Abstract
Background and Objectives
In South Africa, the prevalence of human papillomavirus (HPV) and associated diseases, such as cervical cancer and genital warts, is among the highest in the world. This study evaluates the cost-effectiveness of bivalent, quadrivalent, and nonavalent HPV vaccination for 9- to 14-year-old girls from the South African healthcare system perspective.
Methods
A Markov model portraying the natural HPV disease progression from high-risk infection to cervical intraepithelial neoplasia (CIN) I, CIN II/III, or cervical cancer and from low-risk infection to genital warts was built. Transition probability, utility, and efficacy data were sourced from peer-reviewed literature. Vaccination costs were calculated based on the World Health Organization (WHO) guidelines. The model was populated with a cohort of 520,000 9-year-old girls to calculate incremental cost-effectiveness ratios (ICER) in South African Rand (R) per quality-adjusted life-years (QALYs) gained for each vaccination strategy.
Results
All HPV vaccination strategies dominate the no vaccine strategy. Compared with the bivalent vaccine, the nonavalent strategy increases QALYs by 0.14 and costs by R1793 (ICER: R13,013 per QALY) per person, while the quadrivalent vaccination provides −0.02 incremental QALYs and R1748 costs (ICER: −R116,397 per QALY). Consequently, at the South African willingness-to-pay threshold of R23,630 per QALY, nonavalent vaccination is the preferred strategy, with a probability of 90.2%. Scenario analysis demonstrated that results are influenced by vaccine coverage, efficacy, and duration of efficacy.
Conclusions
The introduction of nonavalent for bivalent HPV vaccination is a cost-effective intervention in South Africa. HPV vaccination should be part of a multifaceted public health strategy entailing screening, condoms, and education of all stakeholders to reduce the significant burden of sexual transmitted diseases in South Africa. Sex-neutral and catch-up vaccinations are subjects for further research.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Human papillomavirus (HPV) infections and associated diseases, such as genital warts and cervical cancer, cause a significant burden to patients and the healthcare system in South Africa. |
Based on a Markov model, we found that nonavalent HPV vaccination is cost effective compared with bivalent HPV vaccination (incremental cost-effectiveness ratio [ICER]: South African Rand (R) 13,013 per quality-adjusted life-year [QALY]) for 9- to 14-year-old girls at the South African willingness-to-pay threshold of R23,630 per QALY. |
Targeted policies aimed at improving vaccination coverage rates are substantial to further reduce ICERs for HPV vaccines in South Africa. |
1 Introduction
Human papillomavirus (HPV) infections cause a significant burden of disease in South Africa. With prevalence rates of 67% among certain populations of young women [1], diseases caused by HPV are among the highest in the world—cervical cancer is the most frequent cancer type among women aged 14–44 years (incidence rate per year: 0.04%) [2]. Even though three screenings with Papanicolaou (Pap) smears are recommended for women older than 30 years, in 10-year intervals, their coverage rate remains below 20% [3]. Consequently, in 2014 a national school vaccination program was introduced to vaccinate girls who are over the age of 9 years, in fourth grade, with the bivalent HPV vaccine (Cervarix®) [4]. The bivalent vaccine is primarily effective against the high-risk cervical cancer causing HPV types 16 and 18, and the aim of the program is to prophylactically reduce the incidence and prevalence of HPV and associated cancerous diseases [5]. The quadrivalent vaccine (Gardasil®) is additionally effective against HPV types 6 and 11, which cause genital warts, and both vaccines have some degree of cross-protective efficacy against other high-risk HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59) [6,7,8]. The nonavelent vaccine (Gardasil 9®) not only offers protection against HPV types 6, 11, 16, and 18 but also provides more substantial protection against HPV types 31, 33, 45, 52, and 58 than the cross-protection offered by the bivalent and quadrivalent vaccines [9].
The majority of Western countries have already included multivalent HPV vaccines in their vaccination schedule, as studies established their superior efficacy and cost-effectiveness in their respective nations [10,11,12,13,14]. The World Health Organization (WHO) recommends a two-dose vaccination regimen (bivalent, quadrivalent, or nonavalent) with doses administered 6 months apart [15]. While studies have demonstrated the cost-effectiveness of the bivalent vaccine compared with cervical cancer screening alone [16], to date no cost-effectiveness analysis of the quadrivalent and nonavalent vaccines in South Africa exists. This study will aim to fill this gap by conducting a cost-effectiveness analysis comparing the bivalent, quadrivalent, and nonavalent vaccines for 9- to 14-year-old girls from the perspective of the South African healthcare system.
2 Data and Methods
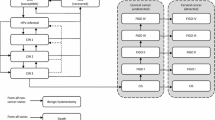
A Markov model was built in Microsoft Excel (2016; Microsoft Corporation, Redmond, WA, USA) to assess the cost-effectiveness of non-vaccinated compared with bivalent, quadrivalent, and nonavalent vaccination schemes for 9-year-old girls in South Africa (Fig. 1) [17, 18]. The model displays the different health states in the progression of HPV-related disease. A healthy individual may be infected with either high-risk HPV (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59) or low-risk HPV (primarily types 6 and 11). Patients with high-risk infections may then develop cervical cancer, whereas patients with low-risk infections may develop genital warts. Cervical cancer health states model the pathologic disease progression: ‘cervical intraepithelial neoplasia (CIN) I’, ‘CIN II/III’, and ‘cervical cancer’. Other cancer types (head and neck, vaginal, and vulvar cancer) were not included due to their relatively low prevalence and incidence rates in South Africa [19]. Patients may be cleared from the infection at any disease stage. Due to the low progression rate of cervical cancer and genital warts at the same time, this simultaneous progression characteristic was neglected in the model but is included in an alternative model structure (electronic supplementary material [ESM] Fig. e1). All states of health may ultimately lead to death.
Markov model structure of cervical cancer and genital warts disease progression. The graph illustrates the Markov model that remodels cervical cancer and genital warts disease progression to evaluate the cost-effectiveness of bivalent, quadrivalent, and nonavalent HPV vaccination. Within the model, each individual transits between health states. A healthy individual may be infected with high-risk HPV (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) and thereafter develop CIN I, CIN II/III, and cervical cancer. Similarly, individuals can also be infected with low-risk HPV (types 6 and 11) and thereafter develop genital warts. Across all disease states, individuals may experience disease regression to the healthy state or eventually die. CIN cervical interstitial neoplasia, HPV human papillomavirus
2.1 Transition Probabilities
Transition probabilities used to populate the model can be found in Table 1. Rates, hazard ratios, odd ratios, and probabilities with a different time horizon than 1 year were converted to 1-year probabilities, similar to previous peer-reviewed methodologies and cost-effectiveness studies [20, 21]. This widely used calculation considers the rate of patients progressing between disease states and the observation’s follow-up period [21]. To adjust for age-related changes in infection rates, sexual behavior, and disease progression, probabilities are split into two age groups: 9–34 years and 35–70 years). Mortality data were obtained from the South African Department of Statistics [22].
2.2 Vaccination Strategy
The current bivalent HPV vaccination program in South Africa consists of a two-dose schedule consistent with WHO recommendations [15]. However, due to all vaccines’ novelty, the ultimate protective duration is not yet known, with recent literature suggesting a duration of at least 10 years [23]. As immunity is likely to wane, affecting the impact of the vaccines on transition probabilities, we used a three-dose schedule that included a booster shot after 20 years, after which lifelong immunity is assumed.
2.3 Costs
Vaccine and booster shot costs are estimated based on WHO guidelines and previous studies (ESM Tables e1 and e2) [24,25,26]. Based on the market costs per dose of South African Rand (R) 54 (bivalent), R68 (quadrivalet), and R1524 (nonavalent), the total cost is calculated using previous data for transportation (10%), waste (5%), distribution (15%), communication (2.5%), and administration expenses (10%) [25]. Training costs for medical staff are not included as personnel were previously trained to administer vaccine shots for the rollout of the bivalent HPV vaccination scheme in 2014. The total cost for the public health system amounts to R140 (bivalent), R175 (quadrivalent), and R3924 (nonavalent) per vaccinated girl. Costs for the booster shots were calculated to be similar to the total vaccination costs and hence include costs for waste, transportation, and distribution.
Direct medical costs are computed for each disease state. Costs for cervical cancer states originate from previous literature on bivalent HPV vaccine economic evaluations (Table 1) [27]. These disease states include expenses for Pap smear screening (R624), treatment (conization: R1386; cryotherapy: R1386; hysterectomy: R15,218; chemoradiation: R120,174; radiotherapy: R117,150), and follow-up. Spending associated with genital warts is based on provider perspective mixed bottom-up and top-down data from Swaziland [28]. Data from Swaziland is justifiably the closest possible approximation due to its similarity to South Africa in terms of social, economic, healthcare, and geographical vectors.
The model also included costs for three cervical cancer screenings for women aged 30, 40, and 50 years, with a coverage rate of 20%. Previous literature estimates that the overall health system cost (including laboratory, consumable, and personal expenses) for one cervical cytology screening summed up to R624 in 2019 [30]. All costs are inflation and purchase price parity adjusted to 2019 R.
2.4 Utilities
Outcomes are measured by quality-adjusted life-years (QALYs) associated with each health state (Table 1). These QALYs represent patient-reported outcome data gathered with standardized and nationally adjusted EQ-5D survey scales. Utility decrements for infection, cervical cancer, and genital warts states were extracted from global studies and peer-reviewed literature. Ultimately, the absorbent state death is valued with a QALY worth 0.
2.5 Other Model Parameters and Vaccine Efficacy
The model has a cycle length of 1 year because the examined cancer types progress slowly and disease treatment schedules fall within a similar timeframe (Table 1) [31]. The simulation runs for 61 years, as it models the vaccination effect of the female cohort born in 2010 (cohort size: 520,000), with a life expectancy of 70 years. HPV infection will start with the median age of sexual debut (18.5 years) for South African girls [32]. Costs and benefits are calculated from the perspective of the South African public healthcare system. A discount rate of 3% is applied to all costs and utilities according to the WHO vaccination guidelines (which also falls within the South African Ministry of Health suggestion of 0–10%) [33]. Vaccination coverage is expected to remain at the current level of 90% [1].
HPV vaccination reduces the probability of progression to HPV infection states by (1−efficacy). HPV type specific efficacy rates were sourced from global phase III clinical trials that were also the basis for each vaccine’s marketing authorization [34,35,36,37]. The overall vaccine efficacy against high-risk/low-risk HPV is calculated by weighting the efficacy against HPV 16, 18/HPV 6, 11 and the cross-protection efficacy against other high-risk/low-risk HPV types with the prevalence of the respective HPV types in South Africa (ESM Table e3) [23, 38]. Consequently, overall efficacy against high-risk infections were estimated at 57.73% for bivalent vaccination, 54.64% for quadrivalent vaccination, and 97.41% for nonavalent vaccination. Efficacy against low-risk infections was calculated at 0% for bivalent vaccination and 74.68% for both quadrivalent and nonavalent vaccination. After the model structure was built, it was subsequently populated with the described data and prevalence rates were cross-checked and adjusted to match actual observed South African epidemiology.
2.6 Outcomes
Main outcomes of interests were the incremental cost-effectiveness ratios (ICERs) per QALY.
2.7 Sensitivity and Willingness-to-Pay Analyses
Univariate sensitivity and scenario analyses were conducted by assessing the impact of 95% confidence intervals displayed in Table 1 of the point estimate input parameters on calculated ICER. A probabilistic sensitivity analysis (PSA) with 1000 iterations evaluated the simultaneous variation of uncertain input variables. Results of the PSA are presented as means with 95% confidence intervals. A willingness-to-pay (WTP) analysis was conducted to evaluate which vaccination strategy is preferred at an estimated threshold of R23,630 per QALY (ESM Table e4).
2.8 Model Structure Sensitivity Analysis
A second model structure helps to account for the uncertainty associated with the first model that is predominantly used in the literature for cervical cancer and genital warts (Fig. 1). The second alternative model (ESM Fig. 1) is based on previous HPV cost-effectiveness models from Singapore and The Netherlands [38, 39]. It accounts for the possibility of simultaneous infections with high- and low-risk HPV types by running two distinct Markov models; however, it structurally underestimates mortality from CIN I–III and cervical cancer and thereby overestimates QALY gained due to the two separate Markov model runs.
3 Results
Base-case results of the cost-effectiveness analysis are presented in Table 2. All HPV vaccination strategies dominate the no vaccine strategy as they provide higher incremental QALYs and save costs. Compared with no vaccine, the bivalent provides 0.15 incremental QALYs at savings of R11,540 (ICER: −R77,115 per QALY), the quadrivalent offers 0.13 incremental QALYs whilst saving R9793 (ICER: −R72,733 per QALY), and the nonavalent strategy yields 0.29 incremental QALYs at savings of R9747 (ICER: −R33,908 per QALY) per person.
The bivalent strategy dominates the quadrivalent strategy given that the later yields 0.02 lower QALYs and R1748 higher costs (ICER: − R116,397 per QALY) per person. Compared with the bivalent vaccine, the nonavalent strategy increases QALYs by 0.14 and costs by R1793 (ICER: R13,013 per QALY) per person. Consequently, the nonavalent’s base-case ICER is below the estimated South African WTP threshold of R23,630 per QALY relative to the bivalent vaccination.
3.1 Univariate Sensitivity and Scenario Analysis
Results of the univariate sensitivity and scenario analysis can be found in ESM Table e5 and ESM Fig. e2. ICER mainly varied by transition probabilities from infection to CIN I and CIN II/III, from CIN I to CIN II/III, and from CIN II/III to cervical cancer, as well as costs associated with cervical cancer and discount rates for costs and utilities. A longer vaccine efficacy duration reduced the estimated ICER, and vice versa. The comparative ICER between the bivalent and nonavalent strategies varied by ± 27%, with ± 5% fluctuations in vaccine coverage rates.
3.2 Probabilistic Sensitivity Analysis
Figure 2 illustrates the results of the PSA with 1000 iterations under the input parameters’ defined distribution (ESM Table e6) per vaccination strategy. The simulation demonstrates that all vaccines provide higher incremental QALYs (bivalent: 0.15 [0.13 to 0.17]; quadrivalent: 0.13 [0.12 to 0.16]; nonavalent 0.29 [0.25 to 0.33]) at cost savings (bivalent: R11,666 [9586 to 13,883]; quadrivalent: R9001 [8169 to 11,817]; nonavalent: R10,012 [6134 to 14,191]) compared with the no vaccination strategy. Similar to base-case results, the PSA confirms that quadrivalent vaccination is dominated by bivalent vaccination. Relative to bivalent vaccination, the nonavalent strategy offered 0.14 QALYs [0.12 to 0.16] and higher expenditures of R1654 [−408 to 3593] at an ICER of R12,031 per QALY [− 2764 to 26,563]. ESM Table e7 shows that results further vary by the input parameters’ defined distribution.
Probabilistic sensitivity analysis for HPV vaccination strategies displayed on a cost-effectiveness plane compared with a no vaccination and b bivalent vaccination. The graph maps incremental QALYs across incremental costs for 1000 iterations of the conducted probabilistic sensitivity analysis. Graph A compares no vaccination with a bivalent, quadrivalent, or nonavalent vaccination strategy, while graph B compares a bivalent strategy with a quadrivalent and nonavalent vaccination strategy. Costs are displayed in 2019 R. HPV human papillomavirus, QALYs quality-adjusted life-years, R South African Rand, WTP willingness to pay
3.3 Willingness-to-Pay Analysis
Results of the WTP analysis are presented in Fig. 3. In this graph, the cost-effectiveness acceptability curve maps the probability that a certain vaccination strategy is preferred over others in South Africa across varying WTP ratios. Above a WTP threshold of R11,750 per QALY, nonavalent vaccination is the preferred strategy, while below this threshold bivalent vaccination is preferred. At the South African WTP threshold of R23,630 per QALY, nonavalent vaccination is the preferred strategy, with a probability of 90.2%.
Cost-effectiveness acceptability curve for bivalent, quadrivalent, and nonavalent HPV vaccination. In this graph, the cost-effectiveness acceptability curve maps the probability a certain vaccination strategy is preferred over others in South Africa across varying WTP ratios. The red line represents the bivalent vaccination strategy, the orange line represents the quadrivalent vaccination strategy, and the blue line represents the nonavalent vaccination strategy. At the South African WTP threshold of R23,630 per QALY, nonavalent vaccination is the preferred strategy, with a probability of 90.2%. HPV human papillomavirus, QALYs quality-adjusted life-years, WTP willingness to pay, R South African Rand
3.4 Model Structure Sensitivity Analysis
Results for the alternative model structure are displayed in ESM Table e8. Similar to the first model, all HPV vaccines dominate the no vaccination strategy. Quadrivalent vaccination is again dominated by the bivalent strategy; however, in the alternative model, nonavalent dominates bivalent, with incremental QALYs of 0.08 and savings of R13,398 (ICER: − R169,239 per QALY) and, consequently, quadrivalent vaccination also.
4 Discussion
This study presents the first cost-effectiveness analysis comparing bivalent, quadrivalent, and nonavalent vaccination programs in South Africa. In our model, bivalent, quadrivalent, and nonavalent vaccines provide higher QALYs and lower costs than no vaccine for 9- to 14-year-old girls. Compared with a bivalent vaccination strategy, the nonavalent vaccine provided an ICER of R13,013 per QALY. This calculated ICER is therefore always within the estimated South African WTP threshold of R23,630 per QALY. To introduce the nonavalent vaccination program for 520,000 girls aged 9–14 years, the South African Department of Health has to lay out an initial investment of R1.8 billion.
The different efficacies of the three vaccines against HPV types result in different degrees of protection against HPV-associated disease, as demonstrated in our model. While all vaccines offer a similar protection against the high-risk HPV types 16 and 18 [9], their efficacy against other HPV types differ. Quadrivalent and nonavalent vaccination protect against genital warts caused by low-risk HPV types 6 and 11, unlike the bivalent vaccine. While genital warts are not as life threatening as cervical cancer, infected patients suffer a significant health burden, as exhibited by the utility decrement of −0.09 QALYs [40]. Additionally, the benefits of genital warts protection are seen more quickly after vaccination, with the benefits of cervical cancer protection taking longer to occur. While the nonavalent vaccine offers the greatest protection against high-risk HPV types 31, 33, 45, 52, and 58, the bivalent vaccine provides greater cross-protection against these types than the quadrivalent vaccine. Combined with lower costs for the bivalent vaccines, this explains why the bivalent scenario dominates the quadrivalent but not the nonavalent scenario.
Consistent with our results, studies in high-income countries, such as Canada and Austria, have found nonavalent vaccination to be the most cost-effective strategy to prevent cervical cancer [10]. Similarly, in Germany, replacement of the quadrivalent vaccine with the new nonavalent vaccine is highly cost effective (ICER: €329 per QALY) [11]. In the US, cost-effectiveness analysis of the nonavalent vaccine suggested that universal vaccination is likely cost saving compared with the quadrivalent vaccine [12]. However, in the underlying model, the price per HPV vaccination between the nonavalent (US$158) and quadrivalent (US$145) vaccines are very close. Similarly, an Australian model concluded that the nonavalent vaccine is cost effective compared with the quadrivalent vaccine if the incremental price is below AU$24 [13]. Correspondingly, in low- and middle-income countries, cost-effectiveness can be reached, with incremental costs below US$9.80 for the nonavalent vaccine relative to the quadrivalent vaccine [14].
However, the nonavalent vaccine was not found to be ubiquitously cost effective, as a study analyzing 16- to 26-year-old girls in China concluded that the nonavalent strategy was not cost effective relative to bivalent or quadrivalent strategies [41]. Their different outcome from our analysis is likely explained by differences in HPV prevalence rates (China 19% vs. South Africa 44–85%), and nonavalent vaccine prices (South Africa R1024 vs. China R9700) [41, 42]. In summary, the cost-effectiveness of the nonavalent HPV vaccination is subject to local HPV prevalence rates, vaccine prices, and the target population’s age and sex.
Recently, certain Western countries have issued guidelines to expand HPV vaccination to boys, to increase herd immunity effects, and protect against several other non-cervical HPV diseases. Herd immunity is likely to result in additional utilities not displayed in our model, as well as a reduction in infection and HPV-related cancer rates for men [43]. Future research should consider sex-neutral vaccination in South Africa as boys are not only transmitters of HPV but also suffer significant health burdens from HPV-related disease.
Our results illustrate that the ICER is dependent on vaccination coverage. Additional resources may be deployed to increase coverage beyond school girls, and more effective campaigns may help to increase awareness of HPV infection and associated diseases. In addition, a complex combination of factors contribute to HPV vaccine hesitancy in South Africa, predominantly driven by parental concern and also current vaccination regimens do not include private sector schools [44]. Evidence suggests that integrated approaches, including communication and social mobilization, are likely to improve uptake, with one intervention showing a 40% increase in uptake when caregivers were provided with information [43]. As such, South Africa’s HPV vaccination program could be improved by dialog-based interventions, including social media and mass media, targeting stakeholders in the private and public domains (parents, educators, media, policy makers, and children) to increase vaccination coverage and its cost-effectiveness.
The conducted univariate, probabilistic, and parameter distribution sensitivity analyses (ESM Table e7) demonstrate that ICERs are influenced by input parameters, their associated uncertainty, and their defined distribution. Consequently, decision makers would benefit from the knowledge associated with the actual value of input parameters. Future studies should therefore conduct expected value for perfect information (EVPI) analysis and eventually an expected value for partial parameter information (EVPPI) to inform government decision-making in resource allocation.
An additional consideration in the South African context is HIV. HIV infection and treatment were shown to affect HPV infection and cancer progression [45], and, with prevalence rates of around 20% [46], it is an important factor in any HPV vaccination program. Currently, there is no evidence on how HIV may affect the efficacy of each of the HPV vaccines, and further research on this may affect the outcome of a cost-effectiveness analysis of the different vaccines.
4.1 Limitations
Our study has several limitations. First, the model only considers the effects of HPV vaccination on genital warts and cervical cancer, excluding other HPV-associated cancers such as head and neck, vaginal, anal, and vulvar cancer. As nonavalent HPV vaccination also directly protects against these cancer types, the overall cost-effectiveness of the nonavalent strategy may likely be lower than the estimated ICER of R13,013 per QALY.
Second, the model assumes instant screening and treatment of all patients. In practice, there are significant screening coverage gaps and time lags between screening and treatment. Not all patients in ill-health states can or want to be treated, and coverage rates, treatment costs, and time to treatment are likely to be different for the privately insured population.
Third, this paper only examines the age-specific group of 9- to 14-year-old girls in South Africa. Analyzing the cost-effectiveness of extending HPV vaccination to women aged 20–35 years or 9- to 14-year-old boys could result in valuable findings and is an important gap for future research.
Fourth, an underlying limitation of Markov models is their memoryless function—models cannot distinguish patients in a disease state. Therefore, our model does not distinguish patients after remitting from HPV infections. While this methodology has also been employed in previous cost-effectiveness studies [27], systematic reviews show that patients remain at an elevated risk for cervical cancer after treatment and remittance in earlier HPV infection stages [47]. Moreover, the alternative model structure demonstrates that ICERs are influenced by the Markov model’s underlying assumption about HPV disease progression.
Fifth, the results of this study are specific to South Africa and its cost, utility, and HPV transmission characteristics. For other countries, specific cost-effectiveness studies with respective changes in vaccine pricing, medical costs, utilities, and incidence probabilities must be undertaken. Additionally, random distributions were associated with monetary cost values instead of physical cost drivers as costs were sourced from previous cost-effectiveness studies.
5 Conclusion
The introduction of nonavalent for bivalent HPV vaccination is a cost-effective intervention in South Africa. HPV vaccination should be part of a multifaceted public health strategy entailing screening, condoms, and education of all stakeholders to reduce the significant burden of sexual transmitted diseases in South Africa. Sex-neutral and catch-up vaccinations are subjects for further research.
References
Mbulawa ZZA, van Schalkwyk C, Hu N-C, Meiring TL, Barnabas S, Dabee S, et al. High human papillomavirus (HPV) prevalence in South African adolescents and young women encourages expanded HPV vaccination campaigns. PLoS ONE. 2018;13:e0190166.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-386.
van Schalkwyk SL, Maree JE, Wright SCD. Cervical cancer: the route from signs and symptoms to treatment in South Africa. Reprod Health Matters. 2008;16:9–17.
Delany-Moretlwe S, Kelley KF, James S, Scorgie F, Subedar H, Dlamini NR, et al. Human papillomavirus vaccine introduction in South Africa: implementation lessons from an evaluation of the national school-based vaccination campaign. Glob Health Sci Pract. 2018;6:425–38.
Botha MH, Dochez C. Introducing human papillomavirus vaccines into the health system in South Africa. Vaccine. 2012;30(Suppl 3):C28-34.
Brown DR, Kjaer SK, Sigurdsson K, Iversen O-E, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199:926–35.
Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–10.
Jenkins D. A review of cross-protection against oncogenic HPV by an HPV-16/18 AS04-adjuvanted cervical cancer vaccine: importance of virological and clinical endpoints and implications for mass vaccination in cervical cancer prevention. Gynecol Oncol. 2008;110:S18-25.
Bergman H, Buckley BS, Villanueva G, Petkovic J, Garritty C, Lutje V, et al. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.CD013479/full.
Mahumud RA, Alam K, Keramat SA, Ormsby GM, Dunn J, Gow J. Cost-effectiveness evaluations of the 9-Valent human papillomavirus (HPV) vaccine: Evidence from a systematic review. PLoS ONE. 2020;15:e0233499.
Largeron N, Petry KU, Jacob J, Bianic F, Anger D, Uhart M. An estimate of the public health impact and cost-effectiveness of universal vaccination with a 9-valent HPV vaccine in Germany. Expert Rev Pharmacoecon Outcomes Res. 2017;17:85–98.
Brisson M, Laprise J-F, Chesson HW, Drolet M, Malagón T, Boily M-C, et al. Health and economic impact of switching from a 4-valent to a 9-valent HPV vaccination program in the United States. J Natl Cancer Inst. 2016;108:1–9.
Drolet M, Laprise J-F, Boily M-C, Franco EL, Brisson M. Potential cost-effectiveness of the nonavalent human papillomavirus (HPV) vaccine. Int J Cancer. 2014;134:2264–8.
Kiatpongsan S, Kim JJ. Costs and cost-effectiveness of 9-valent human papillomavirus (HPV) vaccination in two East African countries. PLoS ONE. 2014;9:e106836.
World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017—recommendations. Vaccine. 2017;35:5753–5.
Li X, Stander MP, Van Kriekinge G, Demarteau N. Cost-effectiveness analysis of human papillomavirus vaccination in South Africa accounting for human immunodeficiency virus prevalence. BMC Infect Dis. 2015;15:566.
Brisson M, Van de Velde N, De Wals P, Boily M-C. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine. 2007;25:5399–408.
Haeussler K, Marcellusi A, Mennini FS, Favato G, Picardo M, Garganese G, et al. Cost-effectiveness analysis of universal human papillomavirus vaccination using a dynamic bayesian methodology: the BEST II study. Value Health. 2015;18:956–68.
Bruni L, Albero G, Serrano B, Mena M, Collado J, Gómez D, et al. Human papillomavirus and related diseases in South Africa: Summary Report 22 October 2021. ICO/IARC information centre on HPV and Cancer (HPV Information Centre). 2021. https://hpvcentre.net/statistics/reports/ZAF.pdf. Accessed 5 Dec 2021.
Michaeli DT, Michaeli JC, Boch T, Michaeli T. Cost-effectiveness of lipid-lowering therapies for cardiovascular prevention in Germany. Cardiovasc Drugs Ther. 2022. https://doi.org/10.1007/s10557-021-07310-y.
Jones E, Epstein D, García-Mochón L. A procedure for deriving formulas to convert transition rates to probabilities for multistate markov models. Med Decis Making. 2017;37:779–89.
Republic of South Africa. Department: Statistics South Africa. 2021. http://www.statssa.gov.za/. Accessed 30 Nov 2021.
Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, de Borba PC, Sanchez N, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccines Immunother. 2014;10:2147–62.
World Health Organization. Guidelines for estimating costs of introducing new vaccines into the national immunization system. World Health Organization. World Health Organization. 2002. https://apps.who.int/iris/handle/10665/67342. Accessed 29 Nov 2021.
Moodley I, Tathiah N, Sartorius B. The costs of delivering human papillomavirus vaccination to Grade 4 learners in KwaZulu-Natal, South Africa. S Afr Med J. 2016;106:60.
Botwright S, Holroyd T, Nanda S, Bloem P, Griffiths UK, Sidibe A, et al. Experiences of operational costs of HPV vaccine delivery strategies in Gavi-supported demonstration projects. PLoS ONE. 2017;12:e0182663.
Vijayaraghavan A, Efrusy M, Lindeque G, Dreyer G, Santas C. Cost effectiveness of high-risk HPV DNA testing for cervical cancer screening in South Africa. Gynecol Oncol. 2009;112:377–83.
Ginindza TG, Sartorius B, Dlamini X, Östensson E. Cost analysis of Human Papillomavirus-related cervical diseases and genital warts in Swaziland. PLoS ONE. 2017;12:e0177762.
Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, et al. Human Papillomavirus and Related Diseases in South Africa. Summary Report 17 June 2019. 2019. https://hpvcentre.net/statistics/reports/ZAF.pdf?t=1572341817098. Accessed 29 Oct 2019.
Sinanovic E, Moodley J, Barone MA, Mall S, Cleary S, Harries J. The potential cost-effectiveness of adding a human papillomavirus vaccine to the cervical cancer screening programme in South Africa. Vaccine. 2009;27:6196–202.
Dasbach EJ, Elbasha EH, Insinga RP. Mathematical models for predicting the epidemiologic and economic impact of vaccination against human papillomavirus infection and disease. Epidemiol Rev. 2006;28:88–100.
McGrath N, Nyirenda M, Hosegood V, Newell M-L. Age at first sex in rural South Africa. Sex Transm Infect. 2009;85(Suppl 1):i49-55.
World Health Organization. WHO guide for standardization of economic evaluations of immunization programmes. World Health Organization. 2008. https://apps.who.int/iris/handle/10665/69981. Accessed 29 Nov 2021.
FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27.
Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43.
Harper DM, Franco EL, Wheeler CM, Moscicki A-B, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–55.
Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow S-N, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14.
Lee VJ, Tay SK, Teoh YL, Tok MY. Cost-effectiveness of different human papillomavirus vaccines in Singapore. BMC Public Health. 2011;11:203.
Westra TA, Stirbu-Wagner I, Dorsman S, Tutuhatunewa ED, de Vrij EL, Nijman HW, et al. Inclusion of the benefits of enhanced cross-protection against cervical cancer and prevention of genital warts in the cost-effectiveness analysis of human papillomavirus vaccination in the Netherlands. BMC Infect Dis. 2013;13:75.
Shi J-F, Kang D-J, Qi S-Z, Wu H-Y, Liu Y-C, Sun L-J, et al. Impact of genital warts on health related quality of life in men and women in mainland China: a multicenter hospital-based cross-sectional study. BMC Public Health. 2012;12:153.
Jiang Y, Ni W, Wu J. Cost-effectiveness and value-based prices of the 9-valent human papillomavirus vaccine for the prevention of cervical cancer in China: an economic modelling analysis. BMJ Open. 2019;9:e031186.
Li K, Li Q, Song L, Wang D, Yin R. The distribution and prevalence of human papillomavirus in women in mainland China. Cancer. 2019;125:1030–7.
Tabrizi SN, Brotherton JML, Kaldor JM, Skinner SR, Liu B, Bateson D, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014;14:958–66.
Ngcobo NJ, Burnett RJ, Cooper S, Wiysonge CS. Human papillomavirus vaccination acceptance and hesitancy in South Africa: research and policy agenda. S Afr Med J. 2018;109:13–5.
Minkoff H, Ahdieh L, Massad LS, Anastos K, Watts DH, Melnick S, et al. The effect of highly active antiretroviral therapy on cervical cytologic changes associated with oncogenic HPV among HIV-infected women. AIDS. 2001;15:2157–64.
Hansoti B, Mwinnyaa G, Hahn E, Rao A, Black J, Chen V, et al. Targeting the HIV epidemic in South Africa: the need for testing and linkage to care in emergency departments. EClinicalMedicine. 2019;15:14–22.
Kalliala I, Athanasiou A, Veroniki AA, Salanti G, Efthimiou O, Raftis N, et al. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: a systematic review and meta-analysis of the literature. Ann Oncol. 2020;31:213–27.
Kriekinge GV, Sohn W-Y, Aljunid SM, Soon R, Yong C-M, Chen J, et al. Comparative cost-effectiveness analysis of two different two-dose human papillomavirus vaccines in Malaysia. Asian Pac J Cancer Prev. 2018;19:933–40.
Van de Velde N, Brisson M, Boily M-C. Modeling human papillomavirus vaccine effectiveness: quantifying the impact of parameter uncertainty. Am J Epidemiol. 2007;165:762–75.
Sanders GD, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis. 2003;9:37–48.
Kumamoto Y, Tsukamoto T, Sugiyama T, Akaza H, Noguchi M, Naya A, et al. National surveillance of sexually transmitted diseases of Japan in 2002. Jpn Arch Sex Transm Dis. 2004;15:17–45.
Goldie SJ, Kohli M, Grima D, Weinstein MC, Wright TC, Bosch FX, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst. 2004;96:604–15.
Insinga RP, Dasbach EJ, Elbasha EH, Liaw K-L, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev. 2007;16:709–15.
Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13:28–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declaration of Competing Interest
Daniel Tobias Michaeli, Sophia Stoycheva, Simon Mashudu Marcus, Wenjia Zhang, Julia Caroline Michaeli, and Thomas Michaeli declare no conflicts of interest.
Ethics approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of data and material
All data are included in the manuscript.
Code Availability
Not applicable.
Author Contributions
DM: Conceptualization, methodology, investigation, formal analysis, writing – original draft, review and editing, visualization. SS: Conceptualization, methodology, investigation, writing – review and editing. SM: Conceptualization, methodology, investigation, writing – review and editing. WZ: Conceptualization, methodology, investigation, writing – review and editing. JM: Investigation, formal analysis, writing – review and editing, supervision, project management, resources. TM: Investigation, formal analysis, writing – original draft, writing – review and editing, supervision, project management, resources. All authors attest they meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Michaeli, D.T., Stoycheva, S., Marcus, S.M. et al. Cost-Effectiveness of Bivalent, Quadrivalent, and Nonavalent HPV Vaccination in South Africa. Clin Drug Investig 42, 333–343 (2022). https://doi.org/10.1007/s40261-022-01138-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01138-6