Abstract
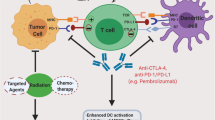
The emergence of immune checkpoint inhibitors (ICIs) has revolutionized the field of oncology. For many cancer types, treatment paradigms have changed, as immunotherapy is increasingly being integrated into frontline standard-of-care treatments and producing meaningful and prolonged responses. This has inspired an avalanche of clinical trials studying ICIs in all types of malignancies, including gynecological cancers. Ovarian and endometrial cancers are characterized by DNA damage repair defects, either via disruption of the homologous recombination DNA repair mechanism in the former or via defects in the mismatch repair (MMR) pathway in the latter, which lead to a high load of neoantigens in both. Cervical cancer is dependent on the expression of human papillomavirus (HPV) proteins, which induce an immune response. Regardless, clinical trials testing ICIs in gynecological malignancies have initially led to disappointing results. Despite durable responses in some patients, overall response rates have been dismal. Nevertheless, in recent years, with the development of better predictive tumor biomarkers, such as microsatellite instability for endometrial cancer and programmed death ligand 1 for cervical cancer, ICIs have found their way into routine treatments for patients with advanced-stage disease. ICI-based combinations, although adding toxicity, have further improved response rates, and new combinations are currently being tested in clinical trials, as are other immunotherapy modalities, such as adoptive cell transfer and HPV-based vaccines. This review summarizes current clinical evidence supporting the use of immunotherapy in gynecological malignancies and describes studies in progress, with a focus on ICIs and predictive response biomarkers.
Similar content being viewed by others
References
Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–86. https://doi.org/10.1158/2159-8290.CD-18-0367.
Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. https://doi.org/10.1056/NEJMoa1003466.
Motzer RJ, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. https://doi.org/10.1056/NEJMoa1510665.
Bellmunt J, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26. https://doi.org/10.1056/NEJMoa1613683.
Socinski MA, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. https://doi.org/10.1056/NEJMoa1716948.
Hodi FS, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–92. https://doi.org/10.1016/S1470-2045(18)30700-9.
Motzer RJ, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90. https://doi.org/10.1056/NEJMoa1712126.
Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. https://doi.org/10.1056/NEJMoa1809615.
Eggermont AMM, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–801. https://doi.org/10.1056/NEJMoa1802357.
Antonia SJ, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–29. https://doi.org/10.1056/NEJMoa1709937.
Oiseth SJ, Aziz MS. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. JCMT. 2017;3(10):250. https://doi.org/10.20517/2394-4722.2017.41.
Ribas A, et al. Tremelimumab (CP-675,206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist. 2007;12(7):873–83. https://doi.org/10.1634/theoncologist.12-7-873.
Perets R, et al. Antitumor activity and safety of MK-1308 (anti-CTLA-4) plus pembrolizumab (pembro) in patients (pts) with non-small cell lung cancer (NSCLC): updated interim results from a phase I study. JCO. 2019;37(15_suppl):2558. https://doi.org/10.1200/JCO.2019.37.15_suppl.2558.
Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–95. https://doi.org/10.1084/jem.20051776.
Horn L, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–9. https://doi.org/10.1056/NEJMoa1809064.
Gandhi L, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005.
Stenger M. First-line atezolizumab plus platinum-based chemotherapy vs chemotherapy alone in advanced urothelial cancer—the ASCO post; 2020. https://ascopost.com/issues/june-25-2020/first-line-atezolizumab-plus-platinum-based-chemotherapy-vs-chemotherapy-alone-in-advanced-urothelial-cancer/. Accessed 13 Sep 2020.
Slater H. Phase III KEYNOTE-361 trial fails to meet primary end points. Cancer Network; 2020. https://www.cancernetwork.com/view/phase-iii-keynote-361-trial-fails-to-meet-primary-end-points. Accessed 13 Sep 2020.
Rini BI, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27. https://doi.org/10.1056/NEJMoa1816714.
Reck M, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. https://doi.org/10.1056/NEJMoa1606774.
Langer CJ, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–508. https://doi.org/10.1016/S1470-2045(16)30498-3.
Mok TSK, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. https://doi.org/10.1016/S0140-6736(18)32409-7.
Paz-Ares L, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39. https://doi.org/10.1016/S0140-6736(19)32222-6.
Schachter J, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853–62. https://doi.org/10.1016/S0140-6736(17)31601-X.
Fuchs CS, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. https://doi.org/10.1001/jamaoncol.2018.0013.
El-Khoueiry AB, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502. https://doi.org/10.1016/S0140-6736(17)31046-2.
O’Neil BH, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS ONE. 2017;12(12):e0189848. https://doi.org/10.1371/journal.pone.0189848.
Antonarakis ES, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. JCO. 2020;38(5):395–405. https://doi.org/10.1200/JCO.19.01638.
Hodi FS, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci. 2008;105(8):3005–10. https://doi.org/10.1073/pnas.0712237105.
Marabelle A, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. JCO. 2020;38(1):1–10. https://doi.org/10.1200/JCO.19.02105.
Liu JF, et al. Safety, clinical activity and biomarker assessments of atezolizumab from a phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol Oncol. 2019a;154(2):314–22. https://doi.org/10.1016/j.ygyno.2019.05.021.
Chung HC, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37(17):1470–8. https://doi.org/10.1200/JCO.18.01265.
Hellmann MD, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–31. https://doi.org/10.1056/NEJMoa1910231.
Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753–8. https://doi.org/10.1158/1078-0432.CCR-18-4070.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. https://doi.org/10.3322/caac.21590.
Bast RC, et al. Critical questions in ovarian cancer research and treatment: report of an American Association for Cancer Research Special Conference. Cancer. 2019;125(12):1963–72. https://doi.org/10.1002/cncr.32004.
Schlienger K, et al. TRANCE- and CD40 ligand-matured dendritic cells reveal MHC class I-restricted T cells specific for autologous tumor in late-stage ovarian cancer patients. Clin Cancer Res. 2003;9(4):1517–27.
Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. https://doi.org/10.1056/NEJMoa020177.
Hwang W-T, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–8. https://doi.org/10.1016/j.ygyno.2011.09.039.
Bachmayr-Heyda A, et al. Prognostic impact of tumor infiltrating CD8+ T cells in association with cell proliferation in ovarian cancer patients—a study of the OVCAD consortium. BMC Cancer. 2013;13:422. https://doi.org/10.1186/1471-2407-13-422.
Adams SF, et al. Intraepithelial T cells and tumor proliferation: impact on the benefit from surgical cytoreduction in advanced serous ovarian cancer. Cancer. 2009;115(13):2891–902. https://doi.org/10.1002/cncr.24317.
Sato E, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–43. https://doi.org/10.1073/pnas.0509182102.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. https://doi.org/10.1038/nature10166.
Strickland KC, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587–98. https://doi.org/10.18632/oncotarget.7277.
Morse CB, et al. Tumor infiltrating lymphocytes and homologous recombination deficiency are independently associated with improved survival in ovarian carcinoma. Gynecol Oncol. 2019;153(2):217–22. https://doi.org/10.1016/j.ygyno.2019.02.011.
Matulonis UA, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080–7. https://doi.org/10.1093/annonc/mdz135.
Hamanishi J, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. JCO. 2015;33(34):4015–22. https://doi.org/10.1200/JCO.2015.62.3397.
Disis ML, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5(3):393. https://doi.org/10.1001/jamaoncol.2018.6258.
Zamarin D, et al. Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an NRG oncology study. JCO. 2020. https://doi.org/10.1200/JCO.19.02059.
Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. https://doi.org/10.1038/nature12477.
Cristescu R, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593. https://doi.org/10.1126/science.aar3593.
Feinberg J, Elvin JA, Bellone S, Santin AD. Identification of ovarian cancer patients for immunotherapy by concurrent assessment of tumor mutation burden (TMB), microsatellite instability (MSI) status, and targetable genomic alterations (GA). Gynecol Oncol. 2018;149:36. https://doi.org/10.1016/j.ygyno.2018.04.081.
Konstantinopoulos PA, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019a;5(8):1141. https://doi.org/10.1001/jamaoncol.2019.1048.
Drew Y, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): Results in germline BRCA-mutated (gBRCA m) platinum-sensitive relapsed (PSR) ovarian cancer (OC). Gynecol Oncol. 2018;149:246–7. https://doi.org/10.1016/j.ygyno.2018.04.555.
Mirza MR, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–64. https://doi.org/10.1056/NEJMoa1611310.
Gelmon KA, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–61. https://doi.org/10.1016/S1470-2045(11)70214-5.
Sandhu SK, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882–92. https://doi.org/10.1016/S1470-2045(13)70240-7.
Home—ClinicalTrials.gov. https://clinicaltrials.gov/. Accessed 27 Apr 2020.
Liu JF, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a phase 2 clinical trial. JAMA Oncol. 2019b. https://doi.org/10.1001/jamaoncol.2019.3343.
Martin-Lluesma S, Graciotti M, Grimm AJ, Boudousquié C, Chiang CL, Kandalaft LE. Are dendritic cells the most appropriate therapeutic vaccine for patients with ovarian cancer? Curr Opin Biotechnol. 2020;65:190–6. https://doi.org/10.1016/j.copbio.2020.03.003.
Phase II study of ipilimumab monotherapy in recurrent platinum-sensitive ovarian cancer—study results—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT01611558. Accessed 25 Apr 2020.
Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. https://doi.org/10.1016/S0140-6736(07)61416-0.
Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–50. https://doi.org/10.1038/nrc798.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Borysiewicz LK, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347(9014):1523–7. https://doi.org/10.1016/s0140-6736(96)90674-1.
Sharma RK, et al. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4–1BB ligand eradicates established tumors. Cancer Res. 2009;69(10):4319–26. https://doi.org/10.1158/0008-5472.CAN-08-3141.
Alvarez RD, et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol Oncol. 2016;140(2):245–52. https://doi.org/10.1016/j.ygyno.2015.11.026.
Basu P, et al. A randomized phase 2 study of ADXS11-001 listeria monocytogenes–listeriolysin O immunotherapy with or without cisplatin in treatment of advanced cervical cancer. Int J Gynecol Cancer. 2018;28(4):764–72. https://doi.org/10.1097/IGC.0000000000001235.
Massarelli E, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(1):67–73. https://doi.org/10.1001/jamaoncol.2018.4051.
Frenel J-S, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35(36):4035–41. https://doi.org/10.1200/JCO.2017.74.5471.
Naumann RW, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II CheckMate 358 trial. JCO. 2019a;37(31):2825–34. https://doi.org/10.1200/JCO.19.00739.
Santin AD, et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol Oncol. 2020;157(1):161–6. https://doi.org/10.1016/j.ygyno.2019.12.034.
Migden MR, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–51. https://doi.org/10.1056/NEJMoa1805131.
Papadopoulos KP, et al. First-in-human study of cemiplimab alone or in combination with radiotherapy and/or low-dose cyclophosphamide in patients with advanced malignancies. Clin Cancer Res. 2020;26(5):1025–33. https://doi.org/10.1158/1078-0432.CCR-19-2609.
Naumann RW, et al. Efficacy and safety of nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: results from CheckMate 358. Ann Oncol. 2019b;30:v898–9. https://doi.org/10.1093/annonc/mdz394.059.
Stevanović S, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res. 2019;25(5):1486–93. https://doi.org/10.1158/1078-0432.CCR-18-2722.
Lheureux S, et al. Association of ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus-related cervical carcinoma. JAMA Oncol. 2018;4(7):e173776. https://doi.org/10.1001/jamaoncol.2017.3776.
Charo LM, Plaxe SC. Recent advances in endometrial cancer: a review of key clinical trials from 2015 to 2019. F1000Res. 2019;8:849. https://doi.org/10.12688/f1000research.17408.1.
Suarez AA, Felix AS, Cohn DE. Bokhman Redux: endometrial cancer ‘types’ in the 21st century. Gynecol Oncol. 2017;144(2):243–9. https://doi.org/10.1016/j.ygyno.2016.12.010.
Cancer Genome Atlas Research Network, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. https://doi.org/10.1038/nature12113.
Rayner E, et al. A panoply of errors: polymerase proofreading domain mutations in cancer. Nat Rev Cancer. 2016;16(2):71–81. https://doi.org/10.1038/nrc.2015.12.
Wang F, et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol. 2019. https://doi.org/10.1001/jamaoncol.2019.2963.
Prendergast EN, et al. Comprehensive genomic profiling of recurrent endometrial cancer: implications for selection of systemic therapy. Gynecol Oncol. 2019;154(3):461–6. https://doi.org/10.1016/j.ygyno.2019.06.016.
Soumerai TE, et al. Clinical utility of prospective molecular characterization in advanced endometrial cancer. Clin Cancer Res. 2018;24(23):5939–47. https://doi.org/10.1158/1078-0432.CCR-18-0412.
Oaknin A, et al. Preliminary safety, efficacy, and pharmacokinetic/pharmacodynamic characterization from GARNET, a phase I/II clinical trial of the anti-PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-h and MSS endometrial cancer. Gynecol Oncol. 2019;154:17. https://doi.org/10.1016/j.ygyno.2019.04.044.
Hasegawa K, et al. “Efficacy and safety of nivolumab (Nivo) in patients (pts) with advanced or recurrent uterine cervical or corpus cancers. JCO. 2018;36(15_suppl):5594–5594. https://doi.org/10.1200/JCO.2018.36.15_suppl.5594.
Konstantinopoulos PA, et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol. 2019b;37(30):2786–94. https://doi.org/10.1200/JCO.19.01021.
Antill YC, et al. “Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: the phase II PHAEDRA trial (ANZGOG1601). JCO. 2019;37(15_suppl):5501–5501. https://doi.org/10.1200/JCO.2019.37.15_suppl.5501.
Ott PA, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol. 2017a;35(22):2535–41. https://doi.org/10.1200/JCO.2017.72.5952.
Makker V, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):711–8. https://doi.org/10.1016/S1470-2045(19)30020-8.
Makker V, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020. https://doi.org/10.1200/JCO.19.02627.
Fleming GF, et al. Clinical activity, safety and biomarker results from a phase Ia study of atezolizumab (atezo) in advanced/recurrent endometrial cancer (rEC). JCO. 2017;35(15_suppl):5585–5585. https://doi.org/10.1200/JCO.2017.35.15_suppl.5585.
Ott PA, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1–positive endometrial cancer: results from the KEYNOTE-028 study. JCO. 2017b;35(22):2535–41. https://doi.org/10.1200/JCO.2017.72.5952.
Fader AN, et al. Preliminary results of a phase II study: PD-1 blockade in mismatch repair–deficient, recurrent or persistent endometrial cancer. Gynecol Oncol. 2016;141:206–7. https://doi.org/10.1016/j.ygyno.2016.04.532.
Mollica V, et al. Immunotherapy and radiation therapy in renal cell carcinoma. CDT. 2020. https://doi.org/10.2174/1389450121666200311121540.
Fife K, Bang A. Combined radiotherapy and new systemic therapies—have we moved beyond palliation? Clin Oncol. 2020. https://doi.org/10.1016/j.clon.2020.07.021.
Neijt JP, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. JCO. 2000;18(17):3084–92. https://doi.org/10.1200/JCO.2000.18.17.3084.
Pectasides D, et al. Carboplatin and paclitaxel in advanced or metastatic endometrial cancer. Gynecol Oncol. 2008;109(2):250–4. https://doi.org/10.1016/j.ygyno.2008.01.028.
Miller D, et al. Late-breaking abstract 1: randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a gynecologic oncology group study. Gynecol Oncol. 2012;125(3):771. https://doi.org/10.1016/j.ygyno.2012.03.034.
Haanen JBAG, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–42. https://doi.org/10.1093/annonc/mdx225.
Acknowledgements
The authors thank Steve Spencer for English editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This manuscript was funded by the women’s health grant at Rambam.
Conflict of interest
AR served on an advisory board for MSD, the manufacturer of pembrolizumab, and has no other conflicts of interest. TT, AA, and RP have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
TT and RP wrote the first draft of the manuscript. All of the authors participated in the conceptual design and writing of the manuscript, as well as critical revisions of important intellectual content, while RP supervised the writing of the manuscript.
Rights and permissions
About this article
Cite this article
Taha, T., Reiss, A., Amit, A. et al. Checkpoint Inhibitors in Gynecological Malignancies: Are we There Yet?. BioDrugs 34, 749–762 (2020). https://doi.org/10.1007/s40259-020-00450-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-020-00450-x