Abstract
Background and Objective
Vancomycin is the most prescribed antibiotic for hospitalized adults with skin and skin structure infections. Vancomycin is associated with acute kidney injury. Iclaprim is an antibiotic under development for the treatment of patients with acute bacterial skin and skin structure infections and is not associated with acute kidney injury. This economic model sought to determine the potential cost saving with iclaprim owing to avoidance of vancomycin-associated acute kidney injury among hospitalized patients with acute bacterial skin and skin structure infections.
Materials and Methods
A hospital cost-minimization model was developed to estimate the overall cost impact of replacing empiric vancomycin with iclaprim among hospitalized adult patients with skin and skin structure infections. The structural model included: vancomycin acquisition; vancomycin assay; incidence of vancomycin-associated acute kidney injury; excess hospital length of stay if acute kidney injury occurred; frequency/cost of specialty physician consults after occurrence of acute kidney injury; and probability/cost of acute dialysis as a result of acute kidney injury. Iclaprim treatment duration was 7 days and iclaprim acquisition cost was varied to determine the upper end of the daily iclaprim price that still conferred cost savings relative to vancomycin. Duration of hospitalization for iclaprim was assumed to be the same as patients with no acute kidney injury.
Results
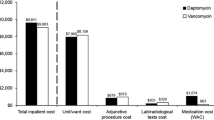
Based on the overall acute kidney injury rate (9.2%), the neutral acquisition price threshold for iclaprim vs. vancomycin was US$1373.47/regimen. Across various subpopulations where acute kidney injury risk ranged between 9.2 and 16.7%, the daily iclaprim acquisition cost that still conferred cost savings was up to US$300/day.
Conclusions
Iclaprim has the potential to reduce the economic burden of acute bacterial skin and skin structure infections in hospitalized patients at risk for vancomycin-associated acute kidney injury when iclaprim acquisition is US$300/day or less.


Similar content being viewed by others
References
Kaye KS, Patel DA, Stephens JM, Khachatryan A, Patel A, Johnson K. Rising United States hospital admissions for acute bacterial skin and skin structure infections: recent trends and economic impact. PLoS One. 2015;10(11):e0143276.
LaPensee K, Fan W. Economic burden of hospitalization with antibiotic treatment for ABSSSI in the US: an analysis of the Premier Hospital Database. In: 17th Annual international meeting of the international society for pharmacoeconomics and outcomes research (ISPOR), Washington DC, 2 June 2012.
Sulham K, LaPensee K, Fan W, Lodise TP. Severity and costs of acute bacterial skin and skin structure infections by treatment setting: an application of the Eron classification to a real-world database. Value Health. 2014;17(3):A282.
Sulham K, Fan W, Werner R. Real-world prescribing patterns for the treatment of acute Bacterial Skin and skin structure infections in the United States: a retrospective database analysis. Value Health. 2015;18(3):A247.
van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734–44.
Jeffres MN. The whole price of vancomycin: toxicities, troughs, and time. Drugs. 2017;77(11):1143–54.
Pritchard L, Baker C, Leggett J, Sehdev P, Brown A, Bayley KB. Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. Am J Med. 2010;123(12):1143–9.
Minejima E, Choi J, Beringer P, Lou M, Tse E, Wong-Beringer A. Applying new diagnostic criteria for acute kidney injury to facilitate early identification of nephrotoxicity in vancomycin-treated patients. Antimicrob Agents Chemother. 2011;55(7):3278–83.
Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52(4):1330–6.
Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49(4):507–14.
Silver SA, Chertow GM. The economic consequences of acute kidney injury. Nephron. 2017;137(4):297–301.
Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–70.
Sincak CA, Schmidt JM. Iclaprim, a novel diaminopyrimidine for the treatment of resistant gram-positive infections. Ann Pharmacother. 2009;43(6):1107–14.
Huang DB, Hawser S, Gemmell CG, Sahm DF. In vitro activity of iclaprim against methicillin-resistant Staphylococcus aureus nonsusceptible to daptomycin, linezolid, or vancomycin: a pilot study. Can J Infect Dis Med Microbiol. 2017;2017:3948626.
Brandt RJ, Henderson BA, Harris SJ, SE Islam, K. Absorption, pharmacokinetics, metabolism, and excretion of iclaprim in healthy humans (A-805). In: Abstracts of the 47th interscience conference on antimicrobial agents and chemotherapy, Chicago (IL), 17 September 2007.
Holland TL, O’Riordan W, McManus A, Shin E, Borghei A, File TM Jr, et al. A phase 3, Randomized, double-blind, multicenter study to EValuate the safety and efficacy of intravenous Iclaprim versus Vancomycin for the trEatment of acute bacterial skin and skin structure infections suspected or confirmed to be due to Gram-positive pathogens: REVIVE-2. Antimicrob Agents Chemother. 2018;62:e02580–7.
Huang DB, O’Riordan W, Overcash JS, Heller B, Amin F, File TM, et al. A phase 3, Randomized, double-blind, multicenter study to EVvaluate the safety and efficacy of intravenous Iclaprim vs Vancomycin for the trEatment of acute bacterial skin and skin structure infections suspected or confirmed to be due to Gram-positive pathogens: REVIVE-1. Clin Infect Dis. 2017;66:1222–9.
Galarraga JE, Mutter R, Pines JM. Costs associated with ambulatory care sensitive conditions across hospital-based settings. Acad Emerg Med. 2015;22(2):172–81.
Vancomycin HCl. Red book online. Micromedex Healthcare Series [database online]. Greenwood Village (CO): Truven Health Analytics; 2018. Available from: www.micromedex.com. Accessed 27 Jan 2018.
Clinical Laboratory Fee Schedule. Centers for Medicare and Medicaid Services. 2016. Available from: https://www.cms.gov/apps/ama/license.asp?file=/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Downloads/17CLAB.zip. Accessed 5 Aug 2018.
Darko W, Medicis JJ, Smith A, Guharoy R, Lehmann DE. Mississippi mud no more: cost-effectiveness of pharmacokinetic dosage adjustment of vancomycin to prevent nephrotoxicity. Pharmacotherapy. 2003;23(5):643–50.
Consumer Price Index inflation calculator. Bureau of Labor Statistics. United States Department of Labor. Available from: https://www.bls.gov/data/inflation_calculator.htm. Accessed 5 Aug 2018.
Sangiovanni R, Stornelli N, Amin R, Huang D, Lodise T, Patel N. Frequency of Vancomycin-Associated Acute Kidney Injury (V-A AKI) and Healthcare Utilization among Veterans’ Affairs patients with skin and skin structure infections (abstract 1287). ePoster presentation at the 28th European Congress of Clinical Microbiology and Infectious Diseases; Madrid; Apr 2018.
Lodise TP, Fan W, Sulham KA. Hospital admission patterns in adult patients with skin and soft tissue infections: identification of potentially avoidable hospital admissions through a retrospective database analysis. Hosp Pract (1995). 2015;43(3):137–43.
Healthcare bluebook. Nashville (TN): CAREOperative LLC; 2018.
Kshirsagar AV, Hogan SL, Mandelkehr L, Falk RJ. Length of stay and costs for hospitalized hemodialysis patients: nephrologists versus internists. J Am Soc Nephrol. 2000;11(8):1526–33.
Alabama Department of Public Health. Reimbursement rate table 2013-2014. Available from: http://www.adph.org/earlydetection/assets/june2013_2014fitwaycptlist.pdf. Accessed 5 Aug 2018.
Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–81.
Cano EL, Haque NZ, Welch VL, Cely CM, Peyrani P, Scerpella EG, et al. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP Database. Clin Ther. 2012;34(1):149–57.
Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29(6):1107–15.
Gomes DM, Smotherman C, Birch A, Dupree L, Della Vecchia BJ, Kraemer DF, et al. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy. 2014;34(7):662–9.
Sangiovanni RJ, Yager J, Carreno JJ, O’Donnell JN, Lowry C, Carlyn C, Patel N. Retreatment with vancomycin and subsequent risk of acute kidney injury (AKI) among Veterans’ Affairs patients who have previously experienced vancomycin-associated AKI (abstract SAT-180). Poster presentation at the American Society of Microbiology Microbe Meeting; New Orleans (LA); 2017.
Langton JM, Reeve R, Srasuebkul P, Haas M, Viney R, Currow D, et al. Health service use and costs in the last 6 months of life in elderly decedents with a history of cancer: a comprehensive analysis from a health payer perspective. Br J Cancer. 2016;114(11):1293–302.
Tanuseputro P, Wodchis WP, Fowler R, Walker P, Bai YQ, Bronskill SE, et al. The health care cost of dying: a population-based retrospective cohort study of the last year of life in Ontario, Canada. PLoS One. 2015;10(3):e0121759.
Tong MC, Wisniewski CS, Wolf B, Bosso JA. Comparison of linezolid and vancomycin for methicillin-resistant Staphylococcus aureus pneumonia: institutional implications. Pharmacotherapy. 2016;36(7):731–9.
Davies SW, Efird JT, Guidry CA, Dietch ZC, Willis RN, Shah PM, et al. Vancomycin-associated nephrotoxicity: the obesity factor. Surg Infect (Larchmt). 2015;16(6):684–93.
Fowler VG Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653–65.
Corrado ML. Integrated safety summary of CANVAS 1 and 2 trials: phase III, randomized, double-blind studies evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother. 2010;65 Suppl. 4:iv67–71.
Wilcox MH, Tack KJ, Bouza E, Herr DL, Ruf BR, Ijzerman MM, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis. 2009;48(2):203–12.
Stryjewski ME, Graham DR, Wilson SE, O’Riordan W, Young D, Lentnek A, et al. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by Gram-positive organisms. Clin Infect Dis. 2008;46(11):1683–93.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Motif BioSciences sponsored work performed by IDRx Solutions LLC to derive the data presented in this article.
Conflict of interest
Nimish Patel and Thomas Lodise have received investigator-initiated research grant support from Motif BioSciences, Inc. David Huang is a paid employee of Motif BioSciences, Inc. Thomas Lodise has received consulting honoraria from Motif BioSciences, Inc.
Rights and permissions
About this article
Cite this article
Patel, N., Huang, D. & Lodise, T. Potential for Cost Saving with Iclaprim Owing to Avoidance of Vancomycin-Associated Acute Kidney Injury in Hospitalized Patients with Acute Bacterial Skin and Skin Structure Infections. Clin Drug Investig 38, 935–943 (2018). https://doi.org/10.1007/s40261-018-0686-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-018-0686-5