Abstract
Background and Objective
The source data of four individual randomised, double-blind, reference- and/or placebo-controlled clinical trials with virtually identical study design were pooled for the present meta-analysis. The main objective was to further evaluate the efficacy and safety of the fixed combination of cinnarizine 20 mg and dimenhydrinate 40 mg in comparison to various other antivertigo treatments in patients suffering from central and/or peripheral vestibular vertigo.
Methods
Adult male and female outpatients were subjected to a 4-week treatment with the fixed combination of cinnarizine 20 mg and dimenhydrinate 40 mg, cinnarizine (20 mg, 50 mg), dimenhydrinate (40 mg, 100 mg), betahistine dimesylate (12 mg), betahistine dihydrochloride (16 mg) and placebo, respectively. The primary efficacy endpoint was the reduction of a validated mean vertigo score (MVS), a composite score of 12 individual vertigo symptoms, the intensities of which were each evaluated by the patients on a 5-point visual analogue scale. For analysis of primary and further secondary efficacy endpoints, baseline-adjusted analysis of covariance (ANCOVA) was used to calculate adjusted least squares means (LSM) with associated two-sided 95% confidence intervals (CIs) for the difference in MVS reductions between treatment groups. Moreover, various sensitivity analyses, responder and subgroup analyses as well as descriptive analyses with respect to safety/tolerability of the treatments were conducted.
Results
Of 795 randomised patients, 779 belonged to the intent-to treat (ITT) and 723 to the per-protocol (PP) population. The main efficacy analysis was based on the ITT population (mean age 52.1 years, 61% female). The mean decrease of the MVS from baseline to Week 4 in the cinnarizine/dimenhydrinate group (−1.10) proved to be significantly larger than in any of the comparator groups. LSM differences for comparators versus the fixed combination ranged between 0.16 (95% confidence interval (CI) 0.03; 0.30, p = 0.017) for cinnarizine 20 mg and 0.60 (95% CI 0.42; 0.78; p < 0.001) for betahistine dimesylate 12 mg in favour of the fixed combination. Furthermore, after 4 weeks of treatment, 74 patients (24.7%) in the cinnarizine/dimenhydrinate group were completely symptom free (MVS = 0), a significantly greater proportion than in any of the comparator groups. Sensitivity analyses showed that baseline characteristics such as age, sex, duration of vertigo and antivertigo pretreatment had only a very minor and clinically non-relevant impact on the efficacy results regarding the primary efficacy outcome. Subgroup analyses with respect to age groups (< 65 years/≥ 65 years) and sex showed no significant differences in efficacy within any of the treatment groups. All treatments were well tolerated. A total of 55 patients (6.9%) reported 75 non-serious adverse events (AEs), and 19 patients (2.4%) discontinued the study prematurely because of AEs. Nearly 95% of the patients (cinnarizine/dimenhydrinate group: 97.9%) rated the tolerability of the study medications as either “good” or “very good”.
Conclusion
The findings of the present meta-analysis indicate that the fixed combination of cinnarizine and dimenhydrinate is a safe and potentially superior treatment option for patients suffering from central and/or peripheral vestibular vertigo, as compared to current standard treatments such as cinnarizine, dimenhydrinate or betahistine given alone in monotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Results of the present meta-analysis of four randomised controlled trials indicate that the fixed-dose combination of cinnarizine 20 mg and dimenhydrinate 40 mg is a safe and effective treatment of vertigo in patients suffering from central and/or peripheral vestibular disorders. |
The findings provide evidence that the fixed combination of cinnarizine/dimenhydrinate is more efficacious than various standard antivertigo treatments such as cinnarizine, dimenhydrinate and betahistine used in monotherapy of patients with vestibular vertigo, in association with a comparable safety profile. |
1 Introduction
Vertigo is caused by dysfunction in the vestibular system, which comprises both central and peripheral components [1]. The peripheral component includes the labyrinth (semicircular canals and otolith system in the inner ear) and the vestibular nerve (vestibular portion of the eighth cranial nerve). The central component includes the vestibular nuclei and their connection with the brainstem, the cerebellum and the cerebrum. It is generally acknowledged that most cases of vertigo likely result from a combined dysfunction involving both peripheral and central vestibular mechanisms, as a pathology of one will inevitably impact the other. Any peripheral vertigo also possesses a central component, as the malfunction of any one peripheral vestibular organ causes a sensory mismatch in the higher centres of equilibrium regulation, and vertigo is triggered by this data conflict [2].
Vertigo includes a heterogeneous group of symptoms often described as a “sensation of motion of either the self or the surroundings”, with each symptom lending clues as to the underlying aetiology for the clinical manifestation of vertigo [3]. Vertigo is known to impede activities of daily life and to interfere with workplace activities [4, 5]. In fact, Neuhauser and colleagues [6] demonstrated in a large community study that up to 40% of individuals with vertigo report interruptions in daily activities and up to 20% avoid leaving the house. Particularly in the elderly, vestibular disorders lead to a significantly increased risk of falls potentially causing serious injuries, and generally increased rates of morbidity and mortality [7]. Vertigo is counted among the geriatric giants, and adult mortality rates in patients with vertigo are similar to other leading causes of death such as cardiovascular disease, cancer or diabetes [8].
Since effective treatment of the underlying disorders is often not possible, either due to the complexity and diversity of the pathogenic mechanisms or particular challenges in establishing unambiguous diagnoses, symptomatic treatment to provide rapid and effective relief of the often disabling symptoms is all the more important. Currently used antivertigo drugs in monotherapy comprise various pharmaceutical classes mainly including cinnarizine, a selective calcium channel antagonist [9, 10] with weak antihistamine properties, dimenhydrinate, an antihistamine with anticholinergic (antimuscarinic) properties [11], and betahistine, a histamine analogue, which is supposed to act as a weak H1 agonist and potent H3 antagonist [12]. Cinnarizine and betahistine primarily act on the peripheral vestibular system, whereas the action of dimenhydrinate is primarily targeted at the central vestibular system. Moreover, a fixed-dose combination (FDC) of 20 mg cinnarizine and 40 mg dimenhydrinate is successfully used in many countries worldwide, which provides an additional option to treat vertigo of both peripheral and central origin. Due to the dual mode of action and synergism of the active substances, the combination allows a dose reduction of the two components compared to the respective monotherapies (cinnarizine 50 mg, dimenhydrinate 100 mg), thereby minimising the potential for drug-related adverse reactions that are dose-dependent. The efficacy and safety of the fixed combination has been demonstrated so far in a total of ten randomised, double-blind, controlled clinical trials [13,14,15,16,17,18,19,20,21,22] as well as in a pooled analysis, which comprised five of the individual studies [23].
The present meta-analysis was undertaken to further corroborate the findings of the single studies based on a broader patient population. Although the results of each of the individual studies have been reported earlier [13,14,15,16], the present analysis is not based on the published data (e.g., pooling of p values), but on the use of the raw individual patient data from the single studies (first level of pooling). This approach is generally preferable over the more traditional meta-analysis using aggregate data from publications and currently considered as “gold standard” in evidence-based medicine [24, 25].
2 Patients and Methods
2.1 Eligibility Criteria and Study Selection
In the present meta-analysis, we included randomised, controlled, clinical trials (RCTs) that investigated the fixed-dose combination (FDC) of cinnarizine 20 mg and dimenhydrinate 40 mg in the treatment of patients with various kinds of vestibular vertigo. RCTs that were available from the database of the studies’ sponsor (Hennig Arzneimittel) by June 2020 were basically taken into consideration before defining the following more specific eligibility criteria: evaluation of the efficacy and safety of the FDC and at least one comparator drug, using a randomised, double-blind, parallel-group design, and a standardised duration of treatment with one tablet given three times daily for 4 weeks, including three examination visits (entry visit, intermediate visit after 1 week, final visit after 4 weeks). A further prerequisite for eligibility was the use of the validated composite outcome scale Mean Vertigo Score (MVS) [26] as primary efficacy endpoint. Based on these stipulated eligibility criteria, and further taking into account that studies in patients with acute vestibular disorders were excluded for methodological reasons, study identification and selection from the available database was rather straightforward.
Individual patient data (IPD) of the selected studies were pooled from the company database and checked independently by two authors for completeness, consistency and plausibility. Comparison with the published reports of the individual studies was done by visual inspection. Any unclear or missing data were sought from the respective original case report forms. Discrepancies or ambiguities were discussed and resolved by consensus.
2.2 Quality of Included Studies and Risk of Bias Assessment
Quality of individual studies and risk of bias (internal validity) were assessed largely based on the Cochrane Collaboration‘s risk of bias assessment tool [27]. Selection bias (sequence generation and allocation sequence concealment), performance bias (blinding of patients and investigators), detection bias (blinding of outcome assessors), attrition bias (handling of incomplete or missing data), and reporting bias (selective outcome reporting) were each judged by means of the categories low, unclear or high risk.
Between-study heterogeneity (diversity) of the included studies was assessed by consideration of both methodological and clinical features (methodological and clinical heterogeneity). Between-study variability concering the primary efficacy endpoint in patients treated with the FDC (statistical heterogeneity) was evaluated by calculation of I2 according to [28]. Interpretation of the I2 statistic is guided by the thresholds suggested in [29].
2.3 Evaluation of Efficacy and Safety
Primary efficacy endpoint was the mean change of a validated MVS, composed of six unprovoked (spontaneous) vertigo symptoms and six vertigo symptoms provoked by various body movements (vertigo triggering factors) from baseline to Week 4 [26]. The intensity of each vertigo symptom was rated by the patient by means of a continuous visual analogue scale (VAS) ranging between 0 = symptom not present to 4 = very strong symptom. Secondary efficacy endpoints comprised the mean change of the MVS from baseline to Week 1, responder rates based on three different criteria fulfilled after 4 weeks of treatment (MVS = 0, MVS ≤ 0.5, and decrease in MVS by ≥ 50%), as well as the patients’ and investigators‘ global efficacy rating by means of a 5-point verbal rating scale (very much improved, much improved, slightly improved, not improved, deteriorated). Furthermore, four vegetative concomitant symptoms (nausea, vomiting, sweating, tachycardia) and further concomitant symptoms (tinnitus, impaired hearing, impaired vision, aural fullness, headache) were registered, each assessed by the patient using the same 5-point VAS as applied for the vertigo symptoms. The four vegetative symptoms were combined into a mean vegetative concomitant symptom score (CSveg). Subgroup analyses were performed for the primary efficacy endpoint (change in MVS after 4 weeks) with respect to the categories age < 65 years/≥ 65 years and sex (male/female).
Safety assessments were based on the proportion of patients reporting adverse events (AEs), which were monitored throughout treatment and registered on occasion of the intermediate and final visits. Furthermore, patients and investigators rated the tolerability of the treatments by means of a 4-point verbal rating scale (very good, good, moderate, poor).
2.4 Statistical Analysis
Efficacy evaluations were primarily based on the intent-to-treat (ITT) population, which included all randomised patients who participated in the study at least until the 1-week follow-up visit. In case of missing 4-week values, imputation of the 1-week value according to the last observation carried forward (LOCF) method was applied. The primary efficacy endpoint (change in MVS after 4-week treatment) was also analysed for the per-protocol (PP) population, which included those patients who did not violate any terms of the protocol that might have had a relevant effect on the efficacy outcome. The safety analysis set comprised all randomised patients who received at least one dose of study medication.
For quantitative primary and secondary efficacy variables, analysis of covariance (ANCOVA) was applied to investigate pairwise differences between treatment groups. The respective baseline values were included in the statistical model (in addition to treatment group). Treatment effects and adjusted least squares (LS) means with associated two-sided 95% confidence intervals (CIs) were calculated. Sensitivity analyses with regard to the primary efficacy endpoint were performed for the ITT population with additional adjustments for age, sex, duration of vertigo and vertigo pretreatment, as well as for the PP population, in order to check for robustness of the results. For supportive assessment, the efficacy variables were additionally analysed descriptively. Pairwise differences between treatment groups for categorical variables, such as responder rates (based on the MVS at the end of Week 4) as well as investigators’ and patients’ global efficacy and tolerability ratings, were assessed using Fisher’s exact test.
Subgroup analyses were performed using ANCOVA (adjusted for baseline MVS). p values were calculated for the differences between the subgroups within each treatment group using a Chi-square test. Comparability of demographic and clinical baseline characteristics between treatment groups was assessed using analysis of variance (ANOVA) in case of quantitative data and Cochran–Mantel–Haenszel test in case of categorical data.
All tests were performed two-sided at a significance level of α = 0.05. Statistical analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA).
3 Results
3.1 Selection and Characteristics of Included Studies
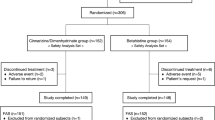
Of the presently available ten double-blind RCTs [13,14,15,16,17,18,19,20,21,22] with the fixed combination as test medication, comprising a total of 1,281 patients, four RCTs fulfilled the prespecified eligibility criteria. Six studies (486 patients) were not considered for the present meta-analysis for the following reasons: three studies included only patients with acute vertigo, either suffering from acute vertigo attacks [18] or unilateral vestibular loss [19] and unilateral vestibular neuritis [20], respectively, with hospitalisation of the patients during the first week of therapy and simultaneous mannitol or corticosteroids infusions as standard treatment. In one study, only patients with confirmed Menière’s disease [21] were included, and duration of treatment was 12 weeks. Two further studies were excluded because a differently composed vertigo score as primary endpoint as well as a discrete 4-point visual analogue scale [17] or a discrete 4-point verbal rating scale [22] were used for the patients‘ rating of the intensity of vertigo symptoms. Exclusion of these two studies avoided transformation of the rating scales and respective adjustment of the primary endpoint, as applied in a previous meta-analysis [23]. The selection process is schematically depicted in Fig. 1.
The individual studies were conducted between January 1993 and April 2015 at 15 study centres, including otolaryngological and neurological university clinics in Austria, Bulgaria, Hungary, Russia, and the Czech Republic. All studies were conducted according to a virtually identical study design, and they investigated the efficacy and safety of the fixed-dose combination (FDC) of 20 mg cinnarizine and 40 mg dimenhydrinate (Studies 1−4) in comparison with cinnarizine 50 mg, dimenhydrinate 100 mg or placebo (Study 1), cinnarizine 20 mg or dimenhydrinate 40 mg (Study 2), betahistine dimesylate 12 mg (Study 3), and betahistine dihydrochloride 16 mg (Study 4), respectively, in patients suffering from central and/or peripheral vestibular vertigo. Study participants, exclusively outpatients, were selected according to very similar inclusion and exclusion criteria and treated with one tablet of study medication three times daily for 4 weeks. Patients were subjected to an entry examination (before start of treatment), an intermediate examination after 1 week (7 ± 2 days), and a final examination after 4 weeks (28 ± 2 days; except Study 1: 28 ± 5 days). The main characteristics of the four included studies are summarised in Table 1. All included studies were conducted in accordance with the principles of Good Clinical Practice (GCP) and the Declaration of Helsinki. Study documents were reviewed and approved by the Ethics Committees of the respective study centres.
3.2 Patient Population: Characteristics and Disposition
The patient population of the four individual studies comprised Caucasian male and female adult outpatients. All patients were suffering from central and/or peripheral vestibular vertigo; in Studies 3 and 4, only patients with peripheral vestibular vertigo were included. Patients with benign paroxysmal positional vertigo (BPPV), confirmed Menière’s disease, bilateral vestibulopathy, acute peripheral vestibular disorders requiring hospitalisation, or non-vestibular vertigo were excluded. More specific exclusion criteria were mostly related to the known contraindications of the actives cinnarizine and dimenhydrinate (e.g., narrow-angle glaucoma, prostate adenoma with residual urine, convulsive fits/epilepsy, Parkinson’s disease, severe renal insufficiency), and betahistine (phaeochromocytoma, gastrointestinal ulcers) in Studies 3 and 4 only. Further exclusion criteria referred to restrictions regarding the use of concomitant medication, such as treatment with aminoglycosidic antibiotics, monoamine oxidase inhibitors, and tricyclic antidepressants, parasympatholytics, glucocorticoids or heparin. The concomitant intake of other antivertigo drugs, apart from the stipulated study medication, was disallowed in all studies. Patients with a history of alcohol or drug abuse as well as pregnant or breast-feeding women were generally excluded.
The required minimum age was 31 years in Studies 1 and 3, 30 years in Study 2, and 18 years in Study 4; the mean age of patients in the individual studies ranged between 49.0 years in Study 3 and 53.5 years in Study 4 (Table 1). Only patients who rated the intensity of at least one of the six spontaneous vertigo symptoms as medium or higher (≥ 2 on the 5-point VAS, see below) on occasion of the entry examination were eligible for enrolment in the studies. No restrictions applied to the study participants with respect to the duration of vertigo or possible pretreatment with antivertigo drugs; the latter, however, had to be discontinued prior to study enrolment, followed by a 1-week washout phase. Written informed consent was obtained from each participant before the start of the study.
The present meta-analysis comprised a total of 795 patients, who were randomised and thus included in the safety analysis (safety data set). The ITT population comprised 779 patients excluding 16 patients, who discontinued the study prematurely before the intermediate visit. Considering all in all 30 dropouts and 42 patients with protocol violations (mostly in Study 1), the PP population included 723 patients. Of a total of 30 (3.8%) dropouts, 19 patients (2.4%) discontinued the study prematurely due to adverse events, the remaining 11 because of other (mostly unknown) reasons. As compared to the published data of the four individual studies, one additional patient in the dimenhydrinate 40 mg group (Study 2) was included, and two patients in the betahistine dimesylate 12 mg group (Study 3) were excluded from the ITT population in the meta-analysis.
Demographic and clinical baseline characteristics of the ITT population, as summarised in Table 2, were largely comparable between treatment groups. The majority of patients were female (61.1%) and the average age was 52.1 years, with no significant differences between treatment groups (p = 0.870 and p = 0.120, respectively). There was also an even distribution between treatment groups with respect to weight (p = 0.078) and height (p = 0.684), but not for body mass index (BMI) (p = 0.006). The majority of patients suffered from peripheral vestibular vertigo (57.3%), mostly included in Study 3 (exclusively “otogenic vertigo” [15]) and Study 4 (categorised as “Menière-like symptom complex”, “other peripheral vertigo” or “labyrinthine dysfunction” [16]). In Studies 1 and 2, diagnoses of central vertigo (12.9%) included cerebral atherosclerosis, transient ischaemic attack, and vascular encephalopathy; the remaining patients (29.8%) presented with signs of both central and peripheral vestibular disorders, including unspecified disorders of the vestibular function and vertebrobasilar insufficiency. Before enrolment into the individual studies, patients suffered from vertigo on average for 32 months (covering a large range within treatment groups), and 274 patients (35.2%) had been pretreated with antivertigo medications; both parameters showed significant differences between treatment groups (p = 0.003 and p = 0.002, respectively). Whereas 43.1% of the patients had concomitant diseases (predominantly hypertension), 37.6% received concomitant medications, mostly cardiovascular drugs (26.7%), such as beta-blocking agents, ACE inhibitors and calcium channel blockers.
3.3 Efficacy
The MVS on occasion of each of the three examination visits during the 4-week treatment is shown for the ITT population in Table 3. Overall reduction of vertigo symptoms after 4 weeks varied between around 73% (fixed combination) and 31% (betahistine dimesylate 12 mg), with the fixed combination showing a significantly stronger reduction than each of the comparators, after both 1 week and 4 weeks. The differences in LSM with respect to the change of the MVS from baseline to Week 4 regarding the fixed combination versus each comparator, together with the 95% CIs, are depicted in Fig. 2. Confidence intervals were all below zero and thus in favour of the fixed combination for all comparisons (p values are given in Table 3). Moreover, the main efficacy results were very similar for the PP population (given in the lower part of Table 3). Sensitivity analyses, using baseline-adjusted ANCOVA for the change in MVS after 4-week treatment, yielded only marginally different results after adjustment for demographic data (age, sex) and baseline clinical characteristics such as duration of vertigo (before enrolment into the individual studies) and pretreatment with antivertigo drugs.
Results of the responder analyses are depicted in Fig. 3. Significant differences were observed for the proportion of patients who were symptom-free at the end of the 4-week treatment (MVS = 0) in favour of the cinnarizine/dimenhydrinate group (24.7%) as compared with any of the comparator groups (0–13.8%). Nearly 70% of the patients treated with the fixed combination had no or only minor vertigo symptoms (MVS ≤ 0.5) at the end of therapy, a significantly better result than only 24.1% in the placebo group and between 29% and 55% in the remaining treatment groups (p < 0.05 in all cases). Furthermore, the percentage of patients who experienced a clinically meaningful improvement of vertigo symptoms by at least 50% was also significantly in favour of the fixed combination (nearly 79%) as compared to the other treatments, except cinnarizine 20 mg (p = 0.132).
Responder rates after 4-week treatment. a Proportion of patients who were symptom-free at the end of the 4-week treatment (MVS = 0). b Proportion of patients with only minor vertigo symptoms at the end of the 4-week treatment (MVS ≤ 0.5). c Proportion of patients with at least 50% reduction in vertigo symptoms at the end of the 4-week treatment. FDC cinnarizine 20 mg + dimenhydrinate 40 mg (n = 299), CZ 20 mg cinnarizine 20 mg (n = 60), CZ 50 mg cinnarizine 50 mg (n = 61), DH 40 mg dimenhydrinate 40 mg (n = 59), DH 100 mg dimenhydrinate 100 mg (n = 59), BH 12 mg betahistine dimesylate 12 mg (n = 29), BH 16 mg betahistine dihydrochloride 16 mg (n = 152), placebo (n = 58), MVS mean vertigo score. *p < 0.05, **p < 0.01, ***p < 0.001, n.s. not significant, fixed combination (FDC) vs. comparator, Fisher‘s exact test
Subgroup analyses with respect to age (< 65/≥ 65 years) and sex were conducted in order to investigate a possible influence of these demographic parameters on the efficacy of the antivertigo treatments, as calculated by the MVS LSM after 4-week treatment. The results, as shown in Table 4, revealed only minor and in no case significant differences between each of the two respective subgroups (age group and male/female, respectively) for any of the eight treatment groups.
The mean vegetative concomitant symptom score CSveg, composed of the symptoms nausea, vomiting, sweating, and tachycardia, distinctly improved under 4-week treatment with the fixed combination (LSM [95% confidence interval (CI)] − 0.95 [− 1.00; − 0.90]). There were no significant differences to dimenhydrinate 40 mg (LSM [95% CI] − 0.96 [− 1.07; − 0.84]) and betahistine dihydrochloride 16 mg (LSM [95% CI] − 0.88 [− 0.95; − 0.80]), but highly significant differences to all other comparators (p < 0.001). In particular, the single symptom nausea, often associated with vertigo, strongly improved under treatment with the fixed combination (LSM [95% CI] −1.62 [− 1.70; − 1.54]) with significant differences to all comparators (p < 0.01) except dimenhydrinate 40 mg (LSM [95% CI] − 1.59 [− 1.77; − 1.41]) and betahistine dihydrochloride 16 mg (LSM [95% CI] − 1.53 [− 1.64; − 1.42]).
The patients’ and investigators’ global ratings of efficacy after 4 weeks were very similar and basically in line with the results for the primary outcome. In the cinnarizine/dimenhydrinate group, 71.0% of the patients rated the overall efficacy as either “very much improved” or “much improved”, as compared to cinnarizine 50 mg (65.4%), cinnarizine 20 mg (63.4%), betahistine dihydrochloride 16 mg (62.8%), placebo (51.9%), dimenhydrinate 40 mg (48.3%), dimenhydrinate 100 mg (43.6%), and betahistine dimesylate 12 mg (31.0%). Whereas 30.7% of the patients treated with the fixed combination rated the global efficacy at the end of treatment as “very much improved”, the respective percentages of patients in the comparator groups ranged between 17.5% (betahistine dihydrochloride 16 mg) and 6.9% (dimenhydrinate 40 mg). After 4 weeks, pairwise comparisons between the fixed combination and each of the comparators using Fisher’s exact test revealed significant differences to cinnarizine 50 mg (p = 0.022), dimenhydrinate 40 mg (p < 0.001), dimenhydrinate 100 mg (p < 0.001), betahistine dimesylate 12 mg (p < 0.001), betahistine dihydrochloride 16 mg (p = 0.012) and placebo (p = 0.029), but no significant difference to cinnarizine 20 mg (p = 0.069).
3.4 Safety and Tolerability
All study medications were well tolerated. Of a total of 795 randomised patients (safety data set), only 55 (6.9%) reported 75 non-serious adverse events (AEs). No serious AEs or deaths were reported. The most common AEs belonged to the system organ class “nervous system disorders”, such as somnolence (3.4%), memory impairment (0.9%), and headache (0.2%), or “gastrointestinal disorders”, such as abdominal pain (0.5%) and dry mouth (0.3%); more details are provided in Table 5. The proportion of patients reporting AEs varied considerably between the four studies. Whereas in Study 3 no AE was reported, nine (5.0%) patients (two FDC, four CH 20 mg, three DH 40 mg) in Study 2, 12 (3.9%) patients (four FDC, eight BH 16 mg) in Study 4, and 34 (13.8%) patients (six FDC, 12 CH 50 mg, 10 DH 100 mg, six placebo) in Study 1 reported at least one AE. A total of 19 patients (2.4%) terminated the treatment prematurely because of AEs (four FDC, two CZ 50 mg, three DH 40 mg, two DH 100 mg, five BH 16 mg, three placebo), ten before the intermediate (1-week) visit and an additional nine patients before the final (4-week) visit; there were no dropouts due to AEs in the cinnarizine 20 mg and betahistine dimesylate groups. The patients‘ tolerability ratings were largely in line with the overall low incidence of AEs. Nearly 95% of the study participants rated the tolerability of the respective treatments as either “good” or “very good” (Table 5), with the highest rate found for betahistine dimesylate (100%), followed by cinnarizine 20 mg (98.4%), the cinnarizine/dimenhydrinate combination and betahistine dihydrochloride (97.9% each), dimenhydrinate 40 mg (96.6%), cinnarizine 50 mg (92.8%), placebo (86.5%), and dimenhydrinate 100 mg (70.9%). Pairwise comparisons between the fixed combination and each comparator revealed non-significant differences in the ratings for cinnarizine 20 mg (p = 0.098) and betahistine dimesylate (p = 0.084), but significantly better ratings than the remaining comparators.
3.5 Risk of Bias Assessment
All included studies were double-blind RCTs. Randomisation was based on a computer-generated block sequence that ensured equal distribution of patients among the treatment groups, separately for each study centre. Patients were randomly assigned to treatment groups in sequential order according to the randomisation code. Treatment allocation was blinded for patients and investigators, as well as for outcome assessors. Randomisation lists remained with the sponsor and investigators received sealed opaque envelopes, to be opened only in case of medical emergency, with decoding information for each single patient to maintain the integrity of the double-blind design for all unaffected patients. Based on these measures, which applied to all individual studies, the risks of selection bias (sequence generation and allocation sequence concealment), performance bias (blinding of patients and investigators), and detection bias (blinding of outcome assessment) are judged as “low” for each of the individual studies included in the meta-analysis.
For 16 patients (2.0% of all randomised patients) who discontinued the study prematurely before the intermediate visit, only baseline values were available. Due to a largely balanced distribution across studies and intervention groups, exclusion of these patients from the ITT analysis is expected to have no clinically relevant impact on the overall efficacy results. The remaining missing outcome data have been imputed using the LOCF (last observation carried forward) method. Thus, the risk of attrition bias due to incomplete outcome data is judged as low. Equally, the risk of reporting bias (selective outcome reporting) is considered low, as all key outcome variables available from the full datasets of the individual studies were included in the meta-analysis to provide a balanced view of benefits and risks. All in all, evaluation based on the Cochrane Collaboration's risk of bias tool indicate a rather low risk of potential biases in the selected studies and thus an acceptable internal validity.
In the present meta-analysis, we applied relatively strict eligibility criteria, resulting in very similar features of the included studies, i.e., identical study design, randomisation, blinding and key outcome measures. Furthermore, patient characteristics such as mean age (range 49.0–52.1 years) and sex distribution (range 59.3–64.0% female patients) were very similar. Therefore, both methodological and clinical between-study heterogeneity should be rather moderate. However, calculation of I2 (73%) revealed that there may be a substantial statistical heterogeneity (50–90% according to [29]) with respect to the mean MVS changes between the FDC groups of the four studies, although the differences (range between − 1.23 and − 0.98) can be considered as not clinically relevant. Furthermore, it should be noted that uncertainty in the value of I2 is substantial when the number of studies is small [29].
4 Discussion
The present meta-analysis was based on the pooled original data of four individual, randomised, double-blind clinical trials with largely identical design features. The findings provide further evidence for the successful treatment of vertigo caused by central and/or peripheral vestibular disorders using a fixed combination of the two low-dosed antivertigo drugs cinnarizine (20 mg) and dimenhydrinate (40 mg).
Treatment with the fixed-combination preparation led to a significantly better improvement of vertigo symptoms than various standard antivertigo treatments currently used in monotherapy, such as cinnarizine (50 mg), dimenhydrinate (100 mg) and two different betahistine formulations (dimesylate 12 mg, dihydrochloride 16 mg), each given three times daily. Further comparators such as placebo as well as the lower-dosed comparators cinnarizine 20 mg and dimenhydrinate 40 mg (same dose as in the combination preparation), which are not standard antivertigo treatments, were included in Studies 1 and 2, respectively, only for regulatory reasons.
The fixed combination contains the two active components cinnarizine and dimenhydrinate, the former a calcium antagonist acting primarily on the vestibular hair cells of the peripheral vestibular system, and the latter an antihistamine that predominantly acts on the vestibular nuclei (located in the medulla oblongata) of the central vestibular system. This dual mode of action is believed to be mainly responsible for the superior efficacy of the fixed combination over the monotherapies with the single actives cinnarizine or dimenhydrinate and the structural histamine analogue betahistine, which is supposed to have a predominantly peripheral-vestibular action. Regarding betahistine, it has to be mentioned that the two different salts do contain substantially different amounts of the active component betahistine. In fact, one tablet of betahistine dihydrochloride 16 mg (recommended daily dose 48 mg) contains more than twice as much betahistine than one tablet of betahistine dimesylate 12 mg (recommended daily dose 36 mg); this might at least partially explain the overall better efficacy (although slightly worse tolerability) of the betahistine dihydrochloride as compared to the dimesylate salt found in the present analysis, which may be particularly recognisable in the results of the responder analysis.
Sensitivity analyses with respect to the primary endpoint indicated no significant influence of age, sex, duration of vertigo or antivertiginous pretreatment before enrolment in the studies on the efficacy results. Both male and female patients appeared to equally benefit from the antivertigo treatments in each of the treatment groups; even though there was a tendency for a slightly better efficacy in men in most treatment groups, the differences were in no case significant. Regarding the fixed combination, these findings are in contrast to recently published data [30], where a superior efficacy in female study participants was reported. Although various design features, patient population and sample size were quite different from our study, this obvious discrepancy needs further investigations. An additional subgroup analysis yielded similar efficacy results in patients aged < 65 and ≥ 65 years, respectively; however, since the number of elderly subjects in some treatment groups was quite low, these results need to be interpreted with caution.
In addition to the primary efficacy analysis, the results of a responder analysis demonstrated clinically relevant improvements of vertigo symptoms by means of three different categories. Treatment with the fixed combination led in about one out of four patients (24.7%) to a complete remission of vertigo, and a further 45.2% of the patients had only minor complaints at the end of the 4-week treatment, both significantly better results than for any of the comparators; in particular, comparison with placebo (3.4% and 24.1%, respectively) clearly indicates a clinically meaningful improvement of the patients‘ vertigo complaints. The clinical relevance of the improvements is also reflected by the patients‘ global efficacy ratings; 71.0% of patients in the cinnarizine/dimenhydrinate group rated the therapeutic success as either “much improved” or “very much improved”, a significantly better rating than for most comparators, except cinnarizine 20 mg.
Limitations of the present meta-analysis, which demand a critical discussion of the reported efficacy results, mainly refer to the nature and diversity of the included individual studies. Only four out of ten currently available RCTs with the fixed combination as test product, comprising 795 (62%) of a total of 1281 patients, were included in the meta-analysis; this is, however, considered as justified, because the remaining six studies had distinctly different design features and patient populations, or used a differently composed primary efficacy endpoint based on a different rating scale. Three studies were excluded because they enroled only patients with acute vestibular disorders [18,19,20], with initial hospitalisation, standard infusions and primary efficacy end point after 1 week’s treatment to account for central compensation processes [19, 20]. The study in patients with Menière’s disease [21] was excluded, because these patients need to be treated for at least several months (primary efficacy endpoint after 12-week therapy) in order to achieve a sufficient improvement of vertigo symptoms. Inclusion of these studies in the present meta-analysis would have caused considerable problems regarding interpretation of the results due to distinctly increased methodological and clinical between-study diversity. Furthermore, the authors decided to exclude two studies [17, 22] that used different rating scales and composite vertigo scores for evaluation of vertigo intensity, in order to avoid re-coding of the primary efficacy endpoint and thus enhance comparability of the results. On the other hand, this restriction led to the situation that each of the seven reference compounds was investigated in only one individual study. In consequence, different baseline values could be expected to have a direct impact on differences between treatment groups. In order to take into account such possible imbalances, baseline-adjusted ANCOVA was applied for the primary efficacy analysis. The ANCOVA approach is considered the preferred method for meta-analyses on continuous outcomes with baseline imbalance based on individual patient data [31, 32].
As increasing age is significantly associated with vertigo due to vestibular disorders, demonstration of effectiveness of antivertigo drugs in patients of advanced age is important. Patients in the fixed combination group of the present meta-analysis had a rather low average age of 52.6 years (range 18–84 years), including only 20.4% elderly subjects (≥ 65 years). Since the patients in the included studies were derived from a tertiary care setting (i.e., university clinics), this causes a certain limitation regarding the generalisability of the findings to elderly patients, who are more commonly in primary and secondary care. Even though the effectiveness of the fixed combination in elderly subjects has been demonstrated by two non-interventional studies [30, 33], further investigations are necessary to verify these results in randomised, controlled studies.
Furthermore, the study population was rather heterogeneous and comprised patients with central, peripheral or combined central/peripheral vertigo, derived from a great variability of possible vestibular disorders. In case of known aetiologies, treatment of the underlying vestibular disorder(s) would certainly be the best possible therapy option. However, causal treatment is often not possible due to the complexity of the underlying pathologies, together with often unavailable suitable equipment or appropriate otoneurological tests, which makes unambiguous differential diagnoses and subsequent more specific treatment regularly difficult [34, 35]. Therefore, symptomatic treatment is all the more important, either as the only therapy option in case of unknown aetiology or as supportive therapy in addition to specific treatment in case of known aetiologies. The fixed combination reduces vertigo symptoms through regulation of vestibular signal transmission, largely independent of the underlying vestibular disorder. In case of peripheral vestibular disorders, however, any treatment with antivertigo drugs should avoid impairment of spontaneous improvement of the patients’ condition due to central compensation processes. Centrally active and potentially sedating antivertigo drugs, such as dimenhydrinate, are known to depress CNS compensatory mechanisms and are thus expected to detrimentally affect vestibular compensation. Although the fixed combination contains dimenhydrinate, albeit in a distinctly lower dose than usually given in monotherapy, it showed no adverse effect on compensation in patients with unilateral vestibular neuritis, when compared to betahistine, a nonsedating drug that may even enhance vestibular compensation [20].
Due to these inherent problems regarding aetiology and treatment of vestibular disorders, a rapid and effective symptomatic treatment of the patients‘ vertigo complaints is of primary concern, in order to facilitate daily activities and enable suitable rehabilitation measures. This fact holds especially for the elderly with often multifactorial disorders, who are subjected to a high risk of falls with potentially serious injuries, and increased rates of morbidity and mortality [7, 8]. In this context, although the findings reported here for a relatively heterogeneous patient population should be interpreted with caution, they may largely mirror the situation in daily clinical practice.
All treatments were well tolerated, based on an overall low incidence of adverse events, only few patients who discontinued the study because of AEs, as well as the patients‘ and investigators' tolerability ratings. A total of 55 patients (6.9%) reported 75 non-serious AEs, and 19 patients (2.4%) discontinued the study prematurely because of AEs. Nearly 95% of the patients (cinnarizine/dimenhydrinate group: 97.9%) rated the tolerability of the study medications as either “good” or “very good”.
5 Conclusions
The present meta-analysis provides further evidence that treatment with the fixed combination of cinnarizine and dimenhydrinate leads to an effective and clinically meaningful improvement of vertigo, together with good tolerability, in patients suffering from vertigo due to various kinds of vestibular disorders. It can therefore be regarded as a useful and even superior therapeutic alternative to other currently used antivertigo drugs.
References
Schuller DE, Schleuning AJ. DeWeese and Saunders’ Otolaryngology-Head and Neck Surgery. 8th ed. St. Louis: Mosby; 1994. p. 495–513.
Brandt T, Dieterich M, Strupp M. Vertigo and dizziness—common complaints. 2nd ed. London: Springer; 2013.
Hanley K, O’Dowd T, Considine N. A systematic review of vertigo in primary care. Br J Gen Pract. 2001;51:666–71.
Kroenke K, Price RK. Symptoms in the community: prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153(21):2474–80.
Yardley L, Burgneay J, Andersson G, et al. Feasibility and effectiveness of providing vestibular rehabilitation for dizzy patients in the community. Clin Otolaryngol. 1998;23:442–8.
Neuhauser HK, Radtke A, von Brevern M, Lezius F, Feldmann M, Lempert T. Burden of dizziness and vertigo in the community. Arch Intern Med. 2008;168(19):2118–24.
Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169:938–44.
Corrales CE, Bhattacharyya N. Dizziness and death: an imbalance in mortality. Laryngoscope. 2016;126:2134–6.
Arab SF, Düwel P, Jüngling E, Westhofen M, Lückhoff A. Inhibition of voltage-gated calcium currents in type II vestibular hair cells by cinnarizine. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:570–5.
Düwel P, Haasler T, Jüngling E, et al. Effects of cinnarizine on calcium and pressure-dependent potassium currents in guinea pig vestibular hair cells. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:441–8.
Soto E, Vega R. Neuropharmacology of vestibular system disorders. Curr Neuropharmacol. 2010;8(1):26–40.
Lacour M. Betahistine treatment in managing vertigo and improving vestibular compensation: clarification. J Vestib Res. 2013;23:139–51.
Pytel J, Nagy G, Toth A, Spellenberg S, Schwarz M, Repassy G. Efficacy and tolerability of a fixed low-dose combination of cinnarizine and dimenhydrinate in the treatment of vertigo: a 4-week, randomized, double-blind, active- and placebo-controlled, parallel-group, outpatient study. Clin Ther. 2007;29(1):84–98.
Hahn A, Novotný M, Shotekov PM, Cirek Z, Bognar-Steinberg I, Baumann W. Comparison of cinnarizine/dimenhydrinate fixed combination with the respective monotherapies for vertigo of various origins. Clin Drug Investig. 2011;31(6):371–83.
Cirek Z, Schwarz M, Baumann W, Novotný M. Efficacy and tolerability of a fixed combination of cinnarizine and dimenhydrinate versus betahistine in the treatment of otogenic vertigo. A double-blind, randomized clinical study. Clin Drug Investig. 2005;25:377–89.
Scholtz AW, Hahn A, Stefflova B, et al. Efficacy and safety of a fixed combination of cinnarizine 20 mg and dimenhydrinate 40 mg vs betahistine dihydrochloride 16 mg in patients with peripheral vestibular vertigo: a prospective, multinational, multicenter, double-blind, randomized, non-inferiority clinical trial. Clin Drug Investig. 2019;39:1045–56. https://doi.org/10.1007/s40261-019-00858-6.
Otto V, Fischer B, Schwarz M, Baumann W, Preibisch-Effenberger R. Treatment of vertebrobasilar insufficiency-associated vertigo with a fixed combination of cinnarizine and dimenhydrinate. Int Tinnitus J. 2008;14:57–67.
Hahn A, Sejna I, Stefflova B, Schwarz M, Baumann W. A fixed combination of cinnarizine/dimenhydrinate for the treatment of patients with acute vertigo due to vestibular disorders. A randomized, reference-controlled, clinical study. Clin Drug Investig. 2008;28:89–99.
Scholtz AW, Schwarz M, Baumann W, Kleinfeldt D, Scholtz HJ. Treatment of vertigo due to acute unilateral vestibular loss with a fixed combination of cinnarizine and dimenhydrinate: a double-blind, randomized, parallel-group clinical study. Clin Ther. 2004;26:866–77.
Scholtz AW, Steindl R, Burchardi N, Bognar-Steinberg I, Baumann W. Comparison of the therapeutic efficacy of a fixed low-dose combination of cinnarizine and dimenhydrinate with betahistine in vestibular neuritis. A randomized, double-blind, non-inferiority study. Clin Drug Investig. 2012;32(6):387–99.
Novotný M, Kostrica R. Fixed combination of cinnarizine and dimenhydrinate versus betahistine dimesylate in the treatment of Menière’s disease: a randomized, double-blind, parallel-group clinical study. Int Tinnitus J. 2002;8:115–23.
Kessler L, Bognar-Steinberg I, Baumann W, Skurczynski W. Treatment of vestibular vertigo: comparison of a fixed combination of cinnarizine 20 mg and dimenhydrinate 40 mg with the 2.5-fold higher dosed active drugs in monotherapy. A prospective, randomized, reference-controlled, two-center, double-blind study. Arch Sensol Neurootol Sci Pract. 2012;7:1–13.
Schremmer D, Bognar-Steinberg I, Baumann W, Pytel J. Efficacy and tolerability of a fixed combination of cinnarizine and dimenhydrinate in treatment of vertigo: analysis of data from five randomized, double-blind clinical studies. Clin Drug Investig. 1999;18(5):355–68.
Stewart LA, Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof. 2002;25(1):76–97.
Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340: c221. https://doi.org/10.1136/bmj.c221.
Rahlfs VW, Zimmermann H. The mean vertigo score (MVS) outcome scale and its use in clinical research for quantifying vestibular disorders. Front Neurol. 2021;12:601749. https://doi.org/10.3389/fneur.2021.601749.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.
Deeks JJ, Higgins JP. Statistical algorithms in review manager 5. Statistical Methods Group of The Cochrane Collaboration. 2010;1–11.
Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane, 2022. www.training.cochrane.org/handbook.
Plescia F, Salvago P, Dispenza F, Messina G, Cannizzaro E, Martines F. Efficacy and pharmacological appropriateness of cinnarizine and dimenhydrinate in the treatment of vertigo and related symptoms. Int J Environ Res Public Health. 2021;18:4787. https://doi.org/10.3390/ijerph18094787.
McKenzie JE, Herbison GP, Deeks JJ. Impact of analysing continuous outcomes using final values, change scores and analysis of covariance on the performance of meta-analytic methods: a simulation study. Res Synth Methods. 2016;7:371–86.
Kebede MM, Peters M, Heise TL, Pischke CR. Comparison of three meta-analytic methods using data from digital interventions on type 2 diabetes. Diabetes Metab Syndr Obes Targets Ther. 2019;12:59–73.
Scholtz AW, Ilgner J, Loader B, Pritschow BW, Weisshaar G. Cinnarizine and dimenhydrinate in the treatment of vertigo in medical practice. Wien Klin Wochenschr. 2015;128(9–10):341–7. https://doi.org/10.1007/s00508-015-0905-5.
Dros J, Maarsingh OR, Beem L, van der Horst HE, ter Riet G, Schellevis FG, van Weert HCPM. Impact of dizziness on everyday life in older primary care patients: a cross-sectional study. Health Qual Life Outcomes. 2011;9:44. https://doi.org/10.1186/1477-7525-9-44.
Maarsingh OR, Stam H, van der Horst HE. A different approach of dizziness in older patients: away from the diagnostic dance between patient and physician. Front Med. 2014;1:50. https://doi.org/10.3389/fmed.2014.00050.
Acknowledgements
We would like to acknowledge the collaboration and commitment of all investigators and their staff at the 15 study centres.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding of this meta-analysis and open access was provided by Hennig Arzneimittel, Flörsheim am Main, Germany.
Conflict of interest
Arne W. Scholtz and Frank Waldfahrer have no conflicts of interest that are directly relevant to the content of this article. Regina Hampel is an employee of GKM Gesellschaft für Therapieforschung mbH, Munich, Germany, and Gerhard Weisshaar is an employee of Hennig Arzneimittel.
Ethics approval
This meta-analysis is based on previously conducted studies. The individual studies were reviewed and approved by the local Ethics Committees at the study sites. All patients provided their written consent to participate in the respective study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analysed during the current meta-analysis are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Authors’ contributions
All authors made substantial contributions to the study conception, design and interpretation of the data. RH performed the aquisition and verification of data, and conducted the statistical analyses. GW drafted the manuscript and all authors took part in revising and critically reviewing the article. All authors read and approved the final manuscript.
Study centres and investigators
Study 1: J. Pytel, G. Nagy, ENT Department, University Medical School Pccs, Hungary; S. Spellenberg, Department of Audiology and Otoneurology, Saint John Hospital, Budapest, Hungary; G. Répassy, A. Tódt, Department of Otorhinolaryngology, University of Debrecen, Hungary. Study 2: Z. Cirek, ENT Department Pilsen of the Charles University Prague, Pilsen, Czech Republic; M. Novotný, ENT Department, University of Olomouc, Czech Republic; P. M. Shotekov, N. Nicoevski, 1st Neurology Clinic, Medical Academy of Sofia, Bulgaria. Study 3: Z. Cirek, ENT Department Pilsen of the Charles University Prague, Pilsen, Czech Republic. Study 4: A. W. Scholtz, ENT Clinic, Medical University of Innsbruck, and ENT Center for Vertigo, Innsbruck, Austria; A. Hahn, ENT Clinic, Charles University of Prague, 3rd Medical Faculty, Prague, Czech Republic; P. Pavlicek, B. Stefflova, ENT Clinic, Regional Hospital Budweis, Czech Republic; D. Medzhidieva, ENT Clinic, Medical University of Sofia—St. Ivan Rilski Hospital, Sofia, Bulgaria; S.V. Ryazantsev, Federal State Institution St. Petersburg Research Institute of Ear, Throat, Nose and Speech, St. Petersburg, Russia; A. Paschinin, North West State Medical University n. a. I.I. Mechnikov of Ministry of Health and Social Development, St. Petersburg, Russia; N. Kunelskaya, Moscow Research—Practical Center of Otolaryngology n. a. L. I. Sverzhevsky, Moscow, Russia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Scholtz, A.W., Waldfahrer, F., Hampel, R. et al. Efficacy and Safety of a Fixed-Dose Combination of Cinnarizine 20 mg and Dimenhydrinate 40 mg in the Treatment of Patients with Vestibular Vertigo: An Individual Patient Data Meta-Analysis of Randomised, Double-Blind, Controlled Clinical Trials. Clin Drug Investig 42, 705–720 (2022). https://doi.org/10.1007/s40261-022-01184-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01184-0