Abstract
Background and Objectives
Midazolam rectal gel is a novel rectal formulation that may be a promising and potential alternative to oral administration for pediatric sedation. The objective of this study was to evaluate the safety, pharmacokinetics, pharmacodynamics, and absolute bioavailability of midazolam rectal gel in healthy Chinese subjects.
Methods
An open-label, single-dose, randomized, two-period, two-treatment, crossover clinical study was conducted in 22 healthy subjects (16 males and six females), each receiving 2.5 mg intravenous midazolam in one period and 5 mg midazolam rectal gel in another period (the dosages here were calculated as active midazolam). Safety, pharmacokinetic, and pharmacodynamic assessments were conducted throughout the study.
Results
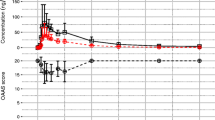
All of the subjects completed both treatment periods. The formulation of rectal gel was well tolerated, with no serious adverse events occurring. After a single rectal dose of 5 mg midazolam rectal gel, it was absorbed rapidly with a median value of time to peak concentration (Tmax) of 1.00 h, and mean values of the peak concentration (Cmax) and area under the concentration–time curve (AUC0–t) of 37.2 ng/mL and 137 h·ng/mL, respectively. The absolute bioavailability of rectal gel was 59.7%. The rectal gel exhibited a relatively delayed onset but a more stable sedative effect and a longer duration when compared with intravenous midazolam.
Conclusion
Midazolam rectal gel may be a feasible alternative with a high level of acceptance in pediatric sedation and enhanced bioavailability compared to an oral formulation. The modeling results may help to disclose out the exposure-response relationship of midazolam rectal gel and support the design of an escalating-doses study and pediatric extrapolation study.
Clinical trial registration
The study was registered at http://www.chinadrugtrials.org.cn (No. CTR20192350).




Similar content being viewed by others
References
Pecking M, Montestruc F, Marquet P, Wodey E, Homery M-C, Dostert P. Absolute bioavailability of midazolam after subcutaneous administration to healthy volunteers. Br J Clin Pharmacol. 2002;54(4):357–62.
Conway A, Rolley J, Sutherland JR. Midazolam for sedation before procedures. Cochrane Database Syst Rev. 2016;2016(5):CD009491.
Kabi F. Midazolam injection, USP Rx only. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208878Orig1s000lbl.pdf. Accessed 3 May 2023.
Balk M, Hentschke H, Rudolph U, Antkowiak B, Drexler B. Differential depression of neuronal network activity by midazolam and its main metabolite 1-hydroxymidazolam in cultured neocortical slices. Sci Rep. 2017;7(1):3503.
Mandema JW, Tuk B, van Steveninck AL, Breimer DD, Cohen AF, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite alpha-hydroxymidazolam in healthy volunteers. Clin Pharmacol Ther. 1992;51(6):715–28.
Singh SK, Dixit T. Pharmacogenomics in anesthesia. In: Handbook of pharmacogenomics and stratified medicine. Elsevier Inc.; 2014. p. 815–33. https://doi.org/10.1016/B978-0-12-386882-4.00035-9.
Sigel E, Steinmann ME. Structure, function, and modulation of GABAA receptors. J Biol Chem. 2012;287:40224–31.
Salman S, Tang EKY, Cheung LC, Nguyen MN, Sommerfield D, Slevin L, et al. A novel, palatable paediatric oral formulation of midazolam: pharmacokinetics, tolerability, efficacy and safety. Anaesthesia. 2018;73:1469–77.
Geiger CM, Sorenson B, Whaley PA. Stability of midazolam in syrspend SF and syrspend SF cherry. Int J Pharm Compd. 2013;17(4):344–6.
Johnston KR, Govel LA, Andritz MH. Gastrointestinal effects of sorbitol as an additive in liquid medications. Am J Med. 1994;97(2):185–91.
Nelson T, Xu Z. Pediatric dental sedation: challenges and opportunities. Clin Cosmet Investig Dent. 2015;7:97–106.
Payne K, Mattheyse FJ, Liebenberg D, Dawes T. The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol. 1989;37(3):267–72.
Duan YX, Li Q, Zheng AP, Zhu XW. Pharmacokinetics and the absolute bioavailability of midazolam rectal gel in rabbits. J Int Pharm Res. 2013;40(3):338–43.
Administration USF and D. Draft guidance for industry—general considerations for pediatric studies for drugs and biological products. 2014. https://www.fda.gov/media/90358/download. Accessed 05 May 2023.
Thorpy MJ, Ahmed I. Chapter 2—Approach to the patient with a sleep disorder. In: Barkoukis TJ, Matheson JK, Ferber R, Doghramji KBT-T in SM, editors. Philadelphia: W.B. Saunders; 2012. p. 10–27. https://www.sciencedirect.com/science/article/pii/B9781437717037100027.
Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10(4):244–51.
Bower AL, Ripepi A, Dilger J, Boparai N, Brody FJ, Ponsky JL. Bispectral index monitoring of sedation during endoscopy. Gastrointest Endosc. 2000;52(2):192–6.
Kim TK, Niklewski PJ, Martin JF, Obara S, Egan TD. Enhancing a sedation score to include truly noxious stimulation: the Extended Observer’s Assessment of Alertness and Sedation (EOAA/S). BJA Br J Anaesth. 2015;115(4):569–77.
Pastis NJ, Yarmus LB, Schippers F, Ostroff R, Chen A, Akulian J, et al. Safety and efficacy of Remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155(1):137–46.
Litman RS, Cohen DE, Sclabassi RJ. Chapter 9—Pediatric anesthesia equipment and monitoring. In: Motoyama EK, Davis PJ, editors. Smith’s anesthesia for infants child. 7th Ed. Philadelphia: Mosby; 2006. p. 272–318. https://www.sciencedirect.com/science/article/pii/B978032302647550014X
Agrawal D, Feldman HA, Krauss B, Waltzman ML. Bispectral index monitoring quantifies depth of sedation during emergency department procedural sedation and analgesia in children. Ann Emerg Med. 2004;43(2):247–55.
Nieuwenhuijs D, Coleman EL, Douglas NJ, Drummond GB, Dahan A. Bispectral index values and spectral edge frequency at different stages of physiologic sleep. Anesth Analg. 2002;94(1):125–9.
Krzyzanski W, Jusko WJ. Application of moment analysis to the sigmoid effect model for drug administered intravenously. Pharm Res. 1997;14(7):949–52.
Mukashyaka MC, Wu C-L, Ha K, Zhang J, Wood J, Foley S, et al. Pharmacokinetic/pharmacodynamic modeling of a cell-penetrating peptide phosphorodiamidate morpholino oligomer in mdx mice. Pharm Res. 2021;38(10):1731–45.
Nickels KC. Less effective and more expensive: is it time to move on from rectal diazepam? Epilepsy Curr. 2018;18(1):27–8.
Dieckmann RA. Rectal diazepam for prehospital pediatric status epilepticus. Ann Emerg Med. 1994;23(2):216–24.
Nie Q, Hui P, Ding H, Wang Z. Rectal chloral hydrate sedation for computed tomography in young children with head trauma. Medicine (Baltimore). 2021;100(9): e25033.
Lam SHF, Li DR, Hong CE, Vilke GM. Systematic review: rectal administration of medications for pediatric procedural sedation. J Emerg Med. 2018;55(1):51–63.
Belo S, Touchard J, Secretan P-H, Vidal F, Boudy V, Cisternino S, et al. Stability of pentobarbital hydrogel for rectal administration in pediatric procedural sedation. Hosp Pharm. 2021;56(4):332–7.
Shao F, Zhang H, Xie L, Chen J, Zhou S, Zhang J, et al. Pharmacokinetics of ginkgolides A, B and K after single and multiple intravenous infusions and their interactions with midazolam in healthy Chinese male subjects. Eur J Clin Pharmacol. 2017;73(5):537–46.
Wermeling DP, Record KA, Archer SM, Rudy AC. A pharmacokinetic and pharmacodynamic study, in healthy volunteers, of a rapidly absorbed intranasal midazolam formulation. Epilepsy Res. 2009;83(2–3):124–32.
Nordt SP, Clark RF. Midazolam: a review of therapeutic uses and toxicity. J Emerg Med. 1997;15(3):357–65.
de Boer AG, Moolenaar F, de Leede LGJ, Breimer DD. Rectal drug administration: clinical pharmacokinetic considerations. Clin Pharmacokinet. 1982;7(4):285–311.
Zhu J, Zhao Y, Wang L, Zhou C, Zhou S, Chen T, et al. Physiologically based pharmacokinetic/pharmacodynamic modeling to evaluate the absorption of midazolam rectal gel. Eur J Pharm Sci. 2021;167:106006.
Peeters MYM, Prins SA, Knibbe CAJ, Dejongh J, Mathôt RAA, Warris C, et al. Pharmacokinetics and pharmacodynamics of midazolam and metabolites in nonventilated infants after craniofacial surgery. Anesthesiology. 2006;105(6):1135–46.
FDA. Guidance for industry: exposure–response relationships—study design, data analysis and regulatory applications. FDA Guidance. 2003. https://www.fda.gov/media/71277/download. Accessed 05 May 2023.
Mathur S, Patel J, Goldstein S et al. Bispectral index. Treasure Isl. StatPearls Publishing. 2022. https://www.ncbi.nlm.nih.gov/books/NBK539809/. Accessed 05 May 2023.
Chudomel O, Herman H, Nair K, Moshé SL, Galanopoulou AS. Age- and gender-related differences in GABAA receptor-mediated postsynaptic currents in GABAergic neurons of the substantia nigra reticulata in the rat. Neuroscience. 2009;163(1):155–67.
Ignjatovic V, Lai C, Summerhayes R, Mathesius U, Tawfilis S, Perugini MA, et al. Age-related differences in plasma proteins: how plasma proteins change from neonates to adults. PLoS ONE. 2011;6(2): e17213.
van Groen BD, Nicolaï J, Kuik AC, Van Cruchten S, van Peer E, Smits A, et al. Ontogeny of hepatic transporters and drug-metabolizing enzymes in humans and in nonclinical species. Pharmacol Rev. 2021;73(2):597–678.
Acknowledgements
The authors would like to thank the healthy subjects involved in the study and their families.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
Sufeng Zhou, Jinying Zhu, Xiaodi Sun, Lijun Xie, Yuqing Zhao, Sijia Ding, Lu Wang, Juan Chen, Bei Zhu, Chen Zhou, and Feng Shao are employees of the First Affiliated Hospital with Nanjing Medical University. Aiping Zheng is an employee of the Institute of Pharmacology and Toxicology of Academy of Military Medical Sciences. Yajuan Li is an employee of Xinjiang Tefeng Pharmaceutical Company, Ltd. The authors report no other conflicts of interest.
Funding
This study was funded by Xinjiang Tefeng Pharmaceutical Company, Ltd.
Availability of data and material
The data are not available in a repository, but reasonable requests can be directed to Feng Shao at jsphshaofeng@hotmail.com.
Ethical approval
This study was conducted according to Good Clinical Practice (GCP) following the tenets of the Declaration of Helsinki. The study was registered at chinadrugtrials.org.cn (CTR20192350). The protocol and informed consent form were approved by the Ethics Committee of the First Affiliated Hospital with Nanjing Medical University (Nanjing, China).
Informed consent
All subjects provided written informed consent before any study-related procedures were conducted.
Consent for publication
Not applicable.
Code availability
Not applicable
Author contributions
Conceptualization: FS, CZ, SZ. Methodology, material preparation and data collection: SZ, XS, LX, SD, LW, JC, BZ, AZ. Data analysis: JZ. Writing-original draft preparation: JZ. Writing-review and editing: SZ, JZ. Resources: YL. Supervision: FS. All authors approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, S., Zhu, J., Sun, X. et al. Safety, Pharmacokinetics, and Pharmacodynamics of Midazolam Gel After Rectal Administration in Healthy Chinese Subjects. Clin Drug Investig 43, 421–433 (2023). https://doi.org/10.1007/s40261-023-01276-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01276-5