Abstract
Background and Objective
In order to integrate the existing and inconsistent information from clinical trials and real-world practice on chronic lymphocytic leukemia (CLL) treated with ibrutinib, this analysis aimed to describe the prescription pattern of new users of ibrutinib affected by CLL, focusing on discontinuation, severe adverse events (AEs) and change of treatment, and to assess the integrated healthcare expenditure from the Italian National Health System (INHS) perspective.
Methods
Starting from the ReS database, adults with at least a supply of ibrutinib (ATC code L01XE27) were selected from 01/01/2016 to 12/31/2017. Those without any ibrutinib supply in the year before the index prescription were considered new users. Out of them, only patients with at least a primary or secondary in-hospital diagnosis of CLL (ICD-9-CM code 204.1*) from 01/01/2013 to 12/31/2018 were further broken down according to the ibrutinib’s line treatment (first line—FL; second or later line—SLL) and analysed. They were characterized by sex and age in the selection period. Mean annual consumption (defined daily doses [DDD]), treatment discontinuation, changes of therapy, interruptions and healthcare costs in charge of the INHS were assessed during two follow-up years.
Results
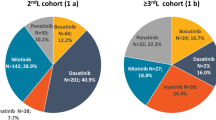
Out of more than 5 million inhabitants of the ReS database, 69 new ibrutinib users and diagnosed with CLL in 2016 (incidence: 1.6 × 100,000) and 41 in 2017 (incidence: 0.9 × 100,000) were selected. Of these, 21 (19.1%) were FL ibrutinib users and 89 (80.9%) were SLL ones, mostly males and with mean ages (±SD) of 65 ± 14 and 70 ± 10, respectively. The mean annual consumption among FL users decreased from 222.2 DDD per patient treated to 216.0 DDD, while increased among SLL patients from 238.6 DDD to 260.1 DDD, in the first and second follow-up year, respectively. The discontinuation rate was about 40% in the first year, similarly among FL and SLL users. SLL patients discontinued more frequently (52.8% vs 20.0%) in the second year. Very few AEs were recorded. The 62.5% of FL and 55.6% of SLL users discontinuing ibrutinib in 1-year follow-up, while one SLL patient (5.3%) in the second year changed therapy. The 20.0% and 15.9% of all new users in first and second year interrupted ibrutinib. The total integrated cost of FL patients was €55,732 reducing by about €15,000, while it was €58,716 for SLL ones decreasing by €6,000, respectively, in the first and in the second year. Pharmaceuticals were the key cost driver (ibrutinib accounted for more than 77%).
Conclusions
This analysis on Italian administrative data provided results about prescription patterns of ibrutinib FL and SLL new users with CLL, focusing on discontinuation, treatment change and healthcare costs over 2-year follow-up, and contributed to improve the knowledge on this hard-to-treat disease.



Similar content being viewed by others
References
Eichhorst B, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23–33.
Salvi G, et al. Chronic lymphocytic leukaemia: census of patients treated in Italian haematology units. Mediterr J Hematol Infect Dis. 2015;7(1):e2015056.
Gribben JG, et al. Optimising outcomes for patients with chronic lymphocytic leukaemia on ibrutinib therapy: European recommendations for clinical practice. Br J Haematol. 2018;180(5):666–79.
Xie J, et al., Real-world treatment patterns and adverse events in patients with Chronic Lymphocytic Leukemia treated with Ibrutinib in the UK: a preliminary analysis. Blood. 2019;134(1): 5885–5885.
Brown JR. How I treat CLL patients with ibrutinib. Blood. 2018;131(4):379–86.
Gaidano G, Rossi D. The mutational landscape of chronic lymphocytic leukemia and its impact on prognosis and treatment. Hematol Am Soc Hematol Educ Program. 2017;2017(1):329–37.
Parikh SA. Chronic lymphocytic leukemia treatment algorithm 2018. Blood Cancer J. 2018;8(10):93.
Harkins RA, Patel SP, Flowers CR. Cost-effectiveness of new targeted agents in the treatment of chronic lymphocytic leukemia. Cancer J. 2019;25(6):418–27.
National Comprehensive Cancer Network, NCCN Clinical Practice Guidelines in Oncology. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Version 2.2021. 2021.
O'Brien S.M., Clinical implications of the 2018 iwCLL Guidelines update. Clin Adv Hematol Oncol. 2018;16 Suppl 15(8):1–16.
Byrd JC, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–506.
Sorensen SV, et al. The cost-effectiveness of Ibrutinib in treatment of relapsed or refractory Chronic Lymphocytic Leukemia. Health Econ Outcome Res Open Access 2016;2:4.
Zhou H, et al. Ibrutinib in chronic lymphocytic leukemia: clinical applications, drug resistance, and prospects. Onco Targets Ther. 2020;13:4877–92.
Agenzia Italiana del Farmaco, Determina 10 agosto 2018. GU n. 207 del 6-9-2018; 2018.
Eyre TA, et al. Comparative analysis of targeted novel therapies in relapsed, refractory chronic lymphocytic leukaemia. Haematologica. 2021;106(1):284–7.
Calabria S, et al. Patterns of prescription, hospitalizations and costs of herpes zoster in patients at risk, from a large Italian claims database. Glob Reg Health Technol Assess. 2020;7:66–71.
Maggioni AP, et al. Four-year trends in oral anticoagulant use and declining rates of ischemic stroke among 194,030 atrial fibrillation patients drawn from a sample of 12 million people. Am Heart J. 2020;220:12–9.
Piccinni C, et al. A real-world study on unmet medical needs in triptan-treated migraine: prevalence, preventive therapies and triptan use modification from a large Italian population along two years. J Headache Pain. 2019;20(1):74–74.
WHO. ATC/DDD Index 2020. 2020; Available from: https://www.whocc.no/atc_ddd_index/.
Italian Ministry of Labour, of Health and of Social Policy, Classificazione delle malattie, dei traumatismi, degli interventi chirurgici e delle procedure diagnostiche e terapeutiche. Versione italiana della ICD9-CM [Classification of diseases, traumas, surgeries, diagnostic and therapeutic procedures. Italian version of the ICD-9CM]; 2007.
Italian Ministry of Labour, of Health and of Social Policy, Nomenclatore prestazioni di assistenza specialistica ambulatoriale. Allegato 4. [Tariffs of outpatient specialist services. Annex 4]. DPCM 12 gennaio 2017.
European Medicine Agency, Summary of product characteristics of Imbruvica; 2020.
Byrd JC, et al. Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase Ib/II PCYC-1102 study. Clin Cancer Res. 2020;26(15):3918–27.
Ghia P, Cuneo A. Ibrutinib in the real world patient: many lights and some shades. Haematologica. 2016;101(12):1448–50.
Islam P, Mato AR. Utilizing real-world evidence (RWE) to improve care in chronic lymphocytic leukemia: challenges and opportunities. Curr Hematol Malig Rep. 2020;15(4):254–60.
Lew TE, Anderson MA, Seymour JF. Promises and pitfalls of targeted agents in chronic lymphocytic leukemia. Cancer Drug Resist. 2020;3(3):415–44.
Mato AR, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–9.
UK Cll Forum. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563–72.
O’Brien SM, et al. Outcomes with ibrutinib by line of therapy and post-ibrutinib discontinuation in patients with chronic lymphocytic leukemia: phase 3 analysis. Am J Hematol. 2019;94(5):554–62.
Hallek M, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–60.
Patel KK, et al. Cost-effectiveness of first-line vs third-line ibrutinib in patients with untreated chronic lymphocytic leukemia. Blood. 2020;136(17):1946–55.
Cuneo A, et al. Efficacy of bendamustine and rituximab in unfit patients with previously untreated chronic lymphocytic leukemia. Indirect comparison with ibrutinib in a real-world setting. A GIMEMA-ERIC and US study. Cancer Med. 2020;9(22):8468–79.
Cuneo A, et al. Efficacy of bendamustine and rituximab as first salvage treatment in chronic lymphocytic leukemia and indirect comparison with ibrutinib: a GIMEMA, ERIC and UK CLL FORUM study. Haematologica. 2018;103(7):1209–17.
Nabhan C, et al. Cost-effectiveness comparison between ibrutinib, chemotherapy, and chemoimmunotherapy in front-line treatment of chronic lymphocytic leukemia (CLL). Blood. 2018;132(Supplement 1):4757–4757.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was not supported by any specific grant.
Conflict of interest
Authors declare no conflict of interests /competing interests.
Ethics approval
Ethical approval was not sought for the present study, because it was based on the reuse of anonymous administrative data and conducted for institutional purposes, in agreement with the Italian health facilities (regions and local health units).
Consent to participate
Consent to participate was not sought for the present study.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated and analysed during the current study are not publicly available, because they come from anonymized administrative databases in property of Italian Regional and Local Health Facilities, which were analysed by Fondazione ReS under specific agreements.
Code Availability
Oracle SQL Developer version 18.3.0 and later is the software used in order to conduct all the analyses.
Author contributions
Each author has approved the submitted version and has agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ronconi, G., Dondi, L., Calabria, S. et al. Real-world Prescription Pattern, Discontinuation and Costs of Ibrutinib-Naïve Patients with Chronic Lymphocytic Leukemia: An Italian Healthcare Administrative Database Analysis. Clin Drug Investig 41, 595–604 (2021). https://doi.org/10.1007/s40261-021-01044-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01044-3