Abstract
Background and Objectives
Intravenous immunoglobulin (IVIG) therapy for acute-stage Kawasaki disease (KD) is the first-line treatment for preventing the development of coronary artery aneurysms (CAA). Corticosteroids (prednisolone) and infliximab are often used in patients at a high risk of CAA or those with CAA at diagnosis; however, there are only a few reports of non-responders to corticosteroids as an adjuvant therapy or rescue alternative to IVIG. In this study, we compared the therapeutic effects of primary and secondary prednisolone with IVIG for KD.
Methods
We established the following three protocols: A was a secondary rescue prednisolone protocol; B was no prednisolone and second-line infliximab protocol, and C was the primary prednisolone protocol. The indication for prednisolone administration was based on the following: primary prednisolone administration, Kobayashi score; and secondary administration, Shizuoka score.
Results
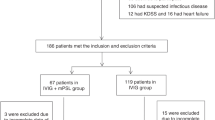
Four hundred and sixty-nine patients were enrolled in the three protocols. A comparison between primary and secondary prednisolone and IVIG, as the first-line therapy revealed that the number of first non-responders in C group was 7 (8.3%), which was significantly lower than the 50 (20.9%) in A group. There was a significant difference in the first and second non-responders among the three groups, and the number of non-responders in A group was 6 (2.5%), which was significantly lower than the 13 (9.9%) in B group (p < 0.001, by Bonferroni test). The multivariate logistic regression analysis showed that IVIG non-responders among the protocol groups had an adjusted odds ratio of 6.47. Fifteen IVIG non-responders were administered infliximab as a second-line therapy, and of them, 9 (60%) showed therapy resistance. CAA occurred in 21 patients (4.6%). There was no significant difference among each protocol group.
Conclusions
The number of IVIG non-responders in the group with prednisolone administration was lower than that in the group without prednisolone administration. Secondary rescue infliximab therapy for IVIG non-responders resulted in a lower defervescence effect than the secondary rescue IVIG with prednisolone administration. Further prospective randomized studies are needed to identify factors useful for preventing IVIG non-responders and determine the optimal rescue therapy for preventing CAA.



Similar content being viewed by others
References
McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–99.
Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1982;2:1359.
Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–7.
Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96:1057–61.
Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997;131:888.
Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105:E78.
Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. 1998;17:1144–8.
Han RK, Silverman ED, Newman A, McCrindle BW. Management and outcome of persistent or recurrent fever after initial intravenous gamma globulin therapy in acute Kawasaki disease. Arch Pediatr Adolesc Med. 2000;154:694–9.
Durongpisitkul K, Soongswang J, Laohaprasitiporn D, Nana A, Prachuabmoh C, Kangkagate C. Immunoglobulin failure and retreatment in Kawasaki disease. Pediatr Cardiol. 2003;24:145–8.
Kato H, Koike S, Yokoyama T. Kawasaki disease: effect of treatment on coronary artery involvement. Pediatrics. 1979;63:175e9.
Shinohara M, Sone K, Tomomasa T, Morikawa A. Corticosteroids in the treatment of the acute phase of Kawasaki disease. J Pediatr. 1999;135:411–3.
Jibiki T, Terai M, Kurosaki T, Nakajima H, Suzuki K, Inomata H, Terashima I, Honda T, Yasukawa K, Hamada H, Kohno Y. Efficacy of intravenous immune globulin therapy combined with dexamethasone for the initial treatment of acute Kawasaki disease. Eur J Pediatr. 2004;163:229–33.
Sundel RP, Baker AL, Fulton DR, Newburger JW. Corticosteroids in the initial treatment of Kawasaki disease: report of a randomized trial. J Pediatr. 2003;142:611–6.
Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, RAISE study group investigators, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012;379:1613–20.
Guidelines for Medical Treatment of Acute Kawasaki disease. Report of the Research Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery (2012 revised version). Pediatr Int. 2014;56:135–58.
Chen S, Dong Y, Kiuchi MG, Wang J, Li R, Ling Z, Zhou T, Wang Z, Martinek M, Pürerfellner H, Liu S, Krucoff MW. Coronary artery complication in Kawasaki Disease and the importance of early intervention: a systematic review and meta-analysis. JAMA Pediatr. 2016;170:1156–63.
Iwashima S, Kimura M, Ishikawa T, Ohzeki T. Importance of C-reactive protein level in predicting non-response to additional intravenous immunoglobulin treatment in children with Kawasaki disease: a retrospective study. Clin Drug Investig. 2011;31:191–9.
Kimura M, Harazaki M, Fukuoka T, Asakura I, Sakai H, Kamimaki T, Ohkawara I, Akiyama N, Tsurui S, Iwashima S, Shimomura M, Morishita H, Meguro T, Seto S. Targeted use of prednisolone with the second IVIG dose for refractory Kawasaki disease. Pediatr Int. 2017;59:397–403.
Burns JC, Mason WH, Hauger SB, Janai H, Bastian JF, Wohrley JD, Balfour I, Shen CA, Michel ED, Shulman ST, Melish ME. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr. 2005;146:662–7.
Son MB, Gauvreau K, Ma L, Baker AL, Sundel RP, Fulton DR, Newburger JW. Treatment of Kawasaki disease: analysis of 27 US pediatric hospitals from 2001 to 2006. Pediatrics. 2009;124:1–8.
Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, Ishii M, Harada K. Revision of diagnostic guidelines for Kawasaki disease. Pediatr Int. 2005;47:232–4.
Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, Matsuishi T. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149:237–40.
Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, Kobayashi T, Morikawa A. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–12.
Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, Kogaki S, Hara J. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166:131–7.
Miyata K, Kaneko T, Morikawa Y, Sakakibara H, Matsushima T, Misawa M, Takahashi T, Nakazawa M, Tamame T, Tsuchihashi T, Yamashita Y, Obonai T, Chiga M, Hori N, Komiyama O, Yamagishi H, Miura M, Post RAISE Group. Efficacy and safety of intravenous immunoglobulin plus prednisolone therapy in patients with Kawasaki disease (Post RAISE): a multicentre, prospective cohort study. Lancet Child Adolesc Health. 2018;2:855–62.
Suzuki H, Terai M, Hamada H, Honda T, Suenaga T, Takeuchi T, Yoshikawa N, Shibuta S, Miyawaki M, Oishi K, Yamaga H, Aoyagi N, Iwahashi S, Miyashita R, Onouchi Y, Sasago K, Suzuki Y, Hata A. Cyclosporin A treatment for Kawasaki disease refractory to initial and additional intravenous immunoglobulin. Pediatr Infect Dis J. 2011;30:871–6.
Research Committee on Kawasaki Disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Tokyo, Japan: Ministry of Health and Welfare; 1984. ((in Japanese)).
Kobayashi T, Fuse S, Sakamoto N, Mikami M, Ogawa S, Hamaoka K, Arakaki Y, Nakamura T, Nagasawa H, Kato T, Jibiki T, Iwashima S, Yamakawa M, Ohkubo T, Shimoyama S, Aso K, Sato S, Saji T, Z Score Project Investigators. A new Z-Score curve of the coronary arterial internal diameter using the lambda-mu-sigma method in a pediatric population. J Am Soc Echocardiogr. 2016;29:794–801.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Wardle AJ, Connolly GM, Seager MJ, Tulloh RM. Corticosteroids for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev. 2017;1:CD011188.
Goto M, Miyagawa N, Kikunaga K, Miura M, Hasegawa Y. High incidence of adrenal suppression in children with Kawasaki disease treated with intravenous immunoglobulin plus prednisolone. Endocr J. 2015;62:145–51.
Zhang L, Priestch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: effects on growth. Cochrane Database Syst Rev. 2014;7:CD009471.
Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991;115:787–96.
Son MB, Gauvreau K, Burns JC, Corinaldesi E, Tremoulet AH, Watson VE, Baker A, Fulton DR, Sundel RP, Newburger JW. Infliximab for intravenous immunoglobulin resistance in Kawasaki disease: a retrospective study. J Pediatr. 2011;158:644–9.
Mori M, Hara T, Kikuchi M, Shimizu H, Miyamoto T, Iwashima S, Oonishi T, Hashimoto K, Kobayashi N, Waki K, Suzuki Y, Otsubo Y, Yamada H, Ishikawa C, Kato T, Fuse S. Infliximab versus intravenous immunoglobulin for refractory Kawasaki disease: a phase 3, randomized, open-label, active-controlled, parallel-group, multicenter trial. Sci Rep. 2018;8:1994.
Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990;56:29–36.
Hirono K, Kemmotsu Y, Wittkowski H, Foell D, Saito K, Ibuki K, Watanabe K, Watanabe S, Uese K, Kanegane H, Origasa H, Ichida F, Roth J, Miyawaki T, Saji T. Infliximab reduces the cytokine-mediated inflammation but does not suppress cellular infiltration of the vessel wall in refractory Kawasaki disease. Pediatr Res. 2009;65:696–701.
Ogihara Y, Ogata S, Nomoto K, Ebato T, Sato K, Kokubo K, Kobayashi H, Ishii M. Transcriptional regulation by infliximab therapy in Kawasaki disease patients with immunoglobulin resistance. Pediatr Res. 2014;76:287–93.
Tremoulet AH, Jain S, Jaggi P, Jimenez-Fernandez S, Pancheri JM, Sun X, Kanegaye JT, Kovalchin JP, Printz BF, Ramilo O, Burns JC. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:1731–8.
Dionne A, Burns JC, Dahdah N, Tremoulet AH, Gauvreau K, de Ferranti SD, Baker AL, Son MB, Gould P, Fournier A, Newburger JW, Friedman KG. Treatment intensification in patients with Kawasaki disease and coronary aneurysm at diagnosis. Pediatrics. 2019;143:e20183341.
Nagatomo Y, Muneuchi J, Nakashima Y, Nanishi E, Shirozu H, Watanabe M, Uike K, Nagata H, Hirata Y, Yamamura K, Takahashi Y, Okada S, Suzuki Y, Hasegawa S, Ohga S. Effective infliximab therapy for the early regression of coronary artery aneurysm in Kawasaki disease. Int J Cardiol. 2018;271:317–21.
Fuse S, Kobayashi T, Arakaki Y, Ogawa S, Katoh H, Sakamoto N, Hamaoka K, Saji T. Standard method for ultrasound imaging of coronary artery in children. Pediatr Int. 2010;52:876–82.
Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, Atz AM, Li JS, Takahashi M, Baker AL, Colan SD, Mitchell PD, Klein GL, Sundel RP. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356:663–75.
Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, Atz AM, Printz BF, Baker A, Vetter VL, Newburger JW, Pediatric Heart Network Investigators. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. 2011;158:831–5.
Jakob A, von Kries R, Horstmann J, Hufnagel M, Stiller B, Berner R, Schachinger E, Meyer K, Obermeier V. Failure to predict high-risk Kawasaki disease patients in a population-based study cohort in Germany. Pediatr Infect Dis J. 2018;37:850–5.
Fabi M, Andreozzi L, Corinaldesi E, Bodnar T, Lami F, Cicero C, Tchana B, Landini C, Sprocati M, Bigucci B, Balsamo C, Sogno Valin P, Di Fazzio G, Iughetti L, Valletta E, Marchetti F, Donti A, Lanari M. Inability of Asian risk scoring systems to predict intravenous immunoglobulin resistance and coronary lesions in Kawasaki disease in an Italian cohort. Eur J Pediatr. 2019;178:315–22.
Arane K, Mendelsohn K, Mimouni M, Mimouni F, Koren Y, Brik Simon D, Bahat H, Hanna Helou M, Mendelson A, Hezkelo N, Glatstein M, Berkun Y, Eisenstein E, Butbul YA, Brik R, Hashkes PJ, Uziel Y, Harel L, Amarilyo G. Japanese scoring systems to predict resistance to intravenous immunoglobulin in Kawasaki disease were unreliable for Caucasian Israeli children. Acta Paediatr. 2018;107:2179–84.
Kim BY, Kim D, Kim YH, Ryoo E, Sun YH, Jeon IS, Jung MJ, Cho HK, Tchah H, Choi DY, Kim NY. Non-responders to intravenous immunoglobulin and coronary artery dilatation in Kawasaki disease: predictive parameters in Korean children. Korean Circ J. 2016;46:542–9.
Son MBF, Gauvreau K, Kim S, Tang A, Dedeoglu F, Fulton DR, Lo MS, Baker AL, Sundel RP, Newburger JW. Predicting coronary artery aneurysms in Kawasaki disease at a North American Center: an assessment of baseline z scores. J Am Heart Assoc. 2017;6:e005378.
Davies S, Sutton N, Blackstock S, Gormley S, Hoggart CJ, Levin M, Herberg JA. Predicting IVIG resistance in UK Kawasaki disease. Arch Dis Child. 2015;100:366–8.
Berdej-Szczot E, Małecka-Tendera E, Gawlik T, Firek-Pędras M, Szydłowski L, Gawlik A. Risk factors of immunoglobulin resistance and coronary complications in children with Kawasaki disease. Kardiol Pol. 2017;75:261–6.
Acknowledgements
We thank the following current and ex-members of the Shizuoka Kawasaki Disease Study Group for their cooperation in carrying out the study. We also thank Takaaki Megro, MD, Shizuoka Children`s Hospital; Tutomu Kamimaki, MD, Shizuoka City Shimizu Hospital; Tomoko Kawasaki, MD, Seirei Numazu Hospital; Toshihiro Tanaka, MD, Shizuoka Kosei Hospital; Ichiro Ohkawara, MD, Shizuoka Red Cross Hospital; Nobutaka Shimizu, MD, Yaizu City Hospital; Kou Asakura, MD, Fujieda Muncipal General Hospital, and Mituaki Kimura, PhD, former chairman of the Shizuoka Kawasaki Disease Study Group for their assistance in the treatment of the patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
All authors made substantial contributions to the conception of the study. All authors read and approved the final manuscript. H.S., S.I., S.S., and M.H. interpreted the data and wrote the paper.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for profit sectors.
Ethics approval
The study was reviewed and approved by the ethics committee of Shizuoka Children’s Hospital (ID: R000014887) and all participating institutions.
Consent to participate
Patients and their guardians provided written informed consent before enrolment or informed consent was obtained as an opt-out and inclusion agreement based on the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. Some institutions posted an explanation of this study on their web homepage. When a subject was not willing to participate, his/her data were excluded from the analysis.
Consent for publication
Not applicable.
Availability of data and material
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Code availability
This study was registered as a clinical trial at UMIN (UMIN000025707).
Rights and permissions
About this article
Cite this article
Sakai, H., Iwashima, S., Sano, S. et al. Targeted Use of Prednisolone with Intravenous Immunoglobulin for Kawasaki Disease. Clin Drug Investig 41, 77–88 (2021). https://doi.org/10.1007/s40261-020-00984-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00984-6