Abstract
Background and Objective
Peficitinib, a JAK3-selective inhibitor that blocks the signal transduction and then suppresses immune responses, has been developed for the treatment of patients with moderate to severe active rheumatoid arthritis (RA). We assessed the relative efficacy and safety of once-daily administration of peficitinib (a JAK3-selective inhibitor) 25 mg, 50 mg, 100 mg, and 150 mg in patients with active RA.
Methods
A Bayesian network meta-analysis was conducted to combine direct and indirect evidence from eligible randomized controlled trials (RCTs). The literature search was performed up to May 2019 using MEDLINE, Embase, and the Cochrane Controlled Trials Register.
Results
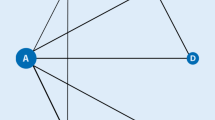
Three RCTs involving 948 patients met the inclusion criteria. There were ten pairwise comparisons, including five direct comparisons and five interventions. The American College of Rheumatology 20% (ACR20) response rate was significantly higher in the peficitinib 150-mg group than in the placebo group (odds ratio (OR): 3.61; 95% credible interval (CrI): 2.35–5.57). Similarly, the ACR20 response rate was significantly higher in the peficitinib 100-mg group than in the placebo group (OR: 2.33, 95% CrI: 1.51–3.56). The peficitinib 50-mg group had a significantly higher ACR20 response rate than the placebo group. However, the ACR20 response rate was not significantly higher in the peficitinib 25-mg group than in the placebo group. The ranking probability based on the surface under the cumulative ranking curve (SUCRA) indicated that peficitinib 150 mg was likely to achieve the best ACR20 response rate (SUCRA = 0.995), followed by peficitinib 100 mg (SUCRA = 0.696), peficitinib 50 mg (SUCRA = 0.558), peficitinib 25 mg (SUCRA = 0.153), and placebo (SUCRA = 0.098). The ACR50 and ACR70 response rates showed a similar distribution pattern to the ACR20 response rate. The difference in the number of patients with adverse events (AEs) among the intervention groups was not statistically significant.
Conclusions
Peficitinib 50, 100, and 150 mg once daily was effective in treating active RA, without causing a significant risk for AEs.






Similar content being viewed by others
References
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–38.
Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–87.
Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–63.
Kubo S, Yamaoka K, Kondo M, Yamagata K, Zhao J, Iwata S, et al. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann Rheum Dis. 2014;73:2192–8.
Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs. 2014;23:1067–77.
Roskoski R Jr. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol Res. 2016;111:784–803.
Saxena R, Mohanty S, Srivastava A, Choudhry VP, Kotwal J. Pathogenetic factors underlying juvenile deep vein thrombosis (DVT) in Indians. Eur J Haematol. 1999;63:26–8.
Chrobak L, Dulicek P. Thrombophilic states. Vnitr Lek. 1998;44:481–6.
Takeuchi T, Tanaka Y, Iwasaki M, Ishikura H, Saeki S, Kaneko Y. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016;75:1057–64.
Smith GA, Uchida K, Weiss A, Taunton J. Essential biphasic role for JAK3 catalytic activity in IL-2 receptor signaling. Nat Chem Biol. 2016;12:373–9.
Genovese MC, Greenwald M, Codding C, Zubrzycka-Sienkiewicz A, Kivitz AJ, Wang A, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932–42.
Kivitz AJ, Gutierrez-Urena SR, Poiley J, Genovese MC, Kristy R, Shay K, et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol. 2017;69:709–19.
Catalá-López F, Tobías A, Cameron C, Moher D, Hutton B. Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int. 2014;34:1489–96.
Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900.
Lee YH, Song GG. Comparative efficacy and safety of secukinumab and adalimumab in patients with active ankylosing spondylitis: a bayesian network meta-analysis of randomized controlled trials. J Rheum Dis. 2017;24:211–9.
Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35:498–502.
Aletaha D, Landewe R, Karonitsch T, Bathon J, Boers M, Bombardier C, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Arthritis Rheum. 2008;59:1371–7.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9 W64.
Brown S, Hutton B, Clifford T, Coyle D, Grima D, Wells G, et al. A Microsoft-Excel-based tool for running and critically appraising network meta-analyses–an overview and application of NetMetaXL. Syst Rev. 2014;3:110.
Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–71.
Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013;33:641–56.
Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98–110.
van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3:285–99.
Lee YH. Association between the neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio and rheumatoid arthritis and their correlations with the disease activity: a meta-analysis. J Rheum Dis. 2018;25:169–78.
Song GG, Lee YH. Causal association between bone mineral density and osteoarthritis: a mendelian randomization study. J Rheum Dis. 2019;26:104–10.
Lee YH, Choi SJ, Ji JD, Song GG. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific grants from any public, commercial, or not-for-profit sector funding agencies.
Conflict of interest
YH Lee and GG Song have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, Y.H., Song, G.G. Comparative Efficacy and Safety of Peficitinib 25, 50, 100, and 150 mg in Patients with Active Rheumatoid Arthritis: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Clin Drug Investig 40, 65–72 (2020). https://doi.org/10.1007/s40261-019-00863-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00863-9