Abstract
Hepatic encephalopathy (HE) is a very prevalent condition in patients with advanced liver disease and has a high recurrence rate. The pathophysiology has a multifactorial origin where hyperammonaemia and inflammation become particularly relevant. There are no HE-specific diagnostic tests, and diagnosis is usually made by taking into account the presence of suggestive and compatible clinical symptoms, the existence of a predisposing liver condition and ruling out other causes with similar clinical manifestations. Once the diagnosis of HE is established, it is essential to carry out an adequate classification based on the underlying liver disease, the intensity of clinical manifestations, the temporal course of the disease and the presence or absence of precipitating factors. Treatment should be aimed at decreasing the duration, intensity and consequences of episodes, preventing recurrence and limiting the impact of the disease in patients and their relatives.
Similar content being viewed by others
Hepatic encephalopathy (HE) is prevalent in patients with advanced liver disease and is associated with substantial morbimortality and costs. |
The HE diagnosis is based on a combination of compatible symptoms in patients with a predisposition (liver failure/portosystemic shunt) and the exclusion of other causes with similar clinical manifestations. |
Once an HE diagnosis is established, adequate classification is essential to provide uniformity in patient care. |
The search for precipitating factors is fundamental for adequate management and improvement in prognosis. |
Specific treatment focuses on targeting and lowering the accumulation of ammonia and targeting inflammation. |
Several factors are known to predispose patients to overt HE, such as a history of previous HE, diabetes, sarcopenia, together with worse liver function, and together with older age, these factors should be carefully evaluated in order to minimise the occurrence of post-TIPS HE. |
1 Introduction
Hepatic encephalopathy (HE) is a very prevalent condition in patients with advanced liver disease, occurring in 10% of patients at the diagnosis of cirrhosis [1], reaching up to 20% in decompensated cirrhosis [2] and up to 50% in patients with transjugular intrahepatic portosystemic shunt (TIPS) [3, 4]. Furthermore, those patients who have had the first episode of HE have a recurrence risk of up to 40% per year [5]. HE is the leading cause of readmission in cirrhotic patients with advanced liver disease, and its prevalence has increased in recent years, which is costly [6, 7].
The impact of the disease on the prognosis of these patients is significant; since it is associated with an increase in mortality and morbidity at 1 year and an increased risk of falls, fractures and suffering a road traffic accident [8].
Also, HE dramatically affects the quality of life of the patient and their caregivers/relatives [9, 10].
2 Hepatic Encephalopathy
2.1 Definition
HE is defined as brain dysfunction in patients with liver failure and/or portosystemic shunts presenting cognitive alterations and personality and mental disorders [4]. A common aspect of HE is a fluctuating course, which may or may not reach the levels of clinical detection. If there is a suspicion of HE and a lack of clinically apparent symptoms, minimal hepatic encephalopathy should be considered, and specific diagnostic tests should be performed to confirm or rule out this condition. In cases where clinical manifestations appear above the clinical detection threshold, episodic HE must be suspected and can be recurrent. Likewise, if these clinical manifestations always appear above the detection level, then persistent HE is present [11]. This definition of HE does not consider the impact of the aetiology of liver disease itself on brain function. Indeed, factors such as alcohol abuse, viral hepatitis, non-alcoholic fatty liver disease (NAFLD) or primary biliary cholangitis [4, 11,12,13] may impact on the manifestations of HE. At present, it is not clear whether the neurological dysfunction secondary to the aetiology of liver disease or comorbidities, such as aging or diabetes, is different [12], and it might be difficult to attribute neurological disturbances to a specific factor in some cases [14].
2.2 Classification
Several international societies recently proposed an adequate categorisation of HE to provide uniformity in terms of clinical research and patient care. Therefore, HE should be classified according to four criteria [11] (Fig. 1):
-
1.
Underlying liver disease: (a) In patients with acute liver disease; (b) in patients with portosystemic shunt without chronic liver disease; and (c) in patients with chronic liver failure with or without portosystemic shunts.
-
2.
Severity of clinical manifestations: The most widely used scale for quantifying the severity of HE is the West Haven criteria. This scale subdivided the clinically manifested HE into four stages (I–IV). However, this classification has interobserver variations, particularly at its milder stages. Therefore, it has been proposed that HE is qualified as overt when at least temporal disorientation and/or asterixis is present (grade ≥ II according to the West Haven criteria). The term covert (as opposed to overt) encompasses grade I and minimal HE, both of which are heterogeneous and with different prognostic implications [15, 16]. Although widely used, the lack of reproducibility across observers made the West Haven criteria weak for clinical trials. For this purpose, other more accurate and validated classifications such as Hepatic Encephalopathy Scoring Algorithm (HESA) or the Hepatic Encephalopathy Grading Instrument (HEGI) are desirable [17,18,19].
-
3.
Time course: HE can be episodic, recurrent (bouts of HE in 6 months or less) or persistent (some degree of manifested HE always present).
-
4.
Precipitating factors: According to their presence, HE can be precipitated (bouts of HE in the setting of a precipitating event that can be usually identified in the great majority of the episodes) or spontaneous (no precipitating event can be found) [20].
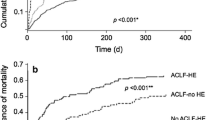
Acute-on-chronic liver failure (ACLF) is a recently described syndrome consistent with decompensation of chronic liver disease characterised by organ failure and poor short-term prognosis. Patients with ACLF have a different pathophysiology characterised by very marked systemic inflammation [21]. Patients with HE and ACLF have a worse prognosis [22] compared to those without ACLF. According to the presence of ACLF in patients with HE, a reclassification within type C is currently under discussion [11, 12].
This review focuses mainly on type C HE (Fig. 1).
2.3 Pathophysiology
HE is not a single clinical entity, and the neurological impairment in patients with liver dysfunction remains incompletely understood. Liver failure causes an increase in the exposure of the brain to several substances that, under normal circumstances, are efficiently metabolised by the liver. Those with a high first-pass metabolism across the liver are the most important. In addition, other factors that are commonly present in patients with liver failure can contribute to deterioration in neurological function by influencing the permeability/integrity of the blood–brain barrier and/or stimulating pathophysiological pathways.
-
Ammonia is mainly produced in the digestive tract as a by-product of protein degradation by the intestinal flora or deamination of glutamine by the intestinal glutaminase [15]. In patients with advanced liver disease with a loss of hepatocyte mass and in those who present portosystemic shunts, ammonia concentration increases at the systemic level, recruiting other organs, such as muscle and kidney, for its clearance [23]. Increased blood ammonia concentration can reach the brain since its dissolved form (NH3) ammonia diffuses freely across cell membranes and as an ion (NH4+) it is transported to the central nervous system through transporters. In the brain, ammonia exerts its deleterious effects through different pathways. To eliminate this increase in ammonia, it is metabolised to glutamine in astrocytes exerting toxic effects, which appears to be involved in the pathogenesis of HE neurological manifestations [24].
-
Inflammation is also involved in the pathophysiology of HE. The inflammatory response has been linked to the development of HE in fulminant hepatic failure and cirrhosis. In addition, inflammatory mediators and oxidative stress may induce blood–brain barrier dysfunction and contribute to neuroinflammation. Also, inflammation may exacerbate the deleterious effects of hyperammonaemia on the brain [25, 26].
-
Biliary manganese excretion is impaired in advanced liver failure, leading to its deposition within the basal ganglia in the brain. This event is believed to participate in the psychomotor impairment associated with HE [27].
-
Hyponatraemia is often present in liver failure. Furthermore, serum sodium concentration correlates with neurological manifestations and predicts recurrence of HE [28].
-
Bile acids, cholesterol metabolism end-products, are elevated in the plasma of patients with advanced liver failure and have been linked to neuroinflammation [29].
Several alterations have been observed in the neuropathology of HE. One of the most distinctive pathological alterations is ammonia-induced astrocyte swelling resulting from ammonia detoxification. The osmotic stress caused by the increased glutamine may lead to cell dysfunction, which may impact neuronal disturbances by different mechanisms, including glutamate reuptake and increased synthesis of neurosteroids, among others (potent GABAA receptor ligands) [30]. In fact, several alterations in neurotransmission, such as increased GABAergic tone and N-methyl-D-aspartate (NMDA) stimulation, have been described [31]. Brain oedema has also been described in HE secondary to changes in the cellular hydration state in response to the increase in the intracellular glutamate concentration [32]. Several cell metabolic pathways can be impaired due to cell oedema, and indeed, increased intracellular lactate has also been described suggesting energy impairment [33].
Other factors, such as altered intestinal microbiota [34], circulatory derangements, nutritional deficits [35], sarcopenia [36], comorbidities (such as alcohol abuse [37] and NAFLD [38]) and other organ failures may also contribute to the process of HE in advanced liver disease [39, 40]. Indeed, it has been described that the composition and functionality of microbiota in HE may modulate the brain function and the disease's clinical course and may be a target for treatment. In addition, muscle mass depletion is a very common event in cirrhosis associated with an increased risk for HE and impact on survival.
2.4 Clinical Manifestations
HE produces a wide spectrum of neurological and psychiatric manifestations. HE alters attention, working memory, psychomotor speed, and visuospatial ability in its lowest expression. As HE progresses, personality changes, such as apathy, irritability and disinhibition, may be reported by the patient’s relatives, and evident consciousness and motor function alterations may occur. Disturbances of the sleep–wake cycle with excessive daytime sleepiness are also frequent [4]. In more severe cases, progressive disorientation, inappropriate behaviour, agitation, stupor and, finally, coma may be observed. However, transient focal neurological deficits or seizures have very rarely been reported in HE [41,42,43].
2.5 Diagnosis
There are no specific HE findings, but there are suggestive symptoms, and generally, the diagnosis is made by exclusion. The diagnosis is made based on three pillars: the presence of compatible symptoms by clinical evaluation (scales are used to grade severity in overt HE); the presence of a predisposing liver condition and its degree (is relevant to assess the degree of liver failure and the role of portosystemic shunts, particularly if liver function is preserved); and ruling out other causes with similar clinical manifestations (Fig. 2). Diagnosing cognitive dysfunction is not difficult. The challenge lies in attributing those alterations to HE when no pathognomonic signs or specific tools are present or available. That is why HE remains a diagnosis of exclusion. The most common disorders to consider in the differential diagnosis of overt HE are complications of diabetes mellitus, alcohol-related disorders, drug-related adverse effects, severe electrolyte disorders, nervous system infection, central psychiatric disorders, nonconvulsive epilepsy, dementia, apnoea and intracranial bleeding or stroke [12, 39].
In the absence of evident clinical manifestations, such as that for minimal HE, neuropsychological tests (Psychometric Hepatic Encephalopathy Score [PHES], Critical Flicker-Frequency Test [CFF], Stroop Test, etc.) and neurophysiological tests (electroencephalography [EEG]) are available for the diagnosis of subclinical cognitive impairment. This makes it possible to quantify persistent cognitive alterations. In addition, pathophysiological tests, including tests of liver function (Child-Pugh, Model for End-Stage Liver Disease [MELD], oral glutamine tolerance test), tools to assess portosystemic shunts (abdominal computed tomography scan) and tests of cerebral structure and composition (brain magnetic resonance imaging [MRI] and MR spectroscopy [MRS]) are valuable instruments to attribute the cognitive abnormalities to a given condition. Of course, none of the tests are specific or perfect since they do not encompass the broad spectrum of the disease. However, they complement each other, so they are usually combined in patients with a difficult diagnosis to help choose treatment options [4, 12, 16, 24]. Of note, there is no direct correlation between plasma NH3 levels and the grade of HE supporting the different individual sensitivities to the same ammonia levels. That is why the diagnostic utility of plasma ammonia levels is limited and mainly relies on its high negative predictive value (0.81) [12].
Once the diagnosis is made, it is essential to carry out an adequate characterisation based on the type of underlying liver disease, clinical manifestations, time course and the presence or absence of precipitating factors (infections, gastrointestinal bleeding, diuretics or electrolyte abnormalities). Treatment of precipitating factors is a mainstay of management and requires their active search and continuous monitoring (Table 1). Inflammation (either associated with infections, translocation or liver injury), gastrointestinal bleeding or electrolyte abnormalities are the most frequent precipitants. Several precipitating factors, e.g. infection and bleeding, are associated with increased mortality, and effective management of those events may improve prognosis in patients with overt HE. Also, rapid resolution of constipation or blood clearance in the gastrointestinal tract improves episodic HE [44, 45].
TIPS is an established treatment for variceal bleeding or ascites in advanced liver failure. HE may be induced or aggravated by this procedure. The management of post-TIPS HE may be challenging; therefore, individualised patient selection taking into account HE risk factors is critical. Minimal HE or previous overt HE, diabetes, sarcopenia, proton pump inhibitors and worse liver function (higher creatinine and bilirubin and lower albumin and sodium) are among the factors predisposing to overt HE in cirrhotic patients [46,47,48,49]. Older age and low portosystemic pressure gradient after TIPS were also identified as risk factors for post-TIPS HE [50,51,52,53,54].
2.6 Treatment
Treatment should be aimed at minimising the duration, intensity and consequence of episodes, preventing recurrence and limiting the impact of the episodes on patients’ and caregivers’ health. Implementation of general measures common to those with other neurological disorders (ensure the airway, haemodynamic stabilisation, prevention of self-injury, adequate nutrition for the neurological situation), together with an active search and exclusion of other causes, is mandatory. Identification and treatment of precipitating factors such as hyponatraemia or infections are also required, together with initiating specific measures to intervene on the pathophysiological mechanisms that can be modified. In Fig. 2, the management algorithm for a patient with possible HE is summarised.
Although many factors are believed to be involved in the development and progression of HE, most treatments focus on targeting and lowering the accumulation of ammonia as well as targeting inflammation (Table 2).
2.6.1 Treatments Targeting Ammonia Production and Removal
Ammonia reduction measures have different mechanisms of action, which include reducing ammonia production from the gut and eliminating plasma ammonia.
-
Among the treatments used, non-absorbable disaccharides favour intestinal transit and lower faecal pH, reducing ammonia production and absorption. A recent meta-analysis showed that non-absorbable disaccharides are effective compared to placebo in the clinical manifestations of HE in prevention and mortality, constituting the treatment of choice in acute HE and preventing recurrences [55].
-
Rifaximin is the most used antibiotic in HE because it shows the best efficacy and safety profile, acting on the functionality of the microbiota. It is highly effective in secondary prevention, showing that when added to non-absorbable disaccharides, it prevents a third episode of encephalopathy (hazard ratio = 0.42; 95% confidence interval 0.28–0.64) [56, 57]. The combination of lactulose plus rifaximin was more effective than lactulose alone to treat episodic overt HE and reduce mortality due to a reduction in sepsis-related deaths in a trial [57]. However, further trials of this combination are still needed for quality evidence.
-
Compared to placebo or no intervention, probiotics appear to promote recovery and may play a role in delaying the development of overt HE improving the quality of life and the plasmatic ammonia concentrations, but with a minor effect on mortality [58].
-
Branched-chain amino acids favour the elimination of plasma ammonia and could improve HE symptoms, but do not seem to affect mortality (low-quality evidence) [59].
-
A recent meta-analysis suggests some benefit from l-ornithine l-aspartate (LOLA), although the included studies show only very low-quality evidence [60].
Patients with recurrent/persistent HE associated with portosystemic shunts could benefit from their occlusion. However, an increase in portal pressure and related complications could develop. So, shunt embolisation can be considered a reasonable option in meticulously selected patients with well-preserved liver function (MELD < 11) [61], in the view of liver transplantation in most cases.
In the setting of post-TIPS hepatic encephalopathy, management with non-absorbable disaccharides and nonabsorbable antibiotics might be insufficient, and the need for alternative pharmacological therapies becomes great. Faecal microbiota transplantation is increasingly being used in treating Clostridium difficile infection, and one trial showed a decrease in HE recurrence compared to the standard of care [62]. So, it may be useful in the near future. Furthermore, a nutritional approach enhancing muscle ammonia detoxification by adding branched-chain amino acids can be considered. In cases where a refractory post-TIPS HE develops, the TIPS modification through the reduction of shunt diameter might be an option. Safety awareness is essential in TIPS modifications, particularly regarding the relapse of portal hypertension complications, making liver transplantation the optimal alternative [63]. Of note, the controlled expansion stents maintain a stable diameter, and the 8-mm sized covered stents reduce post-TIPS HE compared to the 10 mm stent, becoming the stent of choice in recent years [64].
2.6.2 Treatments Targeting Inflammation
Some of these treatments, such as rifaximin and probiotics, in addition to acting through the ammonia pathway, also may act on inflammation. Albumin, a protein with anti-inflammatory, antioxidant and immunomodulatory properties [65], has shown some improvement in survival and clinical symptoms of HE, but these results need to be confirmed in future studies [66].
2.6.3 Liver Transplantation Referral
Overt HE may be an indication for liver transplantation in patients with advanced liver failure. So, these individuals with severe overt HE refractory to maximal therapy should be referred to a liver transplant centre [4].
2.7 Future Horizons
In recent years, an effort has been made to develop new treatments, including faecal transplantation and ornithine phenylacetate, which have been shown to reduce ammonia and improve cognitive function in preclinical and early clinical studies [62, 67]. Some of these strategies already showed encouraging results, but further data are needed to elucidate their potential beneficial effect on HE (Table 2).
3 Conclusions
HE remains one of the most relevant complications in patients with advanced liver disease, contributing to high morbidity, mortality and worse quality of life. Therefore, efforts have been made to define and classify the syndrome to provide uniformity in patients’ care. However, controversies relating to classification remain, such as the difficulty in diagnosing grade I HE, pointing at the use of alternative scales in clinical trials.
Recent advances in the pathophysiology of HE identifying important key players such as interorgan ammonia metabolism and inflammation set the basis for new therapies. Specific treatment focuses mainly on lowering plasma ammonia and targeting inflammatory response, decreasing the duration and intensity of episodes and preventing the recurrence of bouts of HE. In addition, new tools to improve the diagnosis (by clarifying the contribution of aetiology of liver disease or comorbidities) and improving clinical response to treatment would be vital and would cover an area previously unaddressed.
References
Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51(5):1675–82.
D’Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci. 1986;31(5):468–75.
Nolte W, Wiltfang J, Schindler C, et al. Portosystemic hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with cirrhosis: clinical, laboratory, psychometric, and electroencephalographic investigations. Hepatology. 1998;28(5):1215–25.
Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–35.
Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: an open-label randomised controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(885–91):891.e1.
Hirode G, Vittinghoff E, Wong RJ. Increasing burden of hepatic encephalopathy among hospitalized adults: an analysis of the 2010–2014 National Inpatient Sample. Dig Dis Sci. 2019;64:1448–57.
Shaheen A-A, Nguyen HH, Congly SE, Kaplan GG, Swain MG. Nationwide estimates and risk factors of hospital readmission in patients with cirrhosis in the United States. Liver Int. 2019;39:878–84.
Krishnarao A, Gordon FD. Prognosis of hepatic encephalopathy. Clin Liver Dis. 2020;24(2):219–29.
Künzler-Heule P, Beckmann S, Mahrer-Imhof R, Semela D, Händler-Schuster D. Being an informal caregiver for a relative with liver cirrhosis and overt hepatic encephalopathy: a phenomenological study. J Clin Nurs. 2016;25:2559–68.
Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106(9):1646–53.
American Association for the Study of Liver Diseases; European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61(3):642–59.
Rose CF, Amodio P, Bajaj JS, et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J Hepatol. 2020;73(6):1526–47.
Balzano T, Forteza J, Borreda I, et al. Histological features of cerebellar neuropathology in patients with alcoholic and nonalcoholic steatohepatitis. J Neuropathol Exp Neurol. 2018;77(9):837–45.
Córdoba J. New assessment of hepatic encephalopathy. J Hepatol. 2011;54(5):1030–40.
Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7(9):515–25.
Bajaj JS, Cordoba J, Mullen KD, et al; International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN). Review article: the design of clinical trials in hepatic encephalopathy—an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33(7):739–47.
Hassanein T, Blei AT, Perry W, et al. Performance of the hepatic encephalopathy scoring algorithm in a clinical trial of patients with cirrhosis and severe hepatic encephalopathy. Am J Gastroenterol. 2009;104:1392–400.
Rockey DC, Vierling JM, Mantry P, et al. Randomized, double-blind, controlled study of glycerol phenylbutyrate in hepatic encephalopathy. Hepatology. 2014;59:1073–83.
Bajaj JS, Frederick RT, Bass NM, et al. Overt hepatic encephalopathy: development of a novel clinician reported outcome tool and electronic caregiver diary. Metab Brain Dis. 2016;31:1081–93.
Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy–definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–21.
Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N Engl J Med. 2020;382:2137–45.
Cordoba J, Ventura-Cots M, Simón-Talero M, et al; CANONIC Study Investigators of EASL-CLIF Consortium. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol. 2014;60(2):275–81.
Wright G, Noiret L, Damink SWMO, Jalan R. Interorgan ammonia metabolism in liver failure: the basis of current and future therapies. Liver Int. 2011;31:163–75.
Córdoba J, Mínguez B. Hepatic encephalopathy. Sem Liver Dis. 2008;28(1):70–80.
Shawcross DL, Davies NA, Williams R, Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonaemia in cirrhosis. J Hepatol. 2004;40(2):247–54.
Shawcross DL, Sharifi Y, Canavan JB, et al. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol. 2011;54(4):640–9.
Rovira A, Alonso J, Córdoba J. MR imaging findings in hepatic encephalopathy. Am J Neuroradiol. 2008;29:1612–21.
Guevara M, Baccaro ME, Rios J, et al. Risk factors for hepatic encephalopathy in patients with cirrhosis and refractory ascites: relevance of serum sodium concentration. Liver Int. 2010;30(8):1137–42.
McMillin M, Frampton G, Grant S, et al. Bile acid-mediated sphingosine-1-phosphate receptor 2 signaling promotes neuroinflammation during hepatic encephalopathy in mice. Front Cell Neurosci. 2017;11:191.
Butterworth RF. Neurosteroids in hepatic encephalopathy: novel insights and new therapeutic opportunities. J Steroid Biochem Mol Biol. 2016;160:94–7.
Lavoie J, Giguere JF, Layrargues GP, Butterworth RF. Amino acid changes in autopsied brain tissue from cirrhotic patients with hepatic encephalopathy. J Neurochem. 1987;49(3):692–7.
Haussinger D, Kircheis G, Fischer R, Schliess F, vom DS. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema? J Hepatol 2000;32(6):1035–8.
Hadjihambi A, Rose CF, Jalan R. Novel insights into ammonia-mediated neurotoxicity pointing to potential new therapeutic strategies. Hepatology. 2014;60:1101–3.
Davis B, Bajaj J. The human gut microbiome in liver diseases. Semin Liver Dis. 2017;37:128–40.
European Association for the Study of the Liver. EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–93.
Gioia S, Merli M, Nardelli S, et al. The modification of quantity and quality of muscle mass improves the cognitive impairment after TIPS. Liver Int. 2019;39:871–7.
Weissenborn K. Minimal/covert hepatic encephalopathy - impact of comorbid conditions. J Clin Exp Hepatol. 2019;9:109–11.
Filipovi B, Markovi O, Duri V, Filipovi B. Cognitive changes and brain volume reduction in patients with nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol. 2018;2018:9638797.
Butterworth RF. The liver-brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol. 2013;10(9):522–8.
Musgrave H, Hilsabeck RC. Hepatic encephalopathy. In: Ravdin L, Katzen H (eds) Handbook on the neuropsychology of aging and dementia. Clinical handbooks in neuropsychology. Springer, Cham; 2019.
Cadranel JF, Lebiez E, Di Martino V, et al. Focal neurological signs in hepatic encephalopathy in cirrhotic patients: an underestimated entity? Am J Gastroenterol. 2001;96(2):515–8.
Delanty N, French JA, Labar DR, Pedley TA, Rowan AJ. Status epilepticus arising de novo in hospitalized patients: an analysis of 41 patients. Seizure. 2001;10(2):116–9.
Eleftheriadis N, Fourla E, Eleftheriadis D, Karlovasitou A. Status epilepticus as a manifestation of hepatic encephalopathy. Acta Neurol Scand. 2003;107(2):142–4.
Naderian M, Akbari H, Saeedi M, Sohrabpour AA. Polyethylene glycol and lactulose versus lactulose alone in the treatment of hepatic encephalopathy in patients with cirrhosis: A Non-inferiority randomized controlled trial. Middle East J Dig Dis. 2017;9:12–29.
Rahimi RS, Singal AG, Cuthbert JA, Rockey DC. Lactulose vs polyethylene glycol 3350—electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174:1727–33.
Elsaid MI, Rustgi VK. Epidemiology of hepatic encephalopathy. Clin Liver Dis. 2020;24:157–74.
Jepsen P, Watson H, Andersen PK, Vilstrup H. Diabetes as a risk factor for hepatic encephalopathy in cirrhosis patients. J Hepatol. 2015;63:1133–8.
Riggio O, Amodio P, Farcomeni A, et al. A model for predicting development of overt hepatic encephalopathy in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1346–52.
Nardelli S, Gioia S, Ridola L, Farcomeni A, Merli M, Riggio O. Proton pump inhibitors are associated with minimal and overt hepatic encephalopathy and increased mortality in patients with cirrhosis. Hepatology. 2019;70:640–9.
Yao J, Zuo L, An G, et al. Risk factors for hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with hepatocellular carcinoma and portal hypertension. J Gastrointest Liver Dis. 2015;24:301–7.
Nardelli S, Gioia S, Pasquale C, et al. Cognitive impairment predicts the occurrence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2016;111:523–8.
Lewis DS, Lee TH, Konanur M, et al. Proton pump inhibitor use is associated with an increased frequency of new or worsening hepatic encephalopathy after transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol. 2019;30:163–9.
Yin X, Zhang F, Guo H, et al. A nomogram to predict the risk of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients. Sci Rep. 2020;10:1–8.
Nardelli S, Lattanzi B, Torrisi S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol. 2017;15:934–6.
Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016;2016(5):CD003044.
Sharma P, Sharma BC. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(25):2423–4.
Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–63.
Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2(2):CD008716.
Gluud LL, Dam G, Les I, et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2015;2:CD001939.
Goh ET, Stokes CS, Sidhu SS, Vilstrup H, Gluud LL, Morgan MY. L-ornithine l-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2018;5(5):CD012410.
Laleman W, Simon-Talero M, Maleux G, et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57(6):2448–57.
Bajaj J, Salzman N, Acharya C, et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial. Hepatology. 2019;70:1690–703.
Schindler P, Heinzow H, Trebicka J, Wildgruber M. Shunt-induced hepatic encephalopathy in TIPS: current approaches and clinical challenges. J Clin Med. 2020;9(11):3784.
Wang Q, Lv Y, Bai M, et al. Eight millimetre covered TIPS does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal rebleeding. J Hepatol. 2017;67:508–16.
Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58(5):1836–46.
Ashour AA, Atta MA, Sadek KW, et al. Albumin administration in patients with decompensated liver cirrhosis: a meta-analytic update. Eur J Gastroenterol Hepatol. 2021;33(4):479–86.
Rahimi RS, Safadi R, Thabut D, et al. Efficacy and safety of ornithine phenylacetate for treating overt hepatic encephalopathy in a randomized trial. Clin Gastroenterol Hepatol. 2020;16:S1542-3565(20)31432-4.
Acknowledgements
The authors thank Ana Isabel Ortega for writing assistance on behalf of Springer Healthcare Ibérica SL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure statement
This article has been published as part of a journal supplement wholly funded by Eisai.
Funding
CIBEREHD is supported by Instituto de Salud Carlos III, Madrid, Spain. Rita García-Martínez was supported by fellowship JR14/00019 from Fondo de Investigación Sanitaria (Instituto de Salud Carlos III). Eisai funded the writing assistance provided by Springer Healthcare Ibérica SL.
Conflict of interest
The authors declare that there were no conflicts of interest.
Ethics approval
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors have contributed to the conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, and final approval of the version to be published.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
García-Martínez, R., Diaz-Ruiz, R. & Poncela, M. Management of Hepatic Encephalopathy Associated with Advanced Liver Disease. Clin Drug Investig 42 (Suppl 1), 5–13 (2022). https://doi.org/10.1007/s40261-022-01146-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01146-6