Abstract
Background and Objective
A limited number of studies have addressed the protective duration of coronavirus disease 2019 (COVID-19) vaccines following primary and booster doses in Saudi Arabia. Therefore, this study aimed to evaluate the protective duration of primary and booster doses of BNT162b2 and ChAdOx1 COVID-19 vaccine batches in Saudi Arabia.
Methods
A cross-sectional study was conducted from 1 January to 31 December, 2021. The study included 53,354 people infected with severe acute respiratory syndrome coronavirus-2 2 weeks or more after receiving at least a primary vaccination of either the ChAdOx1 or BNT162b2 vaccine.
Results
The total median protective duration of both primary COVID-19 vaccinations was 134 days. Heterologous primary vaccination (ChAdOx1 followed by BNT162b2) showed a significantly higher median protective duration of 142 days. The results show that the total median protective duration of the first booster doses of COVID-19 vaccines was 57 days. ChAdOx1 batch code C1 was found to have the most extended protective duration of 173 days (range 163–192 days).
Conclusions
The current study revealed that the median protective duration of ChAdOx1 and BNT162b2 COVID-19 primary vaccination regimens administered in Saudi Arabia in 2021 was 134 days and that heterologous primary vaccination (ChAdOx1→BNT162b2) exhibited a significantly higher protective duration than other vaccination regimens.
Similar content being viewed by others
The current study revealed that the median protective duration of ChAdOx1 and BNT162b2 coronavirus disease 2019 primary vaccination regimens administered in Saudi Arabia was 134 days in 2021. |
Heterologous primary vaccination (ChAdOx1 followed by BNT162b2) showed a significantly higher median protective duration of 142 days. |
ChAdOx1 batch code C1 was found to have the most extended protective period of 173 days (range 163–192 days). |
1 Introduction
Coronavirus disease 2019 (COVID-19) vaccines are essential preventive pharmacological options to decrease the chance of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among humans [1, 2]. Several approved COVID-19 vaccines have higher efficacy (above 80%) in preventing infection, hospitalization, or death due to COVID-19 [3]. COVID-19 vaccines fall into four categories: mRNA based, adenovirus vector based, protein based, and inactive virus based [4].
Despite being reported to have a high efficacy in preventing SARS-CoV-2 infection, the duration of protection for many COVID-19 vaccines is short-lived. mRNA-based vaccines have been reported to offer about 95% protection after 8 weeks and 67–80% after 28 weeks of vaccination. However, the effectiveness of adenovirus-based vaccines was reported to reach around 75% after 4 weeks and 60% after 20 weeks of vaccination [5].
Regarding the difference in effectiveness between the first and second dose, the first dose of BNT162b2 showed a brief protective duration against COVID-19. Moustsen-Helms et al. found the crude vaccine effectiveness of BNT162b2 was 60% and 96% 14 days after the first and second dose, respectively [6]. Similarly, Andrews et al. found BNT162b2 effectiveness to reach 94% 14 days after the booster dose [7].
Nevertheless, the efficacy of COVID-19 vaccines against some SARS-CoV-2 variants (i.e., omicron) is much lower. Andrews and colleagues revealed that primary doses of the ChAdOx1 vaccine did not effectively prevent the omicron variant [7]. In addition, the BNT162b2 vaccine was documented to protect from the omicron variant by 9% 6 months after vaccination. Moreover, a booster vaccination with BNT162b2 may raise the protection percentage against the omicron variant for only 9 weeks [8].
In the USA, Rosenberg et al. reported that COVID-19 vaccines could not perfectly protect older people from getting infected or hospitalized [9]. In addition, Robles-Fontán showed that receiving the Ad26.COV2.S vaccine was also a factor in not reaching the optimum decline in the number of hospitalizations and deaths caused by SARS-CoV-2 infection [10].
In Saudi Arabia, there is a limited number of studies regarding the protective duration of COVID-19 vaccines after primary and booster vaccinations. Additionally, limited studies globally discuss the difference between COVID-19 vaccine batches concerning the efficacy of lowering the number of cases.
Therefore, this study aimed to evaluate the expected protective duration of COVID-19 vaccines in Saudi Arabia by observing the time gap between the administration of BNT162b2 and ChAdOx1 vaccinations and obtaining a positive polymerase chain reaction (PCR) among SARS-CoV-2 cases.
2 Subjects and Methods
2.1 Study Design and Population
A cross-sectional study was conducted in Saudi Arabia from 1 January to 31 December, 2021. The study included 53,354 Saudi citizens and residents aged ≥ 12 years who were infected with SARS-CoV-2 2 weeks or more after receiving at least a primary vaccination of either the BNT162b2 or ChAdOx1 vaccine. The study excluded people who never tested PCR positive for SARS-CoV-2, did not receive a second dose of a COVID-19 vaccine, or were infected with SARS-CoV-2 2 weeks before COVID-19 primary immunization.
2.2 Outcomes
The main outcome of this study was to determine the time gap between the date of receiving the second and booster doses of COVID-19 vaccines (BNT162b2 and ChAdOx1 vaccines) and the date of a positive nucleic acid amplification (PCR) test for SARS-CoV-2. In addition, this study aimed to compare primary immunization regimens of BNT162b2 and ChAdOx1 vaccines and their batches (second doses) concerning the protective duration against COVID-19.
2.3 Data Collection Tools and Analysis
The study was based on secondary data on BNT162b2 and ChAdOx1 vaccinations obtained from the Saudi Ministry of Health Data Governance Team. The database includes vaccine type, batch code, time of vaccination, PCR-positive result, and the number of COVID-19 vaccine doses.
Official COVID-19 testing centers, supervised by the Saudi Ministry of Health, confirm a COVID-19 diagnosis from nasopharyngeal or oropharyngeal swabs taken from the upper respiratory tract. According to the Saudi Center for Disease Prevention and Control, a confirmed COVID-19 case is defined as a person who has a positive PCR from a clinical laboratory authorized by the Saudi Public Health Authority. A person who meets the suspected case definition and tests positive by an antigen detection rapid diagnostic test authorized by the Saudi Food and Drugs Authority and validated by the Saudi Public Health Authority is also considered a COVID-19 case [11].
For the statistical analysis of the outcome data, the Kruskal–Wallis test and Mann–Whitney U test were performed using SPSS version 25. The data were organized and saved using the Microsoft Excel 2016 program. A p-value < 0.05 was considered statistically significant.
The included participants were divided into four groups: group 1 received two doses of the BNT162b2 vaccine [homologous vaccination] (N = 34,744); group 2 received two doses of ChAdOx1 [homologous vaccination] (N = 8435); group 3 received both vaccines (BNT162b2 followed by ChAdOx1) [heterologous vaccination] (N = 2892), group 4 received ChAdOx1 followed by the BNT162b2 vaccine [heterologous vaccination] (N = 7283). Additional sub-groups of people who received three doses of COVID-19 vaccines [booster dose] (N = 1916) and those who received the most frequent COVID-19 vaccine batches (N = 14,457) were also studied. Those who received booster doses were divided into three groups: the BioNTech group received the BioNTech vaccine as a homolog primary series and the BioNTech vaccine as the booster dose; the ChAdOx1 group received the ChAdOx1 vaccine as a homolog primary series and the ChAdOx1 vaccine as the booster dose; the randomly mixed group received either theBioNTech booster dose after a homolog ChAdOx1 or a heterologous ChAdOx1/BioNTech primary series vaccination or theChAdOx1 booster dose after a homolog BioNTech or a heterologous ChAdOx1/BioNTech primary series vaccination). The median protective duration and the interquartile range were determined and compared between the studied groups.
2.4 Ethical Consideration
The study was reviewed and approved by the Institutional Review Board Committee of the King Fahad Medical City (IRB Log Number: 22-195E). The confidentiality and anonymity of the participants’ data were preserved. This study depended on anonymous secondary data; therefore, no consent for participation was required.
3 Results
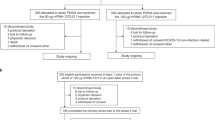
There were 53,354 COVID-19 cases reported between 1 January and 31 December, 2021 among adolescents and adults in Saudi Arabia. These cases were infected with SARS-CoV-2 2 weeks or more after receiving at least a primary vaccination of either the BNT162b2 or ChAdOx1 vaccine. The study included all the reported cases that met the inclusion criteria (N = 53,354) in the analysis. Out of the total included cases, those who received three doses of the COVID-19 vaccine (N = 1916) and received commonly administered BNT162b2 and ChAdOx1 batches (N = 14,457) were analyzed solely as a part of the sub-analysis (Fig. 1).
Almost two-thirds of the COVID-19 cases (65%) received the BNT162b2 homologous primary vaccination, while 16% of the cases received the ChAdOx1 homologous primary vaccination. Other groups of COVID-19 cases received the heterologous primary vaccination (14% received ChAdOx1 followed by BNT162b2 while 5% received BNT162b2 followed by ChAdOx1). The total median protective duration of all types of primary COVID-19 vaccinations was 134 days (Table 1). Heterologous primary vaccination (ChAdOx1 followed by BNT162b2) showed a significantly higher median protective duration (142 days) compared with other primary vaccinations (135 days for BNT162b2 homologous, 130 days for ChAdOx1 homologous, and 102 days for heterologous BNT162b2 followed by ChAdOx1, respectively, p < 0.001) (Fig. 2A).
Concerning sex, the overall total median protective duration after different primary vaccinations was significantly higher among male than female individuals (136 vs 132 days; p < 0.001). The protective duration of the BNT162b2 homologous vaccination was significantly higher among male than female individuals (139 vs 132 days; p < 0.001), while the reverse was reported for the heterologous vaccination [BNT162b2 followed by ChAdOx1] (104 vs 101 days; p = 0.009). However, there was no significant difference in the protective duration concerning sex among those who received a ChAdOx1 homologous or heterologous vaccination (ChAdOx1 followed by BNT162b2).
In respect to the age groups, the total median protective duration of primary COVID-19 vaccinations was significantly higher among elderly individuals compared with adults (181 vs 137 days) and adolescents (181 vs 116 days), respectively (p < 0.001). Among adolescents and adults, the higher protective duration was among those who received the heterologous vaccination (ChAdOx1 followed by BNT162b2) compared with the heterologous vaccination (BNT162b2 followed by ChAdOx1) [141 vs 105 days] and (142 vs 102 days), respectively, with a significant difference between them (p < 0.001). However, the highest median protective duration in elderly individuals was observed among those who received the BNT162b2 homologous vaccination (190 days).
The total number of subjects who received the first booster dose during the study period was 1916. The majority [1800 (94%)] of them received three doses of BNT162b2, while only 5% and 1% received randomly mixed vaccines and three doses of ChAdOx1, respectively.
Table 2 shows that the total median protective duration of all types of first booster COVID-19 vaccines was 57 days, with a higher protective duration for three doses of ChAdOx1 compared with three doses of BNT162b2 (80 vs 57 days) and randomly mixed vaccines (80 vs 51 days), respectively. The median protective duration of the first booster dose was higher among female individuals than male individuals (58 vs 56 days; p = 0.104), with no statistically significant difference between them regarding the type of vaccine.
No significant difference was recorded between male and female individuals regarding the total protective duration following the first booster dose (p = 0.104). In contrast, female individuals recorded a significantly longer protective duration than male individuals following a randomly mixed booster dose (74.0 vs 38.0 days, p = 0.012). The reverse was recorded following the BNT162b2 booster dose (56.0 vs 58.0 days, p = 0.034 respectively). Regarding sex, adults reported a significantly longer protective duration than elderly individuals following the randomly mixed-booster dose only (55 vs 27, p = 0.029, respectively). No significant difference was recorded between both age groups following the booster dose (p > 0.05). Table 2 also shows that administering the booster dose within 5 months of completing the primary vaccination provides a significantly extended protection period (94.5 vs 56 days, p < 0.001).
The protective duration of different batches of studied vaccines is presented in Fig. 2B. ChAdOx1 batch code C1 was found to have the most prolonged protection period reaching 173 days (163–192), followed by BNT162b2 batch code T1, ChAdOx1 batch code C3, ChAdOx1 batch code C2, BNT162b2 batch code T2, and BNT162b2 batch code T3, with the documented median days of 160 (148–166), 152 (143–159), 149 (137–158), 149 (139–154), and 99 (86–106), respectively (p < 0.001).
4 Discussion
The effectiveness of COVID-19 vaccines and their protection duration against SARS-CoV-2 infection have been studied by several researchers globally. mRNA vaccines, including BNT162b2 and Moderna COVID-19 vaccines, effectively prevented COVID-19 by almost 67% and 80% after 196 days of homologous primary vaccination, respectively. The one-dose regimen of the Ad26.COV2.S vaccine was effective by 59% after 140 days of the single dose, while the ChAdOx1 vaccine was effective by around 60% after 90 days of receiving the second dose [5, 12].
In the current study, conducted during the global omicron variant outbreak (January–December 2021), the median protection duration was evaluated following the last vaccination dose of different homologous and heterologous vaccine batches. It was observed that the total median protective duration of all types of primary COVID-19 vaccinations was 134 days. A heterologous vaccination (ChAdOx1 followed by BNT162b2) showed a significantly (p < 0.001) higher median protective duration compared with a homologous primary vaccination of both BNT162b2 and ChAdOx1 and a heterologous primary vaccination (BNT162b2 followed by ChAdOx1). This result was in line with Mayr et al., who found the effectiveness of a heterologous regimen of Ad26.COV2.S followed by the Moderna COVID-19 vaccine was effective by 56.7%, reaching up to 120 days, while the Ad26.COV2.S homologous vaccination was effective by 29.3% for the same period [13].
Concerning non-omicron variants (including the delta variant), Nordström et al. found that mRNA COVID-19 homologous vaccination regimens were more effective than ChAdOx1-based homologous and heterologous regimens after 10–15 weeks of completing primary immunization (≈ 83% vs 50%, respectively). However, they showed an acceptable efficacy (79%) after 12 weeks of receiving a heterologous vaccination that consists of ChAdOx1 followed by the Moderna COVID-19 vaccine [14].
In the present study, the total median protective duration of all types of first booster COVID-19 vaccines was 57 days, with a higher protective duration for the ChAdOx1 vaccine compared with BNT162b2 and randomly mixed vaccines. The decline in the protective duration following the first booster dose compared with the primary vaccination in our study could be attributed to the emerging pandemic of the omicron variant that occurred during the study period.
Among different age groups, the total median protective duration of a randomly mixed booster dose was significantly longer among adults compared with elderly individuals. In comparison, the median protection duration of the primary COVID-19 vaccinations was longer among those aged 60 years and older compared with other age groups (adolescents and adults). Several studies revealed that BNT162b2, Moderna, and ChAdOx1 vaccines’ effectiveness are high among elderly individuals [12, 15, 16]. A common explanation for these outcomes is the priority of vaccine administration among elderly individuals as they are considered a high-risk group for COVID-19, and they could be less exposed to infection by staying at home.
Moreover, the current study also found that overall vaccinated female individuals had a statistically significant shorter duration of protection than male individuals. Flacco et al. also concluded that mRNA vaccines and the ChAdOx1 vaccine showed lower male infection rates during an unspecified evaluation period 14–21 days after the COVID-19 primary vaccination [17].
Bignucolo et al. investigated the influence of sex on the efficacy of COVID-19 vaccines through a systematic review and meta-analysis of clinical trials. The pooled analysis showed a significantly higher efficacy of COVID-19 vaccines in men compared with women regarding the protection of SARS-CoV-2 infection [18]. The sex-related differences in vaccine efficacy could depend on immunological, genetic, and hormonal backgrounds [19,20,21].
In the present study, we could not statistically analyze the median protection days of booster doses between different vaccines because of the small sample size of this group relative to the other groups of primary vaccination. However, one of the comparable findings that could be interpreted is the most effective timing for receiving the booster dose. It was found that administration of the BNT162b2 booster dose within 5 months significantly results in an extended protection period. The latter outcome could be correlated with Menni et al., as they recognized that the effectiveness of the BNT162b2 vaccine in preventing SARS-CoV-2 infection was reduced extensively 5 months after the second dose [22].
To the best of our knowledge, this is the first research to discuss the protective duration of specific COVID-19 batches (second doses) against SARS-CoV-2 infection. Based on Moustsen-Helms et al. and Aran’s studies, the first dose of BNT162b2 has a minimal effect after 3 weeks, while the second dose can raise the protection to 96% [6, 23]. Therefore, the current study only used the batches for second doses to compare the median protective days.
Despite the good efficacy of BNT162b2 during specific periods, two BNT162b2 batches showed significantly shorter protection than the four other COVID-19 batches (including three ChAdOx1 batches). The possible reasons for this variation could be related to the special storage factors, supply chain, operations, warehouse, transportation, and inventory management systems that deal with COVID-19 vaccines. mRNA vaccines could be more affected by these logistic elements owing to their requirement of high-cold storage and issues regarding their thermostability [24].
4.1 Limitations
The small sample size in the sub-group with the first booster dose was one of the study’s limitations, which limited our ability to statistically analyze and compare the efficacy of the first booster dose with the primary vaccination. Additionally, the reported sex-linked variation in the protection period requires the inclusion of sex variables as a core in future studies. Moreover, the study did not include unvaccinated positive cases (control) to calculate the vaccine’s effectiveness.
5 Conclusions
The current study revealed that the median protective duration of total COVID-19 primary vaccination regimens received in Saudi Arabia was 134 days in 2021 and declined to 57 days during the omicron variant outbreak despite the booster doses received. Furthermore, the heterologous primary vaccination (ChAdOx1 followed by BNT162b2) showed a significantly higher protective duration than other vaccination regimens. ChAdOx1 batch code C1 was found to have the most prolonged protection period reaching 173 days (range 163–192 days).
References
Fernandes Q, Inchakalody VP, Merhi M, Mestiri S, Taib N, Moustafa Abo El-Ella D, et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. 2022;54(1):524–40.
Alfaleh A, Alkattan A, Radwan N, Elzohri M, Alzaher A, Ibrahim M, et al. Adverse drug reactions from two COVID-19 vaccines reported in Saudi Arabia. Drugs Ther Perspect. 2022;38(2):84–92.
Nasreen S, Chung H, He S, Brown KA, Gubbay JB, Buchan SA, et al. Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nat Microbiol. 2022;7(3):379–85.
Pascolo S. Vaccines against COVID-19: priority to mRNA-based formulations. Cells. 2021;10(10):2716.
Lin DY, Gu Y, Wheeler B, Young H, Holloway S, Sunny SK, et al. Effectiveness of Covid-19 vaccines over a 9-month period in North Carolina. N Engl J Med. 2022;386(10):933–41.
Moustsen-Helms IR, Emborg HD, Nielsen J, Nielsen KF, Krause TG, Molbak K, et al. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 vaccine in long-term care facility residents and healthcare workers: a Danish cohort study. MedRxiv. 2021. https://doi.org/10.1101/2021.03.08.21252200.
Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28(4):831–7.
Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–46.
Rosenberg ES, Dorabawila V, Easton D, Bauer UE, Kumar J, Hoen R, et al. Covid-19 vaccine effectiveness in New York State. N Engl J Med. 2022;386(2):116–27.
Robles-Fontán MM, Nieves EG, Cardona-Gerena I, Irizarry RA. Effectiveness estimates of three COVID-19 vaccines based on observational data from Puerto Rico. Lancet Reg Health Am. 2022;9: 100212.
Saudi Center for Disease Prevention and Control (Saudi CDC). COVID-19 guidelines 2022. https://covid19.cdc.gov.sa/wp-content/uploads/2022/01/covid-19-coronavirus-disease-guidelines-en.pdf. Accessed 9 Aug 2022.
Whitaker HJ, Tsang RSM, Byford R, Andrews NJ, Sherlock J, Pillai PS, et al. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response amongst individuals in clinical risk groups. J Infect. 2022;84(5):675–83.
Mayr FB, Talisa VB, Shaikh O, Yende S, Butt AA. Effectiveness of homologous or heterologous Covid-19 boosters in veterans. N Engl J Med. 2022;386(14):1375–7.
Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. 2021;11: 100249.
El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385(19):1774–85.
Baum U, Poukka E, Palmu AA, Salo H, Lehtonen TO, Leino T. Effectiveness of vaccination against SARS-CoV-2 infection and Covid-19 hospitalisation among Finnish elderly and chronically ill-An interim analysis of a nationwide cohort study. PLoS ONE. 2021;16(11): e0258704.
Flacco ME, Soldato G, AcutiMartellucci C, Carota R, Di Luzio R, Caponetti A, et al. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: data from an Italian province. Vaccines (Basel). 2021;9(6):628.
Bignucolo A, Scarabel L, Mezzalira S, Polesel J, Cecchin E, Toffoli G. Sex disparities in efficacy in COVID-19 vaccines: a systematic review and meta-analysis. Vaccines (Basel). 2021;9(8):825.
Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38.
Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442–7.
Mauvais-Jarvis F, BaireyMerz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565–82.
Menni C, May A, Polidori L, Louca P, Wolf J, Capdevila J, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022;22(7):1002–10.
Aran D. Estimating real-world COVID-19 vaccine effectiveness in Israel using aggregated counts. MedRxiv. 2021. https://doi.org/10.1101/2021.02.05.21251139.
Uddin MN, Roni MA. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines (Basel). 2021;9(9):1033.
Acknowledgements
The authors thank the Data Governance Team at the Saudi Ministry of Health for their assistance in completing this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study received no funding.
Conflicts of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this article. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethics approval
The study was reviewed and approved by the Institution Review Board Committee of the King Fahad Medical City (IRB Log Number: 22-195E). The confidentiality and anonymity of the participants’ data were preserved.
Consent to participate
This study was dependent on anonymous secondary data, therefore, no consent for participation was needed.
Consent for publication
Not applicable.
Availability of data and material
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
Not applicable.
Authors’ contributions
AF, AK, AZ, and MI contributed to the study conception and design Data cleaning and data analysis were performed by AK, AZ, MI, AH, AF, and KS. The first draft of the manuscript was written by AK, NR, NM, and KA AK, ES, and DH contributed in writing (review and editing) ES and DH contributed in resources. All authors commented on previous versions of the manuscript All authors read, reviewed, and approved the final manuscript.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alfaleh, A., Alkattan, A., Alzaher, A. et al. Protective Duration of ChAdOx1 and BNT162b2 Vaccines Against SARS-CoV-2 Infection. Clin Drug Investig 42, 799–806 (2022). https://doi.org/10.1007/s40261-022-01195-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01195-x