Abstract
Background and Objectives
Ropeginterferon alfa-2b is a novel monopegylated recombinant interferon alfa-2b for the treatment of patients with polycythemia vera. The objectives of this study were to evaluate the pharmacokinetics, pharmacodynamics, safety, and tolerability of ropeginterferon alfa-2b in healthy Japanese subjects compared with Caucasian subjects.
Methods
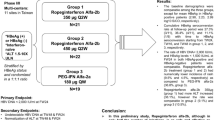
In this multicenter, parallel-group phase I study, a cohort consisting of six Japanese and six Caucasian subjects was designated to receive a single subcutaneous dose of ropeginterferon alfa-2b (100, 200, 300, and 450 µg). Pharmacokinetic and pharmacodynamic parameters, and immunogenicity were evaluated. Safety was assessed throughout the study.
Results
Cohort 4 (450-µg dose) was not initiated because the primary objective of this study was achieved based on the three completed cohorts. A total of 36 enrolled subjects (18 Japanese and 18 Caucasian) in three cohorts were included in the safety, pharmacokinetic, and pharmacodynamic analysis sets. Ropeginterferon alfa-2b exposure in terms of the area under the serum concentration–time curve (AUC) from time zero extrapolated to infinity and the AUC from time zero to the time of the last quantifiable concentration was approximately 1.7-fold and two-fold higher in Japanese subjects than in Caucasian subjects, respectively. Across the same dose range, the maximum serum concentration was approximately 1.25-fold higher in Japanese subjects than in Caucasian subjects. The time to reach the median maximum serum concentration was similar between ethnicities (approximately 96–111 h). The terminal half-life was 48–57 h in Japanese subjects and 31–75 h in Caucasian subjects. The slope of the relationship between dose and drug exposure was greater than 1 in both ethnicities. The dose-dependent induction of beta-2 microglobulin and neopterin expression was observed in both ethnicities, and the two groups showed similar pharmacodynamic parameters. At the end of the study, 22.2% of Japanese subjects and 11.1% of Caucasian subjects developed anti-ropeginterferon alfa-2b-binding antibodies. The neutralizing capacity of these antibodies was not tested. Ropeginterferon alfa-2b up to 300 µg was safe and well tolerated, with no unexpected safety findings based on previous experiences with ropeginterferon alfa-2b and other forms of interferon.
Conclusions
Ropeginterferon alfa-2b exposure was higher in Japanese subjects than in Caucasian subjects. The increase in ropeginterferon alfa-2b exposure was greater than the dose proportion in the dose range of 100–300 µg. Ropeginterferon alfa-2b was safe and well tolerated.
Clinical Trial Registration
ClinicalTrials.gov identifier NCT03546465, registered on 6 June, 2018.




Similar content being viewed by others
References
Spivak JL. Myeloproliferative neoplasms. N Engl J Med. 2017;376:2168–81.
Stein BL, Oh ST, Berenzon D, et al. Polycythemia vera: an appraisal of the biology and management 10 years after the discovery of JAK2 V617F. J Clin Oncol. 2015;33:3953–60.
Griesshammer M, Gisslinger H, Mesa R. Current and future treatment options for polycythemia vera. Ann Hematol. 2015;94:901–10.
Ma X, Vanasse G, Cartmel B, Wang Y, Selinger HA. Prevalence of polycythemia vera and essential thrombocythemia. Am J Hematol. 2008;83:359–62.
Mesa RA, Jamieson C, Bhatia R, et al. NCCN guidelines insights: myeloproliferative neoplasms, version 2.2018. J Natl Compr Cancer Netw. 2017;15:1193–207.
McMullin MF, Harrison CN, Ali S, et al. A guideline for the diagnosis and management of polycythaemia vera: a British Society for Haematology guideline [published correction appears in Br J Haematol. 2019;185:198]. Br J Haematol. 2019;184:176–91.
Tefferi A, Vannucchi AM, Barbui T. Polycythemia vera treatment algorithm 2018. Blood Cancer J. 2018;8:3.
Dingli D, Tefferi A. Hydroxyurea: the drug of choice for polycythemia vera and essential thrombocythemia. Curr Hematol Malig Rep. 2006;1:69–74.
Podoltsev NA, Zhu M, Zeidan AM, et al. The impact of phlebotomy and hydroxyurea on survival and risk of thrombosis among older patients with polycythemia vera. Blood Adv. 2018;2:2681–90.
Silver RT. Interferon-alpha 2b: a new treatment for polycythemia vera. Ann Intern Med. 1993;119:1091–2.
Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006;107:451–8.
Monkarsh SP, Ma Y, Aglione A, et al. Positional isomers of monopegylated interferon alpha-2a: isolation, characterization, and biological activity. Anal Biochem. 1997;247:434–40.
Glue P, Fang JW, Rouzier-Panis R, et al. Pegylated interferon-alpha2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis C Intervention Therapy Group. Clin Pharmacol Ther. 2000;68:556–67.
Quintás-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27:5418–24.
Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer. 2007;110:2012–8.
Crisà E, Cerrano M, Beggiato E, et al. Can pegylated interferon improve the outcome of polycythemia vera patients? J Hematol Oncol. 2017;10:15.
Wagner SM, Melchardt T, Greil R. Ropeginterferon alfa-2b for the treatment of patients with polycythemia vera. Drugs Today (Barc). 2020;56:195–202.
Verger E, Soret-Dulphy J, Maslah N, et al. Ropeginterferon alpha-2b targets JAK2V617F-positive polycythemia vera cells in vitro and in vivo. Blood Cancer J. 2018;8:94.
Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, et al. Ropeginterferon alfa-2b, a novel IFNα-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood. 2015;126:1762–9.
Gisslinger H, Klade C, Georgiev P, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study [published correction appears in Lancet Haematol. 2020;7:e279]. Lancet Haematol. 2020;7:e196–208.
European Medicine Agency. Besremi. https://www.ema.europa.eu/en/medicines/human/EPAR/besremi. Accessed 7 July 2020.
Bruno R, Sacchi P, Maiocchi L, et al. Area-under-the-curve for peginterferon alpha-2a and peginterferon alpha-2b is not related to body weight in treatment-naive patients with chronic hepatitis C. Antivir Ther. 2005;10:201–5.
Zheng L, Li MP, Gou ZP, et al. A pharmacokinetic and pharmacodynamic comparison of a novel pegylated recombinant consensus interferon-α variant with peginterferon-α-2a in healthy subjects. Br J Clin Pharmacol. 2015;79:650–9.
Reddy KR. Development and pharmacokinetics and pharmacodynamics of pegylated interferon alfa-2a (40 kD). Semin Liver Dis. 2004;24(Suppl. 2):33–8.
Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175–87.
Khakoo S, Glue P, Grellier L, et al. Ribavirin and interferon alfa-2b in chronic hepatitis C: assessment of possible pharmacokinetic and pharmacodynamic interactions. Br J Clin Pharmacol. 1998;46:563–70.
Wejstål R, Norkrans G, Weiland O, et al. Lymphocyte subsets and beta 2-microglobulin expression in chronic hepatitis C/non-A, non-B: effects of interferon-alpha treatment. Clin Exp Immunol. 1992;87:340–5.
Bruno R, Sacchi P, Scagnolari C, et al. Pharmacodynamics of peginterferon alpha-2a and peginterferon alpha-2b in interferon-naïve patients with chronic hepatitis C: a randomized, controlled study. Aliment Pharmacol Ther. 2007;26:369–76.
Matsuda F, Torii Y, Enomoto H, et al. Anti-interferon-α neutralizing antibody is associated with nonresponse to pegylated interferon-α plus ribavirin in chronic hepatitis C. J Viral Hepat. 2012;19:694–703.
Antonelli G, Currenti M, Turriziani O, Dianzani F. Neutralizing antibodies to interferon-alpha: relative frequency in patients treated with different interferon preparations. J Infect Dis. 1991;163:882–5.
Perry CM, Jarvis B. Peginterferon-alpha-2a (40 kD): a review of its use in the management of chronic hepatitis C. Drugs. 2001;61:2263–88.
Evon DM, Esserman DE, Howell MA, Ruffin RA. Pegylated interferon pharmacokinetics and self-reported depressive symptoms during antiviral treatment for chronic hepatitis C. Pharmacopsychiatry. 2014;47:195–201.
Wills RJ. Clinical pharmacokinetics of interferons. Clin Pharmacokinet. 1990;19:390–9.
Acknowledgements
We thank Dr. Tetsuji Asao (SunFlare Co., Ltd., Tokyo, Japan) for medical writing services, which were funded by PharmaEssentia Japan KK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by PharmaEssentia Corporation.
Conflicts of interest/competing interests
Narihisa Miyachi and Katsuya Yonezu are employees of PharmaEssentia Japan KK. Oleh Zagrijtschuk and Lisa Kang are employees of PharmaEssentia Corporation USA. Albert Qin is an employee of PharmaEssentia Corporation, Taiwan.
Ethics approval
The protocol and the informed consent documents were approved by the central ethics committee (Alfred Hospital Ethics Committee, Melbourne, VIC, Australia). The study was conducted in accordance with Good Clinical Practices, International Conference on Harmonisation guidelines, applicable regulations, and guidelines governing clinical study conduct and the ethical principles that have their origin in the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all participants before the initiation of any study-specific procedure in this study.
Consent for publication
Not applicable.
Data availability and Material
The study protocol and statistical analysis plan will be shared with those who request data sharing. Requests for the sharing of these documents should be directed to the corresponding author. Requests will be reviewed, and scientifically sound proposals will be approved by the sponsor (PharmaEssentia Corporation). In addition, agreement for data sharing needs to be contracted between data requestors and the sponsor. Data will be shared for 2 years after article publication.
Code availability
Not applicable.
Authors’ contributions
OZ, AQ, and NM designed the study and wrote the protocol. AQ supervised the study. OZ, LK, NM, and KY were responsible for the trial follow-up. LK, AQ, OZ, and NM analyzed/interpreted the data. NM wrote and revised the draft of the manuscript with outside medical writers mentioned in the Acknowledgments section. All authors contributed to and have approved the final version of the manuscript.
Rights and permissions
About this article
Cite this article
Miyachi, N., Zagrijtschuk, O., Kang, L. et al. Pharmacokinetics and Pharmacodynamics of Ropeginterferon Alfa-2b in Healthy Japanese and Caucasian Subjects After Single Subcutaneous Administration. Clin Drug Investig 41, 391–404 (2021). https://doi.org/10.1007/s40261-021-01026-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01026-5