Abstract
Background and Objective
Community-acquired bacterial pneumonia (CABP) affects millions of people each year in the USA. The majority of patients with CABP are treated in the community setting with empirical antimicrobial therapy. Delafloxacin is an anionic fluoroquinolone approved for the treatment of adult patients with CABP. This de novo analysis sought to estimate the budget impact of delafloxacin in the treatment of adult patients with CABP in the outpatient setting from the payer’s perspective.
Methods
A budget impact model (BIM) was developed from the perspective of a US third-party payer to estimate the cost of introducing delafloxacin for the outpatient treatment of CABP over a 1-year time horizon. Population, clinical, and cost inputs were based on the available literature, clinical trial data, and real-world evidence studies. Scenario analyses were conducted to evaluate the potential budget impact among COPD/asthma patients based on the findings from the phase III trial of delafloxacin for CABP, which indicated that patients with COPD or asthma may experience improved effectiveness with delafloxacin compared to moxifloxacin.
Results
In the base-case analysis, with a hypothetical plan of 1,000,000 members, the model estimated that adding delafloxacin to the formulary resulted in a total budget impact of $58,987. This increase was mainly attributed to treatment acquisition costs. In the scenario analysis that was restricted to COPD/asthma patients, adding delafloxacin to the formulary was estimated to result in a total budget impact of $5,042.
Conclusion
The results of the budget impact analyses provide conservative estimates of the impact of adding delafloxacin to outpatient formularies in substitution of moxifloxacin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Total budget impact was primarily driven by an increase in pharmacy costs, with treatment acquisition cost being the main driver of cost increase. Generic antibiotics comprised the largest market share in the comparator model, the costs of which are largely covered by insurance co-pays. |
Compared to the current environment without delafloxacin, a decrease in medical costs was seen in the new environment with delafloxacin. |
The scenario analysis suggests use of delafloxacin in patients with COPD/asthma may minimize its overall budgetary impact while potentially improving outcomes. |
1 Introduction
Community-acquired bacterial pneumonia (CABP) affects millions of people each year in the USA, resulting in substantial morbidity and mortality [1, 2]. While there is considerable focus on the clinical and economic outcomes associated with suspected or documented CABP among hospitalized patients, the majority of patients with mild-to-moderate CABP are treated in the community setting with empirical antimicrobial therapy [3]. According to healthcare utilization estimates published in 2011, suspected or documented CABP accounts for approximately 4.5 million ambulatory visits per year [4]. From 1998 to 2009, approximately 10 million CABP-related antibiotic courses were administered per year in the USA [5]. As the number of at-risk individuals continues to grow with the aging population, the future burden of CABP is expected to be even greater. In addition, the incidence of co-morbid conditions, such as chronic obstructive pulmonary disease (COPD), asthma, congestive heart failure, and diabetes is elevated in older populations and these conditions exacerbate CABP-related morbidity, mortality, and healthcare costs [6, 7].
The recently updated community-acquired pneumonia (CAP) treatment guidelines list a number of preferred antibiotic regimens for outpatient treatment of patients with suspected or documented CAP, depending on the presence of co-morbidities and risk factors for antibiotic-resistant pathogens [8]. While these recommendations were based on best available evidence, many challenges exist with the currently available guideline-concordant outpatient oral antibiotics for patients with CAP. Treatment failure, objectively defined as an antibiotic refill, antibiotic switch, or emergency room (ER)/hospital visit within 30 days of the initial antibiotic prescription, is estimated to occur in one in five patients with suspected or documented CABP who received their care in the outpatient setting [5, 9,10,11]. Among individuals aged ≥ 65 years, studies indicate that the incidence of initial treatment failure is in excess of 25% [5, 11]. There are also growing antibiotic resistance and safety concerns with many of the guideline-recommended outpatient CABP therapies [12,13,14,15,16].
Several antibiotics have recently been approved and these agents greatly expand the treatment options for CABP [17,18,19]. One of the recently approved agents for the treatment of adult patients with CABP is delafloxacin [17]. Delafloxacin is an anionic fluoroquinolone with a broad spectrum that covers Gram-positive, Gram-negative, and atypical species [20]. The minimum inhibitory concentration (MIC) values of delafloxacin are typically four to five dilutions lower relative to other fluoroquinolones against Streptococcus pneumoniae [21]. The structure of delafloxacin confers an increased intracellular penetration and enhanced antibacterial activity in acidic conditions. The increased potency in low pH environments is a potential benefit in CAP patients as the alveolar space is often acidic, especially during the early course of infection [22]. There also appear to be some safety advantages with delafloxacin, specifically no phototoxicity or QT prolongation, relative to other fluoroquinolone antibiotics [23]. In a phase III, randomized, double-blind, comparator-controlled, multicenter global study, delafloxacin was found to be non-inferior to moxifloxacin in the overall population. In pre-specified subgroup analyses, there was a significantly improved early clinical response (ECR) in the delafloxacin arm (93.4%) versus the moxifloxacin group (76.8%) in patients with COPD/asthma [24].
The objective of this de novo analysis was to estimate the budget impact of delafloxacin in the treatment of adult patients with suspected or documented CABP in the outpatient setting from the payer’s perspective. Sensitivity analyses were conducted to assess the impact of key parameters on the model results, and scenario analyses were explored to evaluate the potential budget impact among COPD/asthma patients based on the findings from the phase III trial of delafloxacin for CABP [24].
2 Methods
2.1 Model Description and Structure
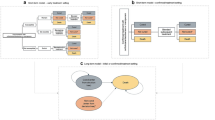
A budget impact model (BIM) was developed from the perspective of a US third-party payer to estimate the cost of introducing delafloxacin for the outpatient treatment of CABP over a 1-year time horizon. An overview of the general model structure is shown in Fig. 1. The model is based on patients with CABP eligible for treatment in an outpatient setting, estimated from a hypothetical population of 1,000,000 members. Since the incidence of members varies by third-party payers, 1,000,000 members was selected as the population in order to facilitate comparison with individual plans. All patients were assumed to be treatment-naïve (i.e., first episode of CABP in a 1-year time horizon and no previous treatment for CABP in 30 days). The target population was stratified by age (18–64 years or ≥ 65 years) and accounted for the presence of COPD or asthma. The budget impact of delafloxacin was calculated by comparing the difference in total costs between the current environment without delafloxacin available for patients with CABP in the outpatient setting and an environment with delafloxacin available for the treatment of adult patients with CABP in the outpatient setting. The model included pharmacy costs and medical costs related to treatment response and non-response.
2.2 Model Inputs and Assumptions
2.2.1 Population
Population parameters and eligible outpatient patient count estimates are shown in Table 1. The base-case scenario assumed a plan size of 1,000,000 members who were assumed to be distributed across private (80% of members) and Medicare (20% of members) coverage. Data from the US Census Bureau were used to estimate the number of members aged 18–64 years and ≥ 65 years across private and Medicare plans [25]. The annual incidence of CABP patients eligible for outpatient treatment (target population) was 0.91% for plan members 18–64 years old (n = 5094) and 1.47% for plan members ≥ 65 years old (n = 3925) [26]. Of the 5,094 patients with CABP between the ages of 18 and 64 years, 1187 (23%) were expected to have underlying COPD or asthma. Of the 3925 patients with CABP aged 65 years and older, 1637 (42%) were expected to have COPD or asthma [5, 11].
2.2.2 Treatment Comparators and Market Share
Comparators (antibiotics, dosing, and treatment duration) selected for the BIM were based on the most recent joint Infectious Diseases Society of America-American Thoracic Society (IDSA-ATS) guidelines for the management of community-acquired pneumonia (Supplemental Table 1) [8]. Comparators included antibiotic monotherapies (omadacycline, lefamulin, levofloxacin, moxifloxacin, doxycycline, amoxicillin and clavulanate potassium extended-release, and azithromycin) and a combination regimen of amoxicillin and azithromycin. Market shares for the comparators for the current environment without delafloxacin are shown in Supplemental Table 2. Market shares in the current and new environments were based on data on file at Melinta. For the environment in which delafloxacin was available, the analysis assumed that delafloxacin gained 1% of total market share from moxifloxacin (i.e., 5–4% total market share) in year 1; other comparators included in the model maintained the same market share across the current and new environment scenarios. We purposefully structured the BIM so that the market share for delafloxacin was gained from moxifloxacin since moxifloxacin was the comparator in its phase III CABP trial [24]. Furthermore, based on the increased safety concerns with fluoroquinolones [12,13,14, 27], we anticipate clinicians will prescribe delafloxacin in patients in whom there is a strong preference to use a fluoroquinolone.
2.3 Clinical Inputs and Assumptions
The model categorized the target CABP population as having a response or non-response to outpatient antibiotic treatment. Treatment non-response was defined as discontinuation of antibiotic therapy either due to lack of efficacy or due to adverse events (AEs). Treatment response was defined as the total number of patients on treatment minus the total number of patients who had therapy discontinued due to lack of efficacy or to AEs [28]. Base-case treatment response rates and treatment discontinuations due to AEs are shown in Table 2. In the base-case scenario, all antibiotic regimens were assumed to have equivalent efficacy based on the ECR endpoint from the pivotal, phase III, non-inferiority randomized clinical trial of delafloxacin versus moxifloxacin [24, 29]. The assumption was that all comparators, including delafloxacin, had equal ECR based on the results of historical non-inferiority clinical trials for all comparators in the model, combined with a lack of systems network meta-analysis that compares CABP treatments [8]. The incidence of AEs leading to discontinuation for antibiotics included in the model were referenced from respective package inserts when discontinuation data were included in the product labelling [17,18,19, 30,31,32,33,34]. For doxycycline hyclate and amoxicillin, the percentage of patients discontinuing therapy due to adverse events was based on randomized clinical trials of the respective agent in patients with CAP [35, 36].
Outcomes associated with treatment non-response and response were derived from a large community commercial healthcare database that examined the outcomes of adult patients with CAP who received their initial care in the outpatient setting (Table 3) [5, 11]. Based on this study, outcomes associated with non-response to treatment included antibiotic retreatment, ER visits, and hospitalizations. The probabilities associated with retreatment, ER visits, and hospitalization were also derived from this real-world evidence study and varied depending on the patient’s age. Patients ≥ 65 years of age experienced a lower probability of antibiotic retreatment (defined as an antibiotic refill or a prescription for a new antibiotic) and ER visits but higher rates of hospitalization compared to patients between the ages of 18 and 64 years.
2.4 Cost Inputs and Assumptions
Only direct medical costs were included in the budget impact model. Direct medical costs included drug costs, drug administration costs, and medical costs related to treatment response or treatment non-response. Drug costs were based on wholesale acquisition costs (WACs) as listed in the RED BOOK 2019 (Supplemental Table 3) [37]. When several generic equivalents were available, the representative National Drug Code (NDC) was based on the lowest-cost option. Provider administration costs based on a 1-h intravenous infusion according to the CMS Physician Fee Schedule (Common Procedural Terminology [CPT] code 96365; $72.80) [38] were included for omadacycline, which requires a parenteral loading dose on the first day of therapy [18]. Branded comparators were assigned a formulary status of tier 2 with a $30 co-payment per prescription. Comparators available as generic products were assigned a formulary status of tier 1 with a $10 co-payment per prescription [39].
Costs of treatment response and non-response are shown in Supplemental Table 4. Treatment response was associated with an additional medical cost of $60.03, as observed in a retrospective analysis of adult patients treated in the outpatient setting for suspected or documented CABP. Non-response to treatment resulted in retreatment of CABP in an outpatient setting, an ER visit, or hospitalization. The cost for each outcome associated with treatment non-response was based on the same retrospective study [5, 11]. Non-medical and indirect costs associated with caregiving and lost productivity, as well as non-antibiotic disease-management costs (including costs associated with underlying COPD or asthma), were not included in the budget impact model. All costs were presented as 2019 US dollars, inflated when appropriate using the medical care services component of the US Consumer Price Index (CPI) (Bureau of Labor Statistics) [25].
2.5 Model Outputs for Base-Case Model
Total costs and aggregated costs by budget category (pharmacy and medical) in the environments with and without delafloxacin were determined. This was calculated as the target population multiplied by anticipated market share of each comparator and then multiplied by the pharmacy and medical costs associated with treatment. The estimated budget impact of delafloxacin (total incremental cost) was based on the difference in total costs between the new and current market environments. Incremental per-treated-member per-month (PTMPM) costs and incremental per-member per-month (PMPM) costs were also calculated.
2.5.1 One-Way Sensitivity Analysis
A one-way sensitivity analysis was conducted on the base-case model to test the uncertainty of the model inputs. For each parameter, the sensitivity analysis tested the drivers of the budget impact results by modifying each input value individually to represent 110% and 90% of its base-case value.
2.6 Scenario Analyses
Two scenario analysis were conducted to evaluate the budgetary impact of differential efficacy of delafloxacin and moxifloxacin in patients with COPD or asthma. The first scenario analysis included all patients in the target population. The second scenario analysis was restricted to only patients with COPD/asthma in the target population. For both scenario analyses, the treatment discontinuation due to lack of efficacy rates inputted for the delafloxacin and moxifloxacin patients with COPD or asthma were obtained from the pivotal phase III CABP trial of delafloxacin compared to moxifloxacin [24]. In this phase III CABP trial, patients with COPD or asthma who received delafloxacin had improved treatment response rates (as measured by ECR) relative to those who received moxifloxacin (93.4% vs. 76.8%, respectively) [24]. Based on the findings from the subgroup analysis of patients with COPD or asthma from the pivotal phase III CABP trial of delafloxacin, treatment discontinuation due to lack of efficacy was modified from 11.1 to 6.6% for delafloxacin and from 11.1 to 23.2% for moxifloxacin for both scenario analyses. Treatment discontinuation due to lack of efficacy for all other comparators remained the same as the base-case input values.
3 Results
In the base-case analysis, with a hypothetical plan of 1,000,000 members, the model estimated that adding delafloxacin to the formulary (90 patients treated with delafloxacin in lieu of moxifloxacin) results in a total budget impact of $58,987 ($0.545 PTMPM; $0.005 PMPM) (Table 4). Total budget impact was primarily driven by an increase in pharmacy costs ($63,461); a decrease in medical costs (− $4474) was observed in the new environment with delafloxacin compared to the current environment without delafloxacin (Fig. 2a and Table 4). The base-case analysis includes 9019 patients with CABP and assumes there is 1% market gain for delafloxacin from moxifloxacin in the new environment. Each subsequent 1% gain in market share for delafloxacin from moxifloxacin in the new environment would result in an additional $58,987 increase in total budget impact. For example, if market share for delafloxacin was increased from 1 to 2%, the total budget impact would be $117,973.
a Estimated total cost in current and new environment scenarios in base case analysis. b Estimated total cost in current and new environment scenarios in scenario analysis 1. c Estimated total cost in current and new environment scenarios in scenario analysis 2. The base case analysis (a) included all CABP patients in the entire target population (n = 9019). Scenario analysis 1 (b) included all CABP patients in the entire target population (n = 9019), but the treatment response rates inputted for the delafloxacin and moxifloxacin patients with COPD or asthma were obtained from pivotal phase III trial of delafloxacin compared to moxifloxacin [24]. Scenario analysis 2 (c) was restricted to the 2824 COPD or asthma patients in the target population. Similar to scenario analysis 1, the treatment response rates inputted for the delafloxacin and moxifloxacin patients with COPD or asthma were obtained from pivotal phase III trial of delafloxacin compared to moxifloxacin [24]. CABP community-acquired bacterial pneumonia, COPD chronic obstructive pulmonary disease
The results of the one-way sensitivity analysis illustrate that the treatment duration of delafloxacin, followed by the cost of delafloxacin and the size of the hypothetical plan population, had the greatest impact on model results (Supplemental Fig. 1). Several other variables were also found to have a similar effect on model results as reflected in the similar model output ranges.
The results of the COPD/asthma scenario analyses are shown in Table 4. The first scenario analysis included all patients in the entire target population (n = 9019), but the treatment response rates inputted for the delafloxacin and moxifloxacin patients with COPD or asthma were obtained the pivotal phase III trial of delafloxacin compared to moxifloxacin. In this COPD/asthma scenario analysis, adding delafloxacin to the formulary was estimated to result in a total budget impact of $45,684 ($0.422 PTMPM; $0.004 PMPM). As in the base-case budget impact analysis, the net budget impact was driven by an increase in pharmacy costs (in the environment where delafloxacin was available for the outpatient treatment of CABP) (Fig. 2b and Table 4). Though medical costs for each market environment were greater in the scenario analysis than in the base-case analysis (driven by a decrease in the probability of treatment response for moxifloxacin), the net increase in total budget impact was smaller than that observed in the base-case budget impact analysis.
The second scenario analysis was restricted to the 2824 COPD/asthma patients in the target population. Like scenario 1 analysis, the treatment response rates inputted for the delafloxacin and moxifloxacin patients with COPD or asthma were obtained from the pivotal phase III trial of delafloxacin compared to moxifloxacin. In this scenario, adding delafloxacin to the formulary (26 patients treated with delafloxacin in lieu of moxifloxacin) was estimated to result in a total budget impact of $5041 ($0.149 PTMPM; $ < 0.000 PMPM) (Fig. 2c and Table 4).
4 Discussion
The purpose of this study was to estimate the budget impact associated with the uptake of delafloxacin for the outpatient treatment of suspected or documented CABP in a 1,000,000-member health plan spanning commercial (80% of members) and Medicare (20% of members) lines of business over a 1-year period. Overall, the base-case analysis indicated that a 1.0% market share gain (90 treated delafloxacin patients) by delafloxacin resulted in a modest increase in the total budget. The total additional cost was estimated to be $58,987, resulting in an additional $0.545 for PTMPM and $0.005 for PMPM. The main cost driver of the budget increase was treatment acquisition cost. In the sensitivity analyses that varied the model inputs by ± 10%, parameters associated with the drug acquisition cost of delafloxacin (i.e., treatment duration, drug cost per day, and patient cost share) were also the ones that most affected the total budget. These findings are not surprising as the comparators included in the model with the largest market share are generic antibiotics and cost nearly nothing to the payer as they are largely covered by the insurance co-pay.
In situations when a drug enters a market place for a clinical syndrome like CABP that is predominately treated with generic drugs, the only way it is possible to negate the observed budget increase due to new drug’s acquisition costs is by offsetting other costs associated with current treatments. In the one-way sensitivity analyses, one of the parameters that most influenced the total budget beyond delafloxacin acquisition costs was the incidence of treatment non-response. It is important to note that the model assumed that all comparators had equal non-treatment response due to lack of efficacy in the base-case analysis. Given the limited number of recent comparative CABP trials, the non-inferiority design of most phase III CABP studies, and the lack of a systems network meta-analysis that compares CABP treatments, we believed it was prudent to assume all comparators had similar efficacy in the base-case analysis [8, 29]. However, one of the noteworthy findings from the delafloxacin versus moxifloxacin phase III study of adult patients with CABP was the ECR observed with delafloxacin (93.4%) versus the moxifloxacin group (76.8%) in the pre-specified COPD/asthma subgroup [24]. In the scenario analysis that was based on the findings from the COPD/asthma subgroup analysis from the phase III study, the estimated budget impact of delafloxacin was ~ 25% less than that observed in the base-case analysis ($45,683 vs. $58,987). It is important to note that the scenario analysis included both COPD/asthma and non-COPD/asthma. When further restricting the analysis to COPD/asthma patients only, the incremental budgetary impact associated with targeted use of delafloxacin COPD/asthma patients was reduced to $5042. Given that the scenario analyses were based on a subgroup analysis from a phase III study, the results should be interpreted with extreme caution and the clinical inputs should be revisited as more phase IV, real-world comparator data become available. However, they reflect the potential value proposition associated with targeted use of delafloxacin in appropriate patient populations where there is a potential clinical benefit.
Several things should be considered when interpreting the findings from this study. This economic model involves a variety of assumptions regarding CABP, treatment patterns, treatment response rates, costs, and market update of delafloxacin. This model represents a simplification of the complex interplay of these factors and is only intended to provide an estimate of the health plan budget impact that may be incurred due to uptake of delafloxacin. Where possible, we used inputs from phase III comparator CABP trials and product labels. The ECR observed in the phase III delafloxacin versus moxifloxacin trial was employed as the input for treatment response rate due to efficacy [36]. The AE discontinuation rates reported in package inserts (PIs) were used as the input for treatment non-response due to AEs [17,18,19, 30,31,32,33,34]. As head-to-head data for all antibiotics used to treat CABP are unavailable, the model assumes that all treatments are equally effective in the base-case scenario. However, rates of discontinuation due to AEs differed across comparators as this input was obtained from each agent’s PI. While AEs leading to discontinuation may not be directly comparable across clinical trials due to subtle differences in trial designs, a systematic review of 16 randomized clinical trials found that fluoroquinolone monotherapy resulted in significantly fewer incidences of treatment discontinuation compared with a beta-lactam/macrolide combination [40]. We believe this finding supports the use of each comparator’s PI for the “AE leading to discontinuation” input as beta-lactam, fluoroquinolones, and macrolides comprised the majority of the market share in the BIM.
While treatment response rates were derived from phase III clinical trials, the associated outcomes with treatment response versus non-response were obtained from a separate data source [5, 11]. It is difficult to rely on healthcare resource utilization-related outcomes associated with treatment response versus non-response in phase III CABP trials. Phase III CABP trials are international and the clinical practices and healthcare reimbursement policies from the represented countries dictate the site of care decisions for study participants. Further mitigating health economic comparisons in most phase III CABP trials are the fixed therapy duration requirements and pre-defined criteria for study follow-up visits and monitoring [41, 42]. Cognizant of this, the subsequent healthcare resource utilization associated with treatment response and non-response were derived from a large community commercial healthcare database that examined the incidence of antibiotic treatment failure and associated outcomes among adult patients with suspected or documented CABP who received their initial care in the outpatient setting (Table 3) [5, 11]. Based on this study, the model included retreatment, ER visits, and hospitalization as clinically relevant outcome parameters after non-response to treatment. We felt the outcomes associated with initial treatment failure reported in this study appropriately reflected real-world clinical practice and expert recommendations for managing non-responding patients. In clinical practice, CABP patients who do not respond to initial therapy due to lack of an ECR or due to intolerability often require another course of antibiotic therapy, usually with another antibiotic regimen. In more severe situations, non-responding CABP patients require intravenous antibiotics and care in the emergency or inpatient setting [8, 29].
A key assumption in the model was that delafloxacin was expected to gain 1.0% share of the total market. Projected market share estimates for delafloxacin were intentionally modest, as is expected for newly approved antimicrobials. Rather than capturing market share from all comparators in the BIM, we purposefully structured the BIM so that the market share was gained from moxifloxacin. Moxifloxacin was the comparator in its phase III CABP trial, thereby making it the logical agent for delafloxacin to gain market share [24]. We felt it was prudent for delafloxacin to gain market share from another fluoroquinolone to clearly highlight its uptake for CABP patients in whom a clinician would want to prescribe a fluoroquinolone. Although the 2019 CAP guidelines still recommend the fluoroquinolones as first-line agents in the outpatient setting among patients with co-morbidities, there is increased recognition of their safety risks. The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have updated the labelling of all fluoroquinolones advising of the serious risk of multiple disabling and potentially irreversible adverse reactions associated with their use. Most recently, the FDA and EMA updated the labelling of all fluoroquinolones to include the increased risk of aortic aneurysm associated with their use and to prescribe fluoroquinolones to patients only when no other treatment options are available [12,13,14, 27]. Despite the increased boxed warning, the CAP guideline “panel believed that fluoroquinolone therapy was justified for adults with co-morbidities and CAP managed in the outpatient setting.” Reasons included the performance of fluoroquinolones in numerous clinical trials, spectrum of activity and low resistance rates in common CAP pathogens, their oral bioavailability and convenience of dosing, and their relative rarity of serious AEs related to their use [8]. The gain of market share exclusively from moxifloxacin also facilitated the scenario analyses in the COPD/asthma subgroup.
We did not factor in a series of costs such as patient satisfaction/quality of life/productivity, risks/complications with use of intravenous medications or hospital readmissions. We did not incorporate mortality into the model. Of note, the study that served as the basis for this outcome associated with treatment non-response found that mortality was greater in the antibiotic failure relative to the non-antibiotic failure group (18.1% vs. 4.6%, respectively) and the differences in 30-day mortality between antibiotic failure groups increased as a function of age [5, 11]. The increased mortality risk associated with treatment failure should be considered when interpreting the findings given the substantial differences in deaths between those that experienced treatment failure versus those that did not. Finally, we did not include patterns of antibiotic resistance in the model since cultures are rarely obtained from patients with CABP in the outpatient setting. We recognize that antibiotic resistance may affect treatment choices and treatment response rate due to efficacy. For now, the results of the BIM analyses should be viewed as conservative estimates of the impact of adding delafloxacin to formularies in substitution of other therapies and the BIM should be considered and assessed as additional data are published on the assumption-based inputs.
In conclusion, there is a vital need for new antibiotics to effectively and safely treat adult patients with CABP in the outpatient setting. The results of the budget impact analysis demonstrate that delafloxacin for the outpatient treatment of CABP may be associated with a modest net budget impact, with a decrease in medical expenditures. Although the results presented here indicate a modest overall impact on the budget, the results of the scenario analyses suggest that targeted use of delafloxacin in patients with COPD/asthma may minimize its overall budgetary impact while potentially improving outcomes. For now, the results of the budget impact analyses should be viewed as conservative estimates of the impact of adding delafloxacin to outpatient formularies in substitution of moxifloxacin. As additional data are published on the assumption-based inputs, the BIM should be considered and assessed.
References
File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122(2):130–41.
National Center for Health Statistics. Pneumonia. Centers for Disease Control and Prevention. https://cdc.gov/nchs/fastats/pneumonia.htm. Published 2017. Accessed 27 Nov 2017.
Llop CJ, Tuttle E, Tillotson GS, LaPlante K, File TM Jr. Antibiotic treatment patterns, costs, and resource utilization among patients with community acquired pneumonia: a US cohort study. Hosp Pract (1995). 2017;45(1):1–8.
Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat 13. 2011;(169):1–38.
Tillotson G, Lodise T, Classi P, Mildvan D, McKinnell JA. Antibiotic treatment failure and associated outcomes among adult patients with community-acquired pneumonia in the outpatient setting: a real-world US Insurance Claims Database Study. Open Forum Infect Dis. 2020;7(3):ofaa065.
Brown JD, Harnett J, Chambers R, Sato R. The relative burden of community-acquired pneumonia hospitalizations in older adults: a retrospective observational study in the United States. BMC Geriatr. 2018;18(1):92.
Wroe PC, Finkelstein JA, Ray GT, et al. Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis. 2012;205(10):1589–92.
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67.
Hess G, Hill JW, Raut MK, et al. Comparative antibiotic failure rates in the treatment of community-acquired pneumonia: results from a claims analysis. Adv Ther. 2010;27(10):743–55.
Ye X, Sikirica V, Schein JR, et al. Treatment failure rates and health care utilization and costs among patients with community-acquired pneumonia treated with levofloxacin or macrolides in an outpatient setting: a retrospective claims database analysis. Clin Ther. 2008;30(2):358–71.
Lodise T, Classi P, Landsman-Blumberg P, Murty S, Tillotson G. Medical and pharmacy costs associated with the treatment of adult patients with community-acquired pneumonia in the outpatient setting. Am J Respir Crit Care Med. 2017;195:A5355.
Antimicrobial Drugs Advisory Committee (AMDAC) and Drug Safety and Risk Management (DSaRM) Advisory Committee. FDA Briefing Information; 2015. https://wayback.archive-it.org/7993/20170405204335/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM472655.pdf. Accessed 30 June 2020.
US Food and Drug Administration. FDA reinforces safety information about serious low blood sugar levels and mental health side effects with fluoroquinolone antibiotics; requires label changes. 2018. www.fda.gov/Drugs/DrugSafety/ucm611032.htm. Accessed 20 Sept 2018.
US Food and Drug Administration. FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients. https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-increased-risk-ruptures-or-tears-aorta-blood-vessel-fluoroquinolone-antibiotics. Accessed 30 June 2020.
Jones RN, Sader HS, Mendes RE, Flamm RK. Update on antimicrobial susceptibility trends among Streptococcus pneumoniae in the United States: report of ceftaroline activity from the SENTRY Antimicrobial Surveillance Program (1998–2011). Diagn Microbiol Infect Dis. 2013;75(1):107–9.
Pfaller MA, Mendes RE, Duncan LR, Flamm RK, Sader HS. In vitro activities of ceftaroline and comparators against Streptococcus pneumoniae isolates from U.S. Hospitals: results from seven years of the AWARE Surveillance Program (2010 to 2016). Antimicrob Agents Chemother. 2018;62(2):e01555-17.
Baxdela (delafloxacin) [package insert]. Lincolnshire: Melinta Therapeutics, Inc; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208610s000,208611s000lbl.pdf.
Nuzyra (omadacycline) [package insert]. Boston: Paratek Pharmaceuticals, Inc.; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209816_209817lbl.pdf.
Xenleta (lefamulin) [package insert]. King of Prussia: Nabriva Therapeutics US, Inc.; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211672s000,211673s000lbl.pdf.
McCurdy S, Keedy K, Lawrence L, et al. Efficacy of delafloxacin versus moxifloxacin against bacterial respiratory pathogens in adults with community-acquired bacterial pneumonia (CABP): microbiology results from the delafloxacin phase 3 CABP trial. Antimicrob Agents Chemother. 2020;64(3):e01949-19.
Pfaller MA, Sader HS, Rhomberg PR, Flamm RK. In vitro activity of delafloxacin against contemporary bacterial pathogens from the United States and Europe, 2014. Antimicrob Agents Chemother. 2017;61(4):e02609-16.
Tulkens PM, Van Bambeke F, Zinner SH. Profile of a novel anionic fluoroquinolone-delafloxacin. Clin Infect Dis. 2019;68(Supplement_3):S213–S222222.
Lodise T, Corey R, Hooper D, Cammarata S. Safety of delafloxacin: focus on adverse events of special interest. Open Forum Infect Dis. 2018;5(10):ofy220.
Salata R, Álvarez-Sala R, Horcajada J, et al. A global phase 3 study of delafloxacin (DLX) compared to moxifloxacin (MOX) in patients with community acquired bacterial pneumonia (CABP). IDweek 2019:POSTER # 2232.
Bureau of Labor Statistics. CPI—all urban consumers. U.S. Medical Care Services. https://data.bls.gov/cgi-bin/surveymost. Accessed 26 Aug 2019.
Park H, Adeyemi AO, Rascati KL. Direct medical costs and utilization of health care services to treat pneumonia in the United States: an analysis of the 2007–2011 medical expenditure panel survey. Clin Ther. 1476e;37(7):1466–1476e1461.
European Medicines Agency. Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics. 2019.
LaPensee K, Mistry R, Lodise T. Budget impact of omadacycline for the treatment of patients with community-acquired bacterial pneumonia in the United States from the hospital perspective. Am Health Drug Benefits. 2019;12(1-Supplement 1):S1–S12.
Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
Avelox (moxifloxacin) [package insert]. Whippany: Bayer HealthCare Pharmaceuticals Inc.; 2019.
Amoxicillin and clavulanate potassium extended release [package insert]. Bridgewater: Dr. Reddy's Laboratories Inc.; 2014.
Levaquin (levofloxacin) [package insert]. Titusville: Janssen Pharmaceuticals, Inc.; 2019.
Zithromax (azithromycin dihydrate) [package insert]. New York: Pfizer, Inc.; 2019.
Vibramycin (doxycycline monohydrate) [package insert]. New York: Pfizer, Inc.; 2019.
Ragnar NS. Atypical pneumonia in the Nordic countries: aetiology and clinical results of a trial comparing fleroxacin and doxycycline. Nordic Atypical Pneumonia Study Group. J Antimicrob Chemother. 1997;39(4):499–508.
Hagberg L, Torres A, van Rensburg D, Leroy B, Rangaraju M, Ruuth E. Efficacy and tolerability of once-daily telithromycin compared with high-dose amoxicillin for treatment of community-acquired pneumonia. Infection. 2002;30(6):378–86.
RED BOOK®. IBM Micromedex®, IBM Watson Health™. Accessed 30 Aug 2019.
Centers for Medicare & Medicaid Services (CMS). Physician Fee Schedule look-up. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed 30 Aug 2019.
Pharmacy Benefit Management Institute (PBMI). 2018. Trends in drug benefit design. https://www.pbmi.com/ItemDetail?iProductCode=BDR_2018&Category=BDR. Accessed 26 Aug 2019.
Raz-Pasteur A, Shasha D, Paul M. Fluoroquinolones or macrolides alone versus combined with beta-lactams for adults with community-acquired pneumonia: systematic review and meta-analysis. Int J Antimicrob Agents. 2015;46(3):242–8.
Lodise TP, Anzueto AR, Weber DJ, et al. Assessment of time to clinical response, a proxy for discharge readiness, among hospitalized patients with community-acquired pneumonia who received either ceftaroline fosamil or ceftriaxone in two phase III FOCUS trials. Antimicrob Agents Chemother. 2015;59(2):1119–26.
U.S. Food and Drug Administration. Community-Acquired Pneumonia-Developing Antimicrobial Drugs for Treatment. Center for Drug Evaluation and Research; 2014.
Acknowledgements
The authors thank N. Theriault for editorial support.
Author information
Authors and Affiliations
Contributions
All authors had a role in study design and conceiving and writing the manuscript. According to the guidelines of the International Committee of Medical Journal Editors (ICMJE, www.icmje.org) all authors met the criteria for authorship and no deserving authors have been omitted.
Corresponding author
Ethics declarations
Funding
Melinta Therapeutics, Inc. provided funding for the research for this article.
Conflict of interest
TL is a consultant and speaker for Melinta Therapeutics. GST is a consultant for Melinta Therapeutics, and has also received consultant fees from Summit Plc, Spero Therapeutics, Shionogi Inc, and KBP Biosciences. JM is a full-time employee of Melinta Therapeutics. AS and DB have no disclosures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lodise, T.P., Tillotson, G.S., Spargo, A. et al. The Role of Delafloxacin in Patients with Community-Acquired Bacterial Pneumonia in the Outpatient Setting: A Budget Impact Model. Clin Drug Investig 40, 961–971 (2020). https://doi.org/10.1007/s40261-020-00938-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00938-y