Abstract
Background
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a class of oral anti-hyperglycemic agents that have been available on the market in Japan since 2014. Although safety information has accumulated alongside the clinical use, the warnings issued by each country based on adverse events associated with the drug are different and examination of the safety of the drug is insufficient.
Objective
This study examined the safety of SGLT2 inhibitors by using a Japanese spontaneous reporting database and focusing on the cautions issued in each country and the disparities within existing research into the occurrence of the adverse events of acute renal failure (ARF), ketoacidosis, and urogenital tract infections (UTIs).
Patients and Methods
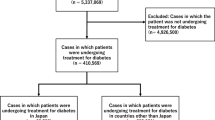
We analyzed data recorded on the Japanese Adverse Drug Event Report database (JADER) between April 2014 and February 2019. We calculated the reporting odds ratio (ROR) and 95% confidence interval (CI) with sex and age as adjustment factors.
Results
JADER contained 366,501 cases with the adverse events of interest; 4322 involved SGLT2 inhibitors. The ROR for SGLT2 inhibitors was calculated as 1.0 (95% CI 0.9–1.2) for ARF, 72.2 (95% CI 59.3–87.8) for ketoacidosis, and 14.0 (95% CI 11.0–17.8) for UTIs. Analysis of only subjects receiving treatment for diabetes showed a similar trend.
Conclusion
The results suggested a correlation between SGLT2 inhibitors and the onset of ketoacidosis and UTIs, but not between SGLT2 inhibitors and ARF. Further verification of the safety of SGLT2 inhibitors, through continued risk assessments and large-scale clinical studies, are necessary.

Similar content being viewed by others
References
FDA. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 15 May 2015. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about. Accessed 19 Dec 2019.
FDA. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). 14 Jun 2016. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin. Accessed 19 Dec 2019.
PMDA. PMDA revises labels of ipragliflozin, luseogliflozin and tofogliflozin. 15 Sep 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150915A001/02.pdf. Accessed 19 Dec 2019.
PMDA. PMDA revises labels dapagliflozin, canagliflozin and empagliflozin. 15 Sep 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150915A001/03.pdf. Accessed 19 Dec 2019.
PMDA. PMDA revises labels ipragliflozin, dapagliflozin, luseogliflozin, canagliflozin, and empagliflozin. 9 Jan 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150109A002/02.pdf. Accessed 19 Dec 2019.
PMDA. PMDA revises a label tofogliflozin. 9 Jan 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150109A002/03.pdf. Accessed 19 Dec 2019.
Committee on the Proper Use of SGLT2 Inhibitors. Recommendations on the proper use of SGLT2 inhibitors. Diabetol Int. 2019;11(1):1–5.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–288.
Donnan JR, Grandy CA, Chibrikov E, et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open. 2019;9:e022577.
Li D, Wang T, Shen S, Fang Z, Dong Y, Tang H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19(3):348–55.
EMA. EudraVigilance. https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance. Accessed 19 Dec 2019.
Government of Canada. Adverse Reaction Database. https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-database/medeffect-canada-caveat-privacy-statement-interpretation-data-search-canada-vigilance-adverse-reaction-online-database.html. Accessed 19 Dec 2019.
Uppsala Monitoring Centre. VigiBase. https://www.who-umc.org/vigibase/vigibase/. Accessed 19 Dec 2019.
PMDA. JADER. (In Japanese). https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0004.html. Accessed 17 Jun 2019.
FDA. FDA Adverse Event Reporting System (FAERS): Latest Quarterly Data Files. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files. Accessed 19 Dec 2019.
Narukawa M. Expectations for pharmacoepidemiology studies on drug risk management. Regul Sci Med Prod. 2016;6:335–43 (in Japanese).
Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis. 2017;27:1108–13.
Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA Adverse Event Reporting System. Diabetologia. 2017;60:1385–9.
Shen J, Yang J, Zhao B. A Survey of the FDA’s Adverse Event Reporting system database concerning urogenital tract infections and sodium glucose cotransporter-2 inhibitor use. Diabetes Ther. 2019;10:1043–50.
Fujita T. Signal detection of adverse drug reactions. Japan J Pharmacoepidemiol/Yakuzai ekigaku. 2009;14:27–36.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Kadowaki T, Nangaku M, Hantel S, Okamura T, von Eynatten M, Wanner C, et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: Results from the EMPA-REG OUTCOME(®) trial. J Diabetes Investig. 2019;10(3):760–70.
Chan JC, Wat NM, So WY, Lam KS, Chua CT, Wong KS, et al. Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes. An Asian perspective from the RENAAL Study. Diabetes Care. 2004;27(4):874–9.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving H-H, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9.
Ado Moumouni AN, Robin P, Hillaire-Buys D, Faillie JL. SGLT-2 inhibitors and ketoacidosis: a disproportionality analysis in the World Health Organization's adverse drug reactions database. Fundam Clin Pharmacol. 2018;32:216–26.
Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849–52.
McGill JB, Subramanian S. Safety of sodium-glucose co-transporter 2 inhibitors. Am J Cardiol. 2019;124:S45–S52.
Yang Y, Chen S, Pan H, Zou Y, Wang B, Wang G, et al. Safety and efficiency of SGLT2 inhibitor combining with insulin in subjects with diabetes: systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96(21):e6944.
Puckrin R, Saltiel MP, Reynier P, Azoulay L, Yu OHY, Filion KB. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018;55(5):503–14.
Cai X, Gao X, Yang W, Chen Y, Zhang S, Zhou L, et al. No disparity of the efficacy and all-cause mortality between Asian and non-Asian type 2 diabetes patients with sodium-glucose cotransporter 2 inhibitors treatment: a meta-analysis. J Diabetes Investig. 2018;9(4):850–61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no specific funding for this study.
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Katsuhara, Y., Ogawa, T. Acute Renal Failure, Ketoacidosis, and Urogenital Tract Infections with SGLT2 Inhibitors: Signal Detection Using a Japanese Spontaneous Reporting Database. Clin Drug Investig 40, 645–652 (2020). https://doi.org/10.1007/s40261-020-00925-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00925-3