 2010;. doi:10.1097/FPC.0b013e32833d7b45.
Zhou K, Pearson ER. Insights from genome-wide association studies of drug response. Annu Rev Pharmacol Toxicol. 2013;53:299–310. doi:10.1146/annurev-pharmtox-011112-140237.
Holden AL, Contreras JL, John S, Nelson MR. The international serious adverse events consortium. Nat Rev Drug Discov. 2014;13(11):795–6. doi:10.1038/nrd4441.
Molokhia M, McKeigue P. EUDRAGENE: European collaboration to establish a case-control DNA collection for studying the genetic basis of adverse drug reactions. Pharmacogenomics. 2006;7(4):633–8. doi:10.2217/14622416.7.4.633.
Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84(3):362–9. doi:10.1038/clpt.2008.89.
Ross CJ, Visscher H, Sistonen J, Brunham LR, Pussegoda K, Loo TT, et al. The Canadian Pharmacogenomics Network for Drug Safety: a model for safety pharmacology. Thyroid. 2010;20(7):681–7. doi:10.1089/thy.2010.1642.
Search Collaborative Group, Link E, Parish S, Armitage J, Bowman L, Heath S, et al. SLCO1B1 variants and statin-induced myopathy–a genomewide study. N Engl J Med. 2008;359(8):789–99. doi:10.1056/NEJMoa0801936.
Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41(7):816–9. doi:10.1038/ng.379.
Cooper GM, Johnson JA, Langaee TY, Feng H, Stanaway IB, Schwarz UI, et al. A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood. 2008;112(4):1022–7. doi:10.1182/blood-2008-01-134247.
Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, Soranzo N, et al. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009;5(3):e1000433. doi:10.1371/journal.pgen.1000433.
Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010;11(1):31–46. doi:10.1038/nrg2626.
Roukos DH. Next-generation sequencing and epigenome technologies: potential medical applications. Expert Rev Med Devices. 2010;7(6):723–6. doi:10.1586/erd.10.68.
Drmanac R, Peters BA, Church GM, Reid CA, Xu X. Accurate whole genome sequencing as the ultimate genetic test. Clin Chem. 2015;61(1):305–6. doi:10.1373/clinchem.2014.224907.
Lacey S, Chung JY, Lin H. A comparison of whole genome sequencing with exome sequencing for family-based association studies. BMC proceedings. 2014;8(Suppl 1 Genetic Analysis Workshop 18Vanessa Olmo):S38. doi:10.1186/1753-6561-8-S1-S38.
Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12(11):745–55. doi:10.1038/nrg3031.
Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med. 2013;369(16):1502–11. doi:10.1056/NEJMoa1306555.
Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet. 2010;11(6):415–25. doi:10.1038/nrg2779.
Linderman MD, Brandt T, Edelmann L, Jabado O, Kasai Y, Kornreich R, et al. Analytical validation of whole exome and whole genome sequencing for clinical applications. BMC Med Genomics. 2014;7:20. doi:10.1186/1755-8794-7-20.
Urban TJ. Whole-genome sequencing in pharmacogenetics. Pharmacogenomics. 2013;14(4):345–8. doi:10.2217/pgs.12.211.
Amur SZI, Abernethy DR, Huang S-M, Lesko LJ. Pharmacogenomics and adverse drug reactions. Pers Med. 2010;7(6):633–42.
Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part I. Clin Pharmacokinet. 2009;48(11):689–723. doi:10.2165/11318030-000000000-00000.
Gordon AS, Tabor HK, Johnson AD, Snively BM, Assimes TL, Auer PL, et al. Quantifying rare, deleterious variation in 12 human cytochrome P450 drug-metabolism genes in a large-scale exome dataset. Hum Mol Genet. 2014;23(8):1957–63. doi:10.1093/hmg/ddt588.
Hammer W, Sjoqvist F. Plasma levels of monomethylated tricyclic antidepressants during treatment with imipramine-like compounds. Life Sci. 1967;6(17):1895–903.
Crews KR, Gaedigk A, Dunnenberger HM, Klein TE, Shen DD, Callaghan JT, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin Pharmacol Ther. 2012;91(2):321–6. doi:10.1038/clpt.2011.287.
Friedrichsdorf SJ, Nugent AP, Strobl AQ. Codeine-associated pediatric deaths despite using recommended dosing guidelines: three case reports. J Opioid Manag. 2013;9(2):151–5. doi:10.5055/jom.2013.0156.
Sistonen J, Madadi P, Ross CJ, Yazdanpanah M, Lee JW, Landsmeer ML, et al. Prediction of codeine toxicity in infants and their mothers using a novel combination of maternal genetic markers. Clin Pharmacol Ther. 2012;91(4):692–9. doi:10.1038/clpt.2011.280.
VanderVaart S, Berger H, Sistonen J, Madadi P, Matok I, Gijsen VM, et al. CYP2D6 polymorphisms and codeine analgesia in postpartum pain management: a pilot study. Ther Drug Monit. 2011;33(4):425–32. doi:10.1097/FTD.0b013e3182272b10.
Kirchheiner J, Schmidt H, Tzvetkov M, Keulen JT, Lotsch J, Roots I, et al. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J. 2007;7(4):257–65. doi:10.1038/sj.tpj.6500406.
Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014;95(4):376–82. doi:10.1038/clpt.2013.254.
Clinical Pharmacogenetics Implementation Consortium (CPIC). Dosing guidelines. https://www.pharmgkb.org/guideline/PA166104996. Accessed Jan 2015.
Steimer W, Zopf K, von Amelunxen S, Pfeiffer H, Bachofer J, Popp J, et al. Amitriptyline or not, that is the question: pharmacogenetic testing of CYP2D6 and CYP2C19 identifies patients with low or high risk for side effects in amitriptyline therapy. Clin Chem. 2005;51(2):376–85. doi:10.1373/clinchem.2004.041327.
Halling J, Weihe P, Brosen K. The CYP2D6 polymorphism in relation to the metabolism of amitriptyline and nortriptyline in the Faroese population. Br J Clin Pharmacol. 2008;65(1):134–8. doi:10.1111/j.1365-2125.2007.02969.x.
Hindorff LA MJ, Morales J, Junkins HA, Hall PN, Klemm AK, Manolio TA. A catalog of published genome-wide association studies. http://www.genome.gov/gwastudies. Accessed Jan 2015.
Ji Y, Schaid DJ, Desta Z, Kubo M, Batzler AJ, Snyder K, et al. Citalopram and escitalopram plasma drug and metabolite concentrations: genome-wide associations. Br J Clin Pharmacol. 2014;78(2):373–83. doi:10.1111/bcp.12348.
Zhou S-F, Liu J-P, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41(2):89–295. doi:10.1080/03602530902843483.
Westlind-Johnsson A, Hermann R, Huennemeyer A, Hauns B, Lahu G, Nassr N, et al. Identification and characterization of CYP3A4*20, a novel rare CYP3A4 allele without functional activity. Clin Pharmacol Ther. 2006;79(4):339–49. doi:10.1016/j.clpt.2005.11.015.
Apellaniz-Ruiz M, Lee MY, Sanchez-Barroso L, Gutierrez-Gutierrez G, Calvo I, Garcia-Estevez L, et al. Whole-exome sequencing reveals defective CYP3A4 variants predictive of paclitaxel dose-limiting neuropathy. Clin Cancer Res. 2015;21(2):322–8. doi:10.1158/1078-0432.CCR-14-1758.
Capron A, Mourad M, De Meyer M, De Pauw L, Eddour DC, Latinne D, et al. CYP3A5 and ABCB1 polymorphisms influence tacrolimus concentrations in peripheral blood mononuclear cells after renal transplantation. Pharmacogenomics. 2010;11(5):703–14. doi:10.2217/pgs.10.43.
Wilke RA, Moore JH, Burmester JK. Relative impact of CYP3A genotype and concomitant medication on the severity of atorvastatin-induced muscle damage. Pharmacogenet Genomics. 2005;15(6):415–21.
Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T, et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013;369(24):2294–303. doi:10.1056/NEJMoa1311386.
Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013;369(24):2283–93. doi:10.1056/NEJMoa1310669.
Stergiopoulos K, Brown DL. Genotype-guided vs clinical dosing of warfarin and its analogues: meta-analysis of randomized clinical trials. JAMA Intern Med. 2014;174(8):1330–8. doi:10.1001/jamainternmed.2014.2368.
Tang Q, Zou H, Guo C, Liu Z. Outcomes of pharmacogenetics-guided dosing of warfarin: a systematic review and meta-analysis. Int J Cardiol. 2014;175(3):587–91. doi:10.1016/j.ijcard.2014.06.031.
Johnson JA, Gong L, Whirl-Carrillo M, Gage BF, Scott SA, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011;90(4):625–9. doi:10.1038/clpt.2011.185.
Chung WH, Chang WC, Lee YS, Wu YY, Yang CH, Ho HC, et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA. 2014;312(5):525–34. doi:10.1001/jama.2014.7859.
Sofi F, Giusti B, Marcucci R, Gori AM, Abbate R, Gensini GF. Cytochrome P450 2C19*2 polymorphism and cardiovascular recurrences in patients taking clopidogrel: a meta-analysis. Pharmacogenomics J. 2011;11(3):199–206. doi:10.1038/tpj.2010.21.
Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–57. doi:10.1001/jama.2009.1232.
Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304(16):1821–30. doi:10.1001/jama.2010.1543.
Hulot JS, Collet JP, Silvain J, Pena A, Bellemain-Appaix A, Barthelemy O, et al. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J Am Coll Cardiol. 2010;56(2):134–43. doi:10.1016/j.jacc.2009.12.071.
Osnabrugge RL, Head SJ, Zijlstra F, Ten Berg JM, Hunink MG, Kappetein AP, et al. A systematic review and critical assessment of 11 discordant meta-analyses on reduced-function CYP2C19 genotype and risk of adverse clinical outcomes in clopidogrel users. Genet Med. 2015;17(1):3–11. doi:10.1038/gim.2014.76.
Bauer T, Bouman HJ, van Werkum JW, Ford NF, ten Berg JM, Taubert D. Impact of CYP2C19 variant genotypes on clinical efficacy of antiplatelet treatment with clopidogrel: systematic review and meta-analysis. BMJ. 2011;343:d4588. doi:10.1136/bmj.d4588.
Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94(3):317–23. doi:10.1038/clpt.2013.105.
Sim SC, Kacevska M, Ingelman-Sundberg M. Pharmacogenomics of drug-metabolizing enzymes: a recent update on clinical implications and endogenous effects. Pharmacogenomics J. 2013;13(1):1–11. doi:10.1038/tpj.2012.45.
Jancova P, Anzenbacher P, Anzenbacherova E. Phase II drug metabolizing enzymes. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslov. 2010;154(2):103–16.
Wang L, Weinshilboum R. Thiopurine S-methyltransferase pharmacogenetics: insights, challenges and future directions. Oncogene. 2006;25(11):1629–38. doi:10.1038/sj.onc.1209372.
Dong XW, Zheng Q, Zhu MM, Tong JL, Ran ZH. Thiopurine S-methyltransferase polymorphisms and thiopurine toxicity in treatment of inflammatory bowel disease. World J Gastroenterol. 2010;16(25):3187–95.
Zelinkova Z, Derijks LJ, Stokkers PC, Vogels EW, van Kampen AH, Curvers WL, et al. Inosine triphosphate pyrophosphatase and thiopurine s-methyltransferase genotypes relationship to azathioprine-induced myelosuppression. Clin Gastroenterol Hepatol. 2006;4(1):44–9. doi:10.1016/j.cgh.2005.10.019.
Hindorf U, Lindqvist M, Peterson C, Soderkvist P, Strom M, Hjortswang H, et al. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55(10):1423–31. doi:10.1136/gut.2005.074930.
Relling MV, Hancock ML, Rivera GK, Sandlund JT, Ribeiro RC, Krynetski EY, et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst. 1999;91(23):2001–8.
Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89(3):387–91. doi:10.1038/clpt.2010.320.
Relling MV, Hancock ML, Boyett JM, Pui CH, Evans WE. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. 1999;93(9):2817–23.
Ford LT, Berg JD. Thiopurine S-methyltransferase (TPMT) assessment prior to starting thiopurine drug treatment; a pharmacogenomic test whose time has come. J Clin Pathol. 2010;63(4):288–95. doi:10.1136/jcp.2009.069252.
Chouchana L, Narjoz C, Roche D, Golmard JL, Pineau B, Chatellier G, et al. Interindividual variability in TPMT enzyme activity: 10 years of experience with thiopurine pharmacogenetics and therapeutic drug monitoring. Pharmacogenomics. 2014;15(6):745–57. doi:10.2217/pgs.14.32.
Hein DW, Doll MA, Fretland AJ, Leff MA, Webb SJ, Xiao GH, et al. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomark Prev. 2000;9(1):29–42.
Wang PY, Xie SY, Hao Q, Zhang C, Jiang BF. NAT2 polymorphisms and susceptibility to anti-tuberculosis drug-induced liver injury: a meta-analysis. Int J Tuberc Lung Dis. 2012;16(5):589–95. doi:10.5588/ijtld.11.0377.
Ng CS, Hasnat A, Al Maruf A, Ahmed MU, Pirmohamed M, Day CP, et al. N-Acetyltransferase 2 (NAT2) genotype as a risk factor for development of drug-induced liver injury relating to antituberculosis drug treatment in a mixed-ethnicity patient group. Eur J Clin Pharmacol. 2014;70(9):1079–86. doi:10.1007/s00228-014-1703-0.
Azuma J, Ohno M, Kubota R, Yokota S, Nagai T, Tsuyuguchi K, et al. NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: a randomized controlled trial for pharmacogenetics-based therapy. Eur J Clin Pharmacol. 2013;69(5):1091–101. doi:10.1007/s00228-012-1429-9.
Sissung TM, Troutman SM, Campbell TJ, Pressler HM, Sung H, Bates SE, et al. Transporter pharmacogenetics: transporter polymorphisms affect normal physiology, diseases, and pharmacotherapy. Discov Med. 2012;13(68):19–34.
Yee SW, Chen L, Giacomini KM. Pharmacogenomics of membrane transporters: past, present and future. Pharmacogenomics. 2010;11(4):475–9. doi:10.2217/pgs.10.22.
Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130(6):1793–806. doi:10.1053/j.gastro.2006.02.034.
Oshiro C, Mangravite L, Klein T, Altman R. PharmGKB very important pharmacogene: SLCO1B1. Pharmacogenet Genomics. 2010;20(3):211–6. doi:10.1097/Fpc.0b013e328333b99c.
Voora D, Shah SH, Spasojevic I, Ali S, Reed CR, Salisbury BA, et al. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J Am Coll Cardiol. 2009;54(17):1609–16. doi:10.1016/j.jacc.2009.04.053.
Donnelly LA, Doney AS, Dannfald J, Whitley AL, Lang CC, Morris AD, et al. A paucimorphic variant in the HMG-CoA reductase gene is associated with lipid-lowering response to statin treatment in diabetes: a GoDARTS study. Pharmacogenet Genomics. 2008;18(12):1021–6. doi:10.1097/FPC.0b013e3283106071.
Ferrari M, Guasti L, Maresca A, Mirabile M, Contini S, Grandi A, et al. Association between statin-induced creatine kinase elevation and genetic polymorphisms in SLCO1B1, ABCB1 and ABCG2. Eur J Clin Pharmacol. 2014;70(5):539–47. doi:10.1007/s00228-014-1661-6.
Zolk O, Fromm MF. Transporter-mediated drug uptake and efflux: important determinants of adverse drug reactions. Clin Pharmacol Ther. 2011;89(6):798–805. doi:10.1038/clpt.2010.354.
Hedley PL, Jorgensen P, Schlamowitz S, Wangari R, Moolman-Smook J, Brink PA, et al. The genetic basis of long QT and short QT syndromes: a mutation update. Hum Mutat. 2009;30(11):1486–511. doi:10.1002/humu.21106.
Vincent GM. The molecular genetics of the long QT syndrome: genes causing fainting and sudden death. Annu Rev Med. 1998;49:263–74. doi:10.1146/annurev.med.49.1.263.
Crediblemeds.org. Combined list of drugs that prolong qt and/or cause torsades de pointes (tdp). 2014. http://www.crediblemeds.org/pdftemp/pdf/CompositeList.pdf. Accessed Jan 2015.
Chambers JC, Zhao J, Terracciano CM, Bezzina CR, Zhang W, Kaba R, et al. Genetic variation in SCN10A influences cardiac conduction. Nat Genet. 2010;42(2):149–52. doi:10.1038/ng.516.
Holm H, Gudbjartsson DF, Arnar DO, Thorleifsson G, Thorgeirsson G, Stefansdottir H, et al. Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet. 2010;42(2):117–22. http://www.nature.com/ng/journal/v42/n2/suppinfo/ng.511_S1.html.
Newton-Cheh C, Eijgelsheim M, Rice KM, de Bakker PI, Yin X, Estrada K, et al. Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat Genet. 2009;41(4):399–406. doi:10.1038/ng.364.
Pfeufer A, Sanna S, Arking DE, Muller M, Gateva V, Fuchsberger C, et al. Common variants at ten loci modulate the QT interval duration in the QTSCD Study. Nat Genet. 2009;41(4):407–14. doi:10.1038/ng.362.
Weeke P, Mosley JD, Hanna D, Delaney JT, Shaffer C, Wells QS, et al. Exome sequencing implicates an increased burden of rare potassium channel variants in the risk of drug-induced long QT interval syndrome. J Am Coll Cardiol. 2014;63(14):1430–7. doi:10.1016/j.jacc.2014.01.031.
Ramirez AH, Shaffer CM, Delaney JT, Sexton DP, Levy SE, Rieder MJ, et al. Novel rare variants in congenital cardiac arrhythmia genes are frequent in drug-induced torsades de pointes. Pharmacogenomics J. 2013;13(4):325–9. doi:10.1038/tpj.2012.14.
Li T, Chang CY, Jin DY, Lin PJ, Khvorova A, Stafford DW. Identification of the gene for vitamin K epoxide reductase. Nature. 2004;427(6974):541–4. doi:10.1038/nature02254.
Rost S, Fregin A, Ivaskevicius V, Conzelmann E, Hortnagel K, Pelz HJ, et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427(6974):537–41. doi:10.1038/nature02214.
Loebstein R, Dvoskin I, Halkin H, Vecsler M, Lubetsky A, Rechavi G, et al. A coding VKORC1 Asp36Tyr polymorphism predisposes to warfarin resistance. Blood. 2007;109(6):2477–80. doi:10.1182/blood-2006-08-038984.
Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68(9):1781–90.
Becquemont L. HLA: a pharmacogenomics success story. Pharmacogenomics. 2010;11(3):277–81. doi:10.2217/pgs.10.38.
Shiina T, Hosomichi K, Inoko H, Kulski JK. The HLA genomic loci map: expression, interaction, diversity and disease. J Hum Genet. 2009;54(1):15–39. doi:10.1038/jhg.2008.5.
Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358(6):568–79. doi:10.1056/NEJMoa0706135.
Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359(9312):1121–2. doi:10.1016/S0140-6736(02)08158-8.
Martin MA, Hoffman JM, Freimuth RR, Klein TE, Dong BJ, Pirmohamed M, et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing: 2014 update. Clin Pharmacol Ther. 2014;95(5):499–500. doi:10.1038/clpt.2014.38.
Martin MA, Klein TE, Dong BJ, Pirmohamed M, Haas DW, Kroetz DL, et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing. Clin Pharmacol Ther. 2012;91(4):734–8. doi:10.1038/clpt.2011.355.
Pavlos R, Mallal S, Phillips E. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2012;13(11):1285–306. doi:10.2217/pgs.12.108.
Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428(6982):486. doi:10.1038/428486a.
Lieberman JA, Yunis J, Egea E, Canoso RT, Kane JM, Yunis EJ. HLA-B38, DR4, DQw3 and clozapine-induced agranulocytosis in Jewish patients with schizophrenia. Arch Gen Psychiatry. 1990;47(10):945–8.
Profaizer T, Eckels D. HLA alleles and drug hypersensitivity reactions. Int J Immunogenet. 2012;39(2):99–105. doi:10.1111/j.1744-313X.2011.01061.x.
Athanasiou MC, Dettling M, Cascorbi I, Mosyagin I, Salisbury BA, Pierz KA, et al. Candidate gene analysis identifies a polymorphism in HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458–63. doi:10.4088/JCP.09m05527yel.
Amar A, Segman RH, Shtrussberg S, Sherman L, Safirman C, Lerer B, et al. An association between clozapine-induced agranulocytosis in schizophrenics and HLA-DQB1*0201. Int J Neuropsychopharmacol. 1998;1(1):41–4. doi:10.1017/S1461145798001023.
Goldstein JI, Jarskog LF, Hilliard C, Alfirevic A, Duncan L, Fourches D, et al. Clozapine-induced agranulocytosis is associated with rare HLA-DQB1 and HLA-B alleles. Nat Commun. 2014;5:4757. doi:10.1038/ncomms5757.
Heap GA, Weedon MN, Bewshea CM, Singh A, Chen M, Satchwell JB, et al. HLA-DQA1-HLA-DRB1 variants confer susceptibility to pancreatitis induced by thiopurine immunosuppressants. Nat Genet. 2014;46(10):1131–4. doi:10.1038/ng.3093.
United States Food and Drug Administration (FDA). Table of pharmacogenomic biomarkers in drug labeling. 2014. http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm. Accessed Jan 2015.
Guo Y, Shi L, Hong H, Su Z, Fuscoe J, Ning B. Studies on abacavir-induced hypersensitivity reaction: a successful example of translation of pharmacogenetics to personalized medicine. Sci China Life Sci. 2013;56(2):119–24. doi:10.1007/s11427-013-4438-8.
Relling MV, Klein TE. CPIC: clinical pharmacogenetics implementation consortium of the pharmacogenomics research network. Clin Pharmacol Ther. 2011;89(3):464–7. doi:10.1038/clpt.2010.279.
Caudle KE, Rettie AE, Whirl-Carrillo M, Smith LH, Mintzer S, Lee MT, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther. 2014;96(5):542–8. doi:10.1038/clpt.2014.159.
Maggo SD, Kennedy MA, Clark DW. Clinical implications of pharmacogenetic variation on the effects of statins. Drug Saf. 2011;34(1):1–19. doi:10.2165/11584380-000000000-00000.
Carr DF, O’Meara H, Jorgensen AL, Campbell J, Hobbs M, McCann G, et al. SLCO1B1 genetic variant associated with statin-induced myopathy: a proof-of-concept study using the clinical practice research datalink. Clin Pharmacol Ther. 2013;94(6):695–701. doi:10.1038/clpt.2013.161.
Mangravite LM, Engelhardt BE, Medina MW, Smith JD, Brown CD, Chasman DI, et al. A statin-dependent QTL for GATM expression is associated with statin-induced myopathy. Nature. 2013;502(7471):377–80. doi:10.1038/nature12508.
Egan A, Colman E. Weighing the benefits of high-dose simvastatin against the risk of myopathy. N Engl J Med. 2011;365(4):285–7. doi:10.1056/NEJMp1106689.
Limaye V, Bundell C, Hollingsworth P, Rojana-Udomsart A, Mastaglia F, Blumbergs P, et al. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve. 2014;. doi:10.1002/mus.24541.
Chua EW, Foulds J, Miller AL, Kennedy MA. Novel CYP2D6 and CYP2C19 variants identified in a patient with adverse reactions towards venlafaxine monotherapy and dual therapy with nortriptyline and fluoxetine. Pharmacogenet Genomics. 2013;23(9):494–7. doi:10.1097/FPC.0b013e328363688d.
McAlpine DE, Biernacka JM, Mrazek DA, O’Kane DJ, Stevens SR, Langman LJ, et al. Effect of cytochrome P450 enzyme polymorphisms on pharmacokinetics of venlafaxine. Ther Drug Monit. 2011;33(1):14–20. doi:10.1097/FTD.0b013e3181fcf94d.
de Leon J. Phenoconversion and therapeutic drug monitoring. Br J Clin Pharmacol. 2015;. doi:10.1111/bcp.12659.
Ford L, Kampanis P, Berg J. Thiopurine S-methyltransferase genotype-phenotype concordance: used as a quality assurance tool to help control the phenotype assay. Ann Clin Biochem. 2009;46(Pt 2):152–4. doi:10.1258/acb.2008.008167.
Rossi AM, Bianchi M, Guarnieri C, Barale R, Pacifici GM. Genotype-phenotype correlation for thiopurine S-methyltransferase in healthy Italian subjects. Eur J Clin Pharmacol. 2001;57(1):51–4.
Schaeffeler E, Fischer C, Brockmeier D, Wernet D, Moerike K, Eichelbaum M, et al. Comprehensive analysis of thiopurine S-methyltransferase phenotype-genotype correlation in a large population of German-Caucasians and identification of novel TPMT variants. Pharmacogenetics. 2004;14(7):407–17.
Shah RR, Smith RL. Addressing phenoconversion: the Achilles’ heel of personalized medicine. Br J Clin Pharmacol. 2015;79(2):222–40. doi:10.1111/bcp.12441.
Shah RR, Smith RL. Inflammation-induced phenoconversion of polymorphic drug metabolizing enzymes: hypothesis with implications for personalized medicine. Drug Metab Dispos. 2015;43(3):400–10. doi:10.1124/dmd.114.061093.
Willig LK, Petrikin JE, Smith LD, Saunders CJ, Thiffault I, Miller NA, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med. 2015;3(5):377–87. doi:10.1016/S2213-2600(15)00139-3.
Fan HC, Gu W, Wang J, Blumenfeld YJ, El-Sayed YY, Quake SR. Non-invasive prenatal measurement of the fetal genome. Nature. 2012;487(7407):320–4. doi:10.1038/nature11251.
Biesecker LG, Green RC. Diagnostic clinical genome and exome sequencing. N Engl J Med. 2014;370(25):2418–25. doi:10.1056/NEJMra1312543.
Wilke RA, Xu H, Denny JC, Roden DM, Krauss RM, McCarty CA, et al. The emerging role of electronic medical records in pharmacogenomics. Clin Pharmacol Ther. 2011;89(3):379–86. doi:10.1038/clpt.2010.260.
Rasmussen-Torvik LJ, Stallings SC, Gordon AS, Almoguera B, Basford MA, Bielinski SJ, et al. Design and anticipated outcomes of the eMERGE-PGx project: a multicenter pilot for preemptive pharmacogenomics in electronic health record systems. Clin Pharmacol Ther. 2014;96(4):482–9. doi:10.1038/clpt.2014.137.
Mizzi C, Peters B, Mitropoulou C, Mitropoulos K, Katsila T, Agarwal MR, et al. Personalized pharmacogenomics profiling using whole-genome sequencing. Pharmacogenomics. 2014;15(9):1223–34. doi:10.2217/pgs.14.102.
Altman RB. Pharmacogenomics: “noninferiority” is sufficient for initial implementation. Clin Pharmacol Ther. 2011;89(3):348–50. doi:10.1038/clpt.2010.310.
Ingelman-Sundberg M DA, Nebert DW. The human cytochrome P450 (CYP) allele nomenclature database. 2015. http://www.cypalleles.ki.se/cyp2d6.htm. Accessed Jan 2015.
Kindmark A, Jawaid A, Harbron CG, Barratt BJ, Bengtsson OF, Andersson TB, et al. Genome-wide pharmacogenetic investigation of a hepatic adverse event without clinical signs of immunopathology suggests an underlying immune pathogenesis. Pharmacogenomics J. 2008;8(3):186–95. doi:10.1038/sj.tpj.6500458.
Singer JB, Lewitzky S, Leroy E, Yang F, Zhao X, Klickstein L, et al. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nat Genet. 2010;42(8):711–4. doi:10.1038/ng.632.
Donaldson P BP, Graham J, Henderson J, Leathart J, Pirmohamed M, Bernal W, Aithal GP, Day CP, Daly AK, editors. Flucloxacillin-induced liver injury: the extended MHC 57.1 haplotype as a major risk factor 59th Annual Meeting of the American Association for the Study of Liver Diseases 2008; San Francisco, CA, USA: Hepatology.
Hung SI, Chung WH, Jee SH, Chen WC, Chang YT, Lee WR, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16(4):297–306. doi:10.1097/01.fpc.0000199500.46842.4a.
Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18(2):99–107. doi:10.1097/FPC.0b013e3282f3ef9c.
Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci. 2005;102(11):4134–9. doi:10.1073/pnas.0409500102.
Loganayagam A, Arenas Hernandez M, Corrigan A, Fairbanks L, Lewis CM, Harper P, et al. Pharmacogenetic variants in the DPYD, TYMS, CDA and MTHFR genes are clinically significant predictors of fluoropyrimidine toxicity. Br J Cancer. 2013;108(12):2505–15. doi:10.1038/bjc.2013.262.
Barbarino JMHC, Klein TE, Altman RB. PharmGKB summary: very important pharmacogene information for UGT1A1. Pharmacogenet Genomics. 2014;24(3):177–83.
Peltonen L, McKusick VA. Genomics and medicine. Dissecting human disease in the postgenomic era. Science. 2001;291(5507):1224–9.
Acknowledgments
Simran D.S. Maggo is a Postdoctoral Fellow supported by the Jim and Mary Carney Charitable Trust, Whangarei, New Zealand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Maggo, S.D.S., Savage, R.L. & Kennedy, M.A. Impact of New Genomic Technologies on Understanding Adverse Drug Reactions. Clin Pharmacokinet 55, 419–436 (2016). https://doi.org/10.1007/s40262-015-0324-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0324-9